Abstract
副黏病毒是一类重要的呼吸道病原体,在儿童感染性疾病中具有重要临床意义。其感染过程中可诱导多种形式的程序性细胞死亡(PCD),这些PCD形式在病毒复制、传播及宿主免疫应答中发挥关键作用,显著影响病毒的生命周期及疾病进展。一方面,PCD通过清除被感染细胞有助于限制病毒扩散并激活宿主免疫应答,从而增强抗病毒防御能力;另一方面,过度或异常的细胞死亡可能导致组织损伤和免疫紊乱,为病毒复制提供有利环境,进而加重病情。如细胞凋亡通过外源性与内源性通路参与感染控制,同时亦可能被病毒操控以增强其扩散能力;细胞焦亡依赖炎症小体激活,诱导裂解性死亡并释放炎症因子;坏死性凋亡通过RIPK1-RIPK3-MLKL通路介导,与细胞焦亡一样在提升先天免疫的同时可能引发炎症失衡;免疫原性细胞死亡通过释放损伤相关分子模式和新抗原激活特异性免疫反应,在抗病毒及抗肿瘤治疗中具有潜在价值;铁死亡通过调控铁代谢与相关转运蛋白参与病毒复制及细胞清除。本文综述副黏病毒感染中细胞凋亡、焦亡、坏死性凋亡、免疫原性细胞死亡及铁死亡的作用,旨在为深入理解副黏病毒的致病过程及抗病毒新策略的开发提供研究思路。
Keywords: 副黏病毒, 程序性细胞死亡, 致病性, 免疫病理学, 感染性疾病, 综述
Abstract
Paramyxoviruses are important respiratory pathogens with substantial clinical relevance in pediatric infectious diseases. During infection, multiple forms of programmed cell death (PCD) may be induced, and this plays pivotal roles in viral replication, dissemination, and host immune responses, thereby profoundly influencing the viral life cycle and disease progression. On one hand, PCD facilitates the clearance of infected cells, restricts viral spread, and activates host immune defenses, thereby enhancing antiviral immunity. On the other hand, excessive or dysregulated cell death may lead to tissue damage and immune imbalance, creating a microenvironment conducive to viral replication and exacerbating disease severity. For instance, apoptosis—mediated by both extrinsic and intrinsic pathways—contributes to infection control but may also be hijacked by viruses to promote dissemination. Pyroptosis, driven by inflammasome activation, triggers lytic cell death and the release of pro-inflammatory cytokines. Necroptosis, mediated by the RIPK1-RIPK3-MLKL signaling axis, and pyroptosis both amplify innate immune responses but may concurrently induce inflammatory dysregulation. Immunogenic cell death (ICD), characterized by the release of damage-associated molecular patterns and neoantigens, activates antigen-specific immune responses and holds therapeutic potential for antiviral and antitumor interventions. Emerging evidence suggests that ferroptosis, through the modulation of iron metabolism and associated transporters, may also participate in viral replication and infected cell clearance. This review comprehensively summarizes the roles of apoptosis, pyroptosis, necroptosis, ICD, and ferroptosis in paramyxovirus infection, aiming to deepen the understanding of paramyxovirus pathogenesis and to provide insights for developing novel antiviral strategies.
Keywords: Paramyxovirus, Programmed cell death, Pathogenesis, Immunopathology, Infectious diseases, Review
副黏病毒是一类不分节段的单负链RNA病毒[1]。常见人类易感的副黏病毒主要包括副流感病毒、麻疹病毒、RSV、腮腺炎病毒和人偏肺病毒等[2]。副黏病毒感染引发的相关疾病在全球范围内呈现高发态势,在儿童群体中的影响尤为显著[3]。从感染谱来看,副黏病毒在儿童呼吸系统感染中最为常见[4-5]。其中,RSV是儿童呼吸道感染的主要病原体之一,可引起轻微感冒乃至严重肺炎等一系列症状[6]。副流感病毒和人偏肺病毒也能引起儿童急性呼吸道感染。麻疹病毒通常引发皮疹和高热,严重时可导致诸如脑炎或亚急性硬化性全脑炎等并发症[7]。腮腺炎病毒感染主要表现为腮腺肿大,部分严重病例则会影响患者生殖系统和神经系统[8]。副黏病毒感染引发的疾病尚无特效治疗手段,尽管麻疹病毒疫苗和腮腺炎病毒疫苗在全球范围内被广泛使用,并取得了一定成效,但仍存在地区覆盖率不均、疫苗有效性随时间下降等问题[2, 8-9]。
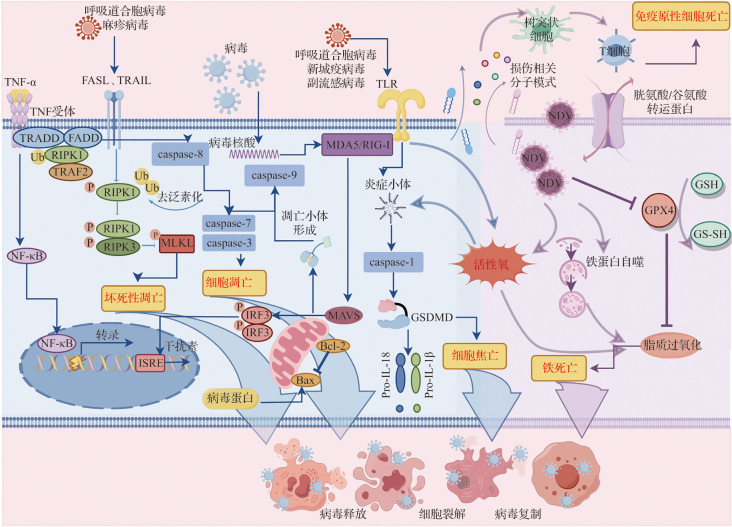
研究表明,副黏病毒感染能够诱导宿主发生多种PCD类型,如图1所示[10-13]。在副黏病毒感染过程中,PCD不仅是宿主细胞对抗病毒的一种防御机制,也可能成为病毒传播和致病性的促进因素[13-14]。一方面,PCD能够通过消灭被感染细胞来防止病毒的复制和传播,并激活机体免疫反应,从而建立并维持宿主抗病毒免疫防御;另一方面,PCD也可能会引起细胞不受控制的损伤和免疫反应紊乱,进而促进病毒传播并导致组织损伤[15-16]。PCD作为宿主抗病毒感染先天免疫反应的关键组成部分,对维护宿主的免疫平衡和生存至关重要。表1列出了副黏病毒在不同宿主细胞中诱导的PCD,可见副黏病毒感染具有普遍诱导宿主PCD的能力[17-91]。因此,深入探讨副黏病毒诱导PCD的机制,有助于理解病毒的致病机制,对于指导临床治疗具有重要意义。本文通过揭示PCD在病毒生命周期中的复杂角色,以期更好地理解病毒复制、传播以及宿主免疫应答之间的相互作用,这对于开发新的抗病毒策略、优化疫苗设计以及制订针对性的免疫治疗方案具有重要的指导价值。
图1. 副黏病毒感染诱导的程序性细胞死亡类型.
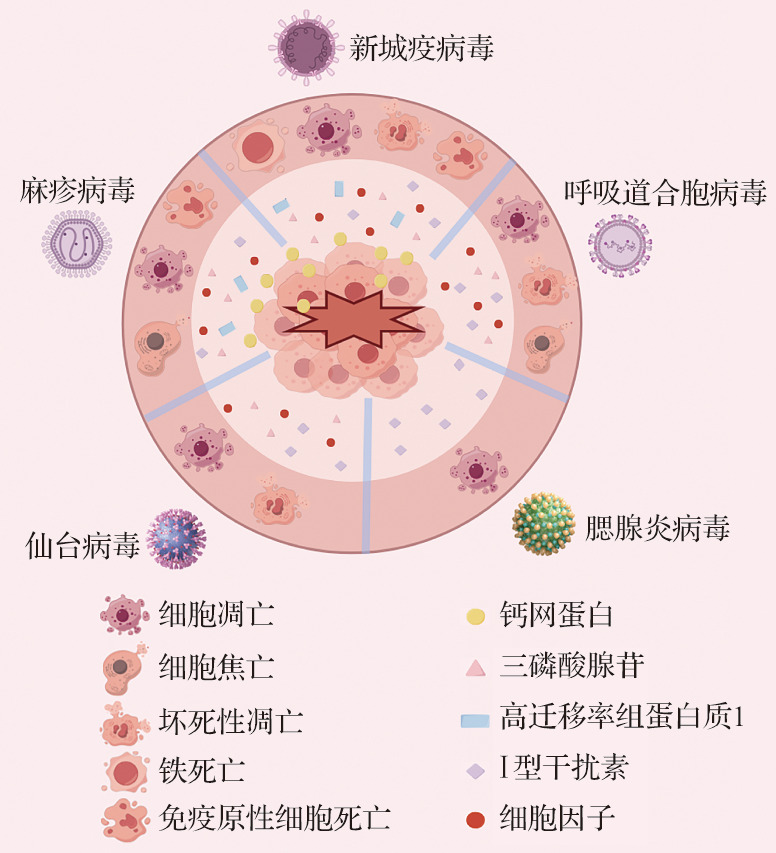
新城疫病毒、呼吸道合胞病毒、腮腺炎病毒、仙台病毒及麻疹病毒等副黏病毒均可诱导活性氧生成,参与细胞凋亡、细胞焦亡、坏死性凋亡及铁死亡等程序性细胞死亡,促使细胞释放钙网蛋白、三磷酸腺苷、高迁移率组蛋白质1、Ⅰ型干扰素及多种细胞因子等关键分子,在调控免疫应答与病毒清除中发挥重要作用.
表1.
副黏病毒在不同宿主细胞中诱导的程序性细胞死亡方式
| 死亡方式 | 病毒类型 | 感染细胞 | 参考文献 |
|---|---|---|---|
| 细胞凋亡 | 麻疹病毒 | HEK-293;宫颈癌(HeLa);乳腺癌(MCF7、MDA-MB-231、SKOV-3);肝癌(HepG2、Hep3B、HuH-6、HuH-7);黑色素瘤(HT144);结直肠癌(HT-29、HCT-116、LS-174T);肺癌(A549、H838、H520);胃癌(BGC-823、SGC-7901);胶质母细胞瘤(U-87 MG、U-251 MG);脑内皮细胞;胰腺癌(PANC-1、MIA-PaCa2);食管鳞状细胞癌(KYSE-30、KYSE-150);肾母细胞瘤(G401、SK-NEP-1);树突状细胞;旁观T细胞;单个核细胞;非洲绿猴肾细胞;THP-1;MAIT | [17-36] |
| 仙台病毒 | HEK-293;宫颈癌(HeLa);前列腺癌(PC3);乳腺癌(MCF7、MDA-MB-231);肝癌(HepG2、Hep3B);肺癌(A549、NCI-H44);胃癌(MG-63);骨肉瘤(HOS);小鼠胚胎成纤维细胞;结直肠癌(CT26、HCT116);非洲绿猴肾细胞;小鼠黑色素瘤(B16-F10);胶质母细胞瘤(U-251 MG)、单个核细胞 | [37-51] | |
| 腮腺炎病毒 | 白血病细胞(U937);生殖细胞;颗粒细胞;神经元;结直肠癌(SW480) | [52-56] | |
| 呼吸道合胞病毒 | 上皮细胞(ACE、A549);小鼠神经母细胞瘤(N2a);神经母细胞瘤(SH-SY5Y);喉癌(Hep-2);肝癌(HuH-7);前列腺癌(PC3);淋巴细胞(CD4+T细胞、CD8+T细胞);巨噬细胞;中性粒细胞 | [57-64] | |
| 新城疫病毒 | 宫颈癌(HeLa);乳腺癌(MCF7、AMJ13);前列腺癌(PC3);肺癌(A549、H460、H1299);神经母细胞瘤(SK-NEP-1);胃癌(SGC-7901、HGC);肝癌(HepG2、HuH-7、Hep3B、SMMC7721);胶质母细胞瘤(U-87 MG、U-251 MG);结直肠癌(CaCo2、HT29);鳞癌(Cal27、HN13);肠腺癌(HuTu80);小鼠白血病细胞;小鼠胶质瘤(GL261);鸡胚成纤维细胞;小鼠胚胎成纤维细胞;单个核细胞;WEHI-3B;CT-16;TC-1 | [65-82] | |
| 细胞焦亡 | 麻疹病毒 | 食管鳞状细胞癌(KYSE-30、KYSE-150) | [30] |
| 呼吸道合胞病毒 | 巨噬细胞 | [83] | |
| 坏死性凋亡 | 仙台病毒 | 小鼠成纤维细胞(L929) | [84] |
| 呼吸道合胞病毒 | 巨噬细胞、上皮细胞 | [57, 83] | |
| 新城疫病毒 | 宫颈癌(HeLa) | [85] | |
| 免疫原性细胞死亡 | 麻疹病毒 | 黑色素瘤(Mel888、Mel624、MeWO、SKMel28);肾母细胞瘤(G401、SK-NEP-1) | [31, 86] |
| 新城疫病毒 | 肺癌(A549、H460);黑色素瘤(A-375、C8161);胶质瘤(GL261);前列腺癌(PC3);胶质母细胞瘤(LN229) | [79, 87-89] | |
| 铁死亡 | 新城疫病毒 | 胶质母细胞瘤(U-251 MG);宫颈癌(HeLa) | [90-91] |
1. 副黏病毒感染后细胞凋亡的作用
副黏病毒感染宿主后,细胞凋亡的激活是宿主对抗病毒复制和扩散的一种早期反应机制。已有大量细胞培养和动物感染模型表明,细胞凋亡在机体抗病毒机制中发挥重要作用[92-94]。而在某些情况下,细胞凋亡可促进病毒从被感染细胞中释放,进而扩散至相邻细胞并引发更为广泛的病毒感染[95]。因此,细胞凋亡在病毒感染过程中具有双面性,既构成宿主防御的一部分,也可被病毒利用以促进其复制和传播[15]。深入探究细胞凋亡在病毒感染中的角色对于开发新的治疗策略和疫苗具有重大意义。
副黏病毒诱导细胞凋亡的机制涉及多个方面,包括病毒核酸和蛋白与宿主细胞的相互作用、激活细胞内信号传导途径,以及引发细胞应激反应等[14, 17, 40, 45]。外源性凋亡在病毒感染诱导细胞死亡信号表达增加后启动,从而可能扩大病毒的感染范围[41, 63]。这种机制有助于病毒逃避宿主的免疫监视,增加病毒的传播效率。研究显示,麻疹病毒感染脑内皮细胞后诱导TRAIL表达,激活外源性凋亡途径,随后导致细胞单层结构破坏和广泛的细胞死亡[96]。值得注意的是,研究者还发现即使只有少量脑内皮细胞被麻疹病毒感染,病毒也能高效复制并造成大面积感染,且这种现象在阻断病毒诱导的外源性凋亡后消失[96]。这表明外源性凋亡在促进病毒感染扩散方面发挥作用。此外,研究显示,RSV感染相关呼吸衰竭患者的支气管肺泡灌洗液中白细胞释放的可溶性TRAIL水平升高[59]。进一步实验表明,在体外培养的无肺部疾病儿童的原代支气管上皮细胞中,表达死亡受体TRAIL-R1和TRAIL-R2的细胞可被可溶性TRAIL诱导凋亡,且该过程可被TRAIL信号通路抑制剂显著抑制[59]。上述表明,TRAIL促凋亡途径在RSV感染导致的肺上皮细胞损伤中发挥重要作用。此外,副黏病毒感染同样可通过外源性凋亡途径诱导免疫细胞发生凋亡继而扩大感染[63-64]。如麻疹病毒可以诱导树突状细胞、旁观T细胞和单个核细胞等免疫细胞发生外源性凋亡,引起免疫细胞耗竭或功能障碍,免疫记忆受损,导致病毒感染进一步扩大[34, 97]。
内源性凋亡主要是宿主细胞的一种防御机制,通过对被感染细胞的快速清除从而限制病原体的复制和扩散[13]。线粒体作为细胞凋亡的调控中心,当副黏病毒感染细胞后,其基质蛋白(M)中BH3与细胞内的Bax相互作用,导致Bax的构象变化从而使线粒体外膜通透性增加,随后诱导caspase依赖性细胞凋亡[55, 67]。此外,病毒感染还会引发DNA损伤、活性氧应激以及干扰素信号通路下游的P53转录激活,这些信号通路协同参与诱导内源性细胞凋亡,细胞以“牺牲自身”的形式阻止病毒的进一步传播,在一定程度上控制病毒的扩散[13]。例如,有研究发现麻疹病毒感染后能够引发儿童的亚急性硬化性全脑炎,这是由于神经细胞在感染麻疹病毒后可识别病毒入侵并启动内源性凋亡程序,从而限制病毒在中枢神经系统中的进一步传播,在一定程度上减缓疾病进展[98]。
总体而言,副黏病毒感染诱发的凋亡是一个涉及宿主细胞和病毒之间精细调控的过程,既有病毒利用宿主机制扩大感染的不利,也有宿主细胞通过凋亡限制病毒传播的防御策略。理解这种复杂的相互作用有助于根据病毒的致病机制开发新的抗病毒策略。
2. 副黏病毒感染后细胞焦亡的作用
细胞焦亡不同于细胞凋亡,其主要特征在于细胞膜的溶解破裂,并伴随炎症因子的释放,为典型裂解型细胞死亡方式[99]。细胞焦亡在机体的天然免疫反应中扮演重要角色,尤其是在对抗感染方面。
大量研究表明,当病毒入侵细胞后,宿主细胞内模式识别受体识别病原体相关分子模式、损伤相关分子模式或病毒核酸,诱导炎症小体的激活并驱动细胞焦亡[100]。目前已知多种炎症小体可被病毒诱导激活,主要包括NOD样受体家族、AIM2炎症小体以及γ干扰素诱导蛋白-16炎症小体[14]。研究显示,副流感病毒、RSV、新城疫病毒和人偏肺病毒均会诱导NLRP3炎症小体的激活,介导caspase-1依赖性焦亡[11, 83, 101-102]。细胞膜上的孔洞使得细胞膜完整性丧失和细胞内容物外流,从而限制病毒的复制和传播。此外,这一过程还伴随炎症因子的释放,激活宿主先天免疫反应,募集免疫细胞清除被感染的细胞和病毒。然而,过度的细胞焦亡可能会导致细胞因子风暴,引发促炎症级联效应从而加剧组织损伤[103]。以巨噬细胞为例,RSV感染后可激活巨噬细胞中的Toll样受体2,并在感染细胞中触发活性氧,介导NLRP3炎症小体依赖性焦亡,其产生的细胞碎片通过积累可导致小气道的物理性阻塞,继而引起典型的毛细支气管炎病变;此外,该过程释放的细胞因子和趋化因子还参与了RSV诱导肺炎的疾病进展[83]。研究显示,RSV还可通过上调CMPK2激活NLRP3炎症小体并释放大量IL-1β和IL-18,导致肺上皮细胞大量死亡,从而诱导更为严重的病毒性肺炎,而靶向CMPK2的药物可显著抑制RSV介导的细胞焦亡,进而控制疾病进展[104]。因此,适度的细胞焦亡可激活宿主先天免疫反应,快速清除被感染细胞并限制病毒的进一步扩散;而失衡的细胞焦亡则会导致免疫反应紊乱,促进病毒传播,从而加剧病毒性疾病的严重程度。
3. 副黏病毒感染后坏死性凋亡的作用
坏死性凋亡是一种受程序性调控的细胞自我破坏形式,常在细胞凋亡通路受阻的情况下被诱导激活。坏死性凋亡具有显著的免疫原性,是宿主抵御病原体入侵的重要防御机制之一[105]。研究发现,小鼠纤维肉瘤细胞系L929在感染仙台病毒SeV后,其转录翻译的蛋白Y1和Y2通过抑制RIPK1泛素化,激活RIG-I诱导坏死性凋亡,促使被感染细胞快速死亡从而限制病毒扩散[84]。同时,RIPK3敲低细胞中病毒滴度升高,可见坏死性凋亡在限制病毒载量方面发挥重要作用[84]。此外,与野生型小鼠比较,RIPK3缺陷小鼠感染仙台病毒后,其病毒特异性CD8+ T细胞浸润增多且病理性改变增加,进一步验证了坏死性凋亡对于病毒清除的重要性[84]。
与细胞焦亡类似,坏死性凋亡也是一种炎症性和裂解型细胞死亡方式,可释放损伤相关分子模式,这些损伤相关分子模式可以激活免疫系统,促进炎症反应,有助于宿主抵抗病毒感染[14]。然而,过度的坏死性凋亡也会加速病毒扩散并导致组织损伤和疾病。研究显示,RSV感染小鼠气道上皮细胞后可显著上调RIPK1和MLKL表达,并诱导HMGB1释放、中性粒细胞炎性浸润和气道上皮细胞坏死性凋亡和脱落;而抑制坏死性凋亡能够减轻小鼠模型中的细支气管炎症状,降低病毒载量,并防止了2型炎症和气道重塑,从而可能减缓后期由病毒或过敏原引起的哮喘进展[57]。可见靶向坏死性凋亡途径可能是一种有效的治疗策略。
4. 副黏病毒感染后免疫原性细胞死亡的作用
免疫原性细胞死亡是指在死亡细胞抗原激活机体免疫系统后所介导的肿瘤细胞或病原体死亡,是一种特殊的调节性细胞死亡[106]。在病毒感染中,细胞通过免疫原性细胞死亡形式释放损伤相关分子模式(如HMGB1和ATP),激活宿主的先天免疫反应,从而对抗病毒感染。此外,免疫原性细胞死亡介导的死亡细胞裂解后可释放新抗原表位,被模式识别受体识别并呈递给T淋巴细胞,激活病毒特异性的T细胞反应[107]。Shalhout等[108]基于溶瘤病毒所介导的免疫原性细胞死亡机制,开发了更有效杀伤肿瘤细胞的免疫疗法。
副黏病毒中的新城疫病毒和麻疹病毒是天然的溶瘤病毒,可通过肿瘤免疫疗法抑制肿瘤生长。研究发现,新城疫病毒感染A549和H460细胞后,细胞内ATP、HMGB1、热休克蛋白70/90被释放,钙网蛋白易位到细胞表面,随后细胞发生免疫原性细胞死亡,具有良好的溶瘤效果;自噬相关基因的耗竭可以显著抑制免疫原性细胞死亡,表明自噬在这一现象中起着关键作用[87]。麻疹病毒在感染黑色素瘤细胞系(Mel888、Mel624、MeWO、SKMel28)后,通过释放促炎性细胞因子和HMGB1来激活自然杀伤细胞和树突状细胞,也能起到良好的溶瘤效果[86]。
上述研究表明,一些副黏病毒通过触发肿瘤细胞的免疫原性细胞死亡,可能成为癌症的潜在治疗方式。免疫原性细胞死亡不仅能导致肿瘤细胞的破坏,还可以通过释放损伤分子激活宿主的免疫系统,从而抑制肿瘤的生长和扩散。
5. 副黏病毒感染后铁死亡的作用
铁死亡作为一种非凋亡形式的PCD,其在病毒感染中的作用正逐渐被发现。研究表明,Ⅰ型单纯疱疹病毒能够诱发铁死亡,并在病毒性脑炎中发挥作用[109]。铁死亡的激活可以通过诱导或抑制不同类型的调节性细胞死亡来促进病毒复制或帮助病毒逃避宿主监视。铁死亡相关代谢包括铁代谢、脂质过氧化和抗氧化代谢,与病毒感染和宿主铁死亡过程密切相关[110]。在病毒感染中,铁是病毒与宿主之间竞争的重要元素,宿主对铁的利用受限可能会促进病毒感染,并加速宿主细胞死亡进程。
目前,副黏病毒研究中关于铁死亡的报道较少。研究发现,新城疫病毒感染神经胶质瘤细胞(U-251 MG)后,引起P53转录激活并逐渐积累,可抑制胱氨酸/谷氨酸转运蛋白阻止胱氨酸的摄取,从而引起细胞内半胱氨酸耗竭;还可下调谷胱甘肽过氧化物酶4表达,使过氧化磷脂堆积,进而诱导细胞铁死亡的发生[90]。可见病毒能利用铁死亡与宿主进行营养剥夺,促进其自身复制和传播。因此,深入理解铁死亡在病毒感染中的具体作用机制对于开发新的治疗策略具有一定价值。
6. 结 语
目前研究已揭示副黏病毒感染过程中PCD呈现出多样性和复杂性,其中细胞凋亡和细胞焦亡占据核心地位(图2)。不同类型的副黏病毒具有各自的细胞嗜性,提示其诱导的细胞死亡机制在不同病毒感染中可能发挥着独特且具有特异性的功能。副黏病毒感染诱导的细胞死亡机制是病毒感染免疫学中的一个关键研究领域,涉及宿主细胞对病原体的复杂反应。在感染过程中,宿主细胞可能经历包括细胞凋亡、细胞焦亡和坏死性凋亡在内的多种PCD形式。细胞死亡在副黏病毒感染中具有双重角色:一方面,它是病毒清除宿主细胞、完成生命周期的必要途径;另一方面,它亦是宿主先天免疫应答的重要组成部分。例如,细胞凋亡通常是一种免疫沉默的、可控的死亡方式,有助于防止过度炎症反应的发生;而细胞焦亡和坏死性凋亡则常伴随炎症因子的释放,能够有效激活并增强宿主的免疫反应。这些不同形式的细胞死亡在感染的不同时期可能发挥不同功能:如早期的细胞凋亡有助于限制病毒的复制,而后期的细胞焦亡和坏死性凋亡则有助于激活宿主的免疫反应。值得注意的是,副黏病毒与宿主细胞之间的相互作用还表现在病毒可能通过编码特定的蛋白质来干扰宿主的细胞死亡途径,从而促进其生存和传播。这种进化适应性策略既凸显了PCD机制的双重生物学意义,也暴露出病毒逃逸的关键靶点。
图2. 副黏病毒感染后程序性细胞死亡的分子机制.
病毒感染可激活宿主的多种识别机制,如TLR、TNF受体以及病毒核酸识别通路MDA5/RIG-I,诱导相应信号通路并启动多种细胞死亡反应. TNF-α与其受体结合激活RIPK1/RIPK3/MLKL轴,介导坏死性凋亡;病毒核酸通过MDA5/RIG-I识别后,激活线粒体MAVS通路,引发Bax介导的线粒体通透性转变,释放细胞色素C并激活caspase-9,诱导caspase-3/7介导的细胞凋亡;炎症小体的激活导致caspase-1活化与GSDMD裂解,引发细胞焦亡;而活性氧积累与铁蛋白自噬共同促进脂质过氧化,触发铁死亡;细胞死亡过程中释放的损伤相关分子模式可被树突状细胞识别,并递呈给T淋巴细胞,诱导继发性免疫原性细胞死亡. TLR:Toll样受体;TNF:肿瘤坏死因子;MDA:黑色素瘤分化相关蛋白;RIG:维甲酸诱导基因;RIPK:受体相互作用蛋白激酶;MLKL:混合谱系激酶结构域样蛋白;MAVS:线粒体抗病毒信号蛋白;Bcl:B细胞淋巴瘤蛋白;Bax:Bcl-2相关X蛋白;caspase:胱天蛋白酶;GSDMD:Gasdermin家族D成员;FASL:Fas配体;TRAIL:TNF相关凋亡诱导配体;TRADD:TNF受体相关死亡结构域蛋白;FADD:死亡受体相关死亡结构域蛋白;Ub:泛素;TRAF:TNF受体相关因子;NF-κB:核因子κB;ISRE:干扰素刺激响应元件;IRF:干扰素调节因子;GPX:谷胱甘肽过氧化物酶;GSH:还原型谷胱甘肽;GS-SH:谷胱甘肽巯基形式.
近年来,针对PCD通路的精准干预已成为抗病毒治疗的新兴方向。在药物开发层面,研究发现凋亡调控因子Bcl-2抑制剂和TRAIL受体激动剂可清除人类免疫缺陷病毒或乙型肝炎病毒感染的细胞,并在副黏病毒持续性感染小鼠模型中展现出潜在的应用价值[111-112]。针对过度炎症反应,靶向坏死性凋亡关键激酶RIPK3的抑制剂成功降低甲型流感小鼠模型的严重肺组织损伤,提示其在副黏病毒相关重症肺炎治疗中的可行性[113]。而一项临床试验表明,细胞焦亡通路中核心蛋白NLRP3的特异性抑制剂能显著缓解2019冠状病毒病的细胞因子风暴,为RSV感染引发的呼吸系统疾病提供了参考范式[114]。在疫苗设计领域,PCD机制的系统应用正在革新免疫策略。焦亡诱导型佐剂(如AS01)通过激活caspase-1促进IL-1β释放,显著增强了疫苗的免疫原性[115]。如AS01佐剂的应用使带状疱疹疫苗Shingrix®的中和抗体滴度较传统疫苗提升了数倍[116]。这一技术路径为副黏病毒亚单位疫苗的效价增强提供了重要参考。此外,2019冠状病毒病ChAdOx1疫苗在感染细胞后,能够诱导细胞释放大量病毒抗原,并激活NLRP3炎症小体通路,从而实现体液免疫与细胞免疫的双重增效[117]。
综上所述,靶向PCD的干预策略展现出显著的抗病毒疗效。然而,细胞死亡机制的复杂性提示深入研究其在病毒感染中的作用对于优化治疗策略至关重要。在感染过程中,多种细胞死亡方式之间的相互作用和平衡对疾病的进展和结果至关重要。适度的细胞死亡可以帮助宿主限制病毒的扩散和复制,而过度的细胞死亡可能导致组织损伤和功能障碍,促进疾病进展。因此,未来的研究需要深入探讨细胞死亡的机制和调控,了解不同细胞死亡方式在病毒感染中的具体作用,及其如何被宿主细胞和病毒利用。通过深入研究副黏病毒感染中的细胞死亡机制,更好地理解病毒与宿主之间的复杂相互作用,并为开发新的治疗策略和干预措施提供科学依据。
Supplementary information
本文附加文件见电子版。
Acknowledgments
研究得到国家自然科学基金(32270848,U23A 20498)支持
Acknowledgments
The study was supported by the National Natural Science Foundation of China (32270848, U23A20498)
[缩略语]
呼吸道合胞病毒(respiratory syncytial virus,RSV);程序性细胞死亡(programmed cell death,PCD);肿瘤坏死因子相关凋亡诱导配体(tumor necrosis factor-related apoptosis-inducing ligand,TRAIL);B细胞淋巴瘤蛋白(B-cell lymphoma,Bcl);Bcl-2同源结构域3(Bcl-2 homology domain 3,BH3);Bcl-2相关X蛋白(Bcl-2-associated X protein,Bax);胱天蛋白酶(cysteine aspartic acid specific protease,caspase);黑色素瘤缺乏因子(absent in melanoma,AIM);NOD样受体热蛋白结构域相关蛋白(NOD-like receptor thermal protein domain associated protein,NLRP);胞苷/尿苷单磷酸激酶(cytidine/uridine monophosphate kinase,CMPK);受体相互作用蛋白激酶(receptor-interacting protein kinase,RIPK);维甲酸诱导基因(retinoic acid-inducible gene,RIG);混合谱系激酶结构域样蛋白(mixed lineage kinase domain-like protein,MLKL);高迁移率组蛋白质(high-mobility group box,HMGB);三磷酸腺苷(adenosine triphosphate,ATP)
利益冲突声明
所有作者均声明不存在利益冲突. 本文由至少两名编委以外的同行专家评审,最终决定由与作者无利益冲突的其他编委作出
Conflict of Interests
The authors declare that there is no conflict of interests. The manuscript was assigned to at least two independent outside reviewers, and the decision was made by other Editorial Board Members who do not have conflicts of interests with the author
作者贡献
刘叶、汪一龙、何志旭和赵正言参与论文选题和设计或参与资料获取、分析或解释,起草研究论文或修改重要智力性内容. 所有作者均已阅读并认可最终稿件,并对数据的完整性和安全性负责. 具体见电子版
医学伦理
研究不涉及人体或动物实验
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors
数据可用性
本研究未生成任何新数据集,所有分析数据均来已公开的来源,并已在文中明确引用
Data Availability
This study did not generate any new datasets, and all data analyzed are from publicly available sources, as cited in the manuscript
参考文献(References)
- 1.COX R M, PLEMPER R K. Structure and organization of paramyxovirus particles[J]. Curr Opin Virol, 2017, 24: 105-114. 10.1016/j.coviro.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DUPREX W P, DUTCH R E. Paramyxoviruses: patho-genesis, vaccines, antivirals, and prototypes for pandemic preparedness[J]. J Infect Dis, 2023, 228(Suppl 6): S390-S397. 10.1093/infdis/jiad123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.TANG J W, LAM T T, ZARAKET H, et al. Global epidemiology of non-influenza RNA respiratory viruses: data gaps and a growing need for surveillance[J/OL]. Lancet Infect Dis, 2017, 17(10): e320-e326. 10.1016/S1473-3099(17)30238-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LI Z J, ZHANG H Y, REN L L, et al. Etiological and epidemiological features of acute respiratory infections in China[J]. Nat Commun, 2021, 12(1): 5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.KRISTENSEN M, DE STEENHUIJSEN PITERS W A A, WILDENBEEST J, et al. The respiratory microbiome is linked to the severity of RSV infections and the persistence of symptoms in children[J]. Cell Rep Med, 2024, 5(12): 101836. 10.1016/j.xcrm.2024.101836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LIU W K, CHEN D H, TAN W P, et al. Paramyxoviruses respiratory syncytial virus, parainfluenza virus, and human metapneumovirus infection in pediatric hospi-talized patients and climate correlation in a subtropical region of Southern China: a 7-year survey[J]. Eur J Clin Microbiol Infect Dis, 2019, 38(12): 2355-2364. 10.1007/s10096-019-03693-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GARG R K, MAHADEVAN A, MALHOTRA H S, et al. Subacute sclerosing panencephalitis[J/OL]. Rev Med Virol, 2019, 29(5): e2058. 10.1002/rmv.2058 [DOI] [PubMed] [Google Scholar]
- 8.HVIID A, RUBIN S, MÜHLEMANN K. Mumps[J]. Lancet, 2008, 371(9616): 932-944. 10.1016/s0140-6736(08)60419-5 [DOI] [PubMed] [Google Scholar]
- 9.PLEMPER R K. Measles resurgence and drug deve-lopment[J]. Curr Opin Virol, 2020, 41: 8-17. 10.1016/j.coviro.2020.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.PIDELASERRA-MARTÍ G, ENGELAND C E. Mecha-nisms of measles virus oncolytic immunotherapy[J]. Cytokine Growth Factor Rev, 2020, 56: 28-38. 10.1016/j.cytogfr.2020.07.009 [DOI] [PubMed] [Google Scholar]
- 11.SCHIRRMACHER V. Molecular mechanisms of anti-neoplastic and immune stimulatory properties of oncolytic Newcastle disease virus[J]. Biomedicines, 2022, 10(3): 562. 10.3390/biomedicines10030562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MATVEEVA O V, KOCHNEVA G V, NETESOV S V, et al. Mechanisms of oncolysis by paramyxovirus Sendai[J]. Acta Naturae, 2015, 7(2): 6-16. 10.32607/20758251-2015-7-2-6-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DANTHI P. Viruses and the diversity of cell death[J]. Annu Rev Virol, 2016, 3(1): 533-553. 10.1146/annurev-virology-110615-042435 [DOI] [PubMed] [Google Scholar]
- 14.VERBURG S G, LELIEVRE R M, WESTERVELD M J, et al. Viral-mediated activation and inhibition of programmed cell death[J/OL]. PLoS Pathog, 2022, 18(8): e1010718. 10.1371/journal.ppat.1010718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.YUAN C, MA Z, XIE J, et al. The role of cell death in SARS-CoV-2 infection[J]. Signal Transduct Target Ther, 2023, 8(1): 357. 10.1038/s41392-023-01580-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.IMRE G. The involvement of regulated cell death forms in modulating the bacterial and viral pathogenesis[J]. Int Rev Cell Mol Biol, 2020, 353: 211-253. 10.1016/bs.ircmb.2019.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.BHASKAR A, BALA J, VARSHNEY A, et al. Expres-sion of measles virus nucleoprotein induces apoptosis and modulates diverse functional proteins in cultured mammalian cells[J/OL]. PLoS One, 2011, 6(4): e18765. 10.1371/journal.pone.0018765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LAL G, RAJALA M S. Combination of oncolytic measles virus armed with BNiP3, a pro-apoptotic gene and paclitaxel induces breast cancer cell death[J]. Front Oncol, 2019, 8: 676. 10.3389/fonc.2018.00676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ZHOU S, LI Y, HUANG F, et al. Live-attenuated measles virus vaccine confers cell contact loss and apoptosis of ovarian cancer cells via ROS-induced silencing of E-cadherin by methylation[J]. Cancer Lett, 2012, 318(1): 14-25. 10.1016/j.canlet.2011.10.038 [DOI] [PubMed] [Google Scholar]
- 20.LIU C H, TAI C J, KUO Y T, et al. Combination of oncolytic measles virus and ursolic acid synergistically induces oncolysis of hepatocellular carcinoma cells[J]. Viruses, 2023, 15(6): 1294. 10.3390/v15061294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LAMPE J, BOSSOW S, WEILAND T, et al. An armed oncolytic measles vaccine virus eliminates human hepa-toma cells independently of apoptosis[J]. Gene Ther, 2013, 20(11): 1033-1041. 10.1038/gt.2013.28 [DOI] [PubMed] [Google Scholar]
- 22.ZHANG S C, WANG W L, CAI W S, et al. Engineered measles virus Edmonston strain used as a novel oncolytic viral system against human hepatoblastoma[J]. BMC Cancer, 2012, 12: 427. 10.1186/1471-2407-12-427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LAINE D, BOURHIS J M, LONGHI S, et al. Measles virus nucleoprotein induces cell-proliferation arrest and apoptosis through NTAIL-NR and NCORE-FcgammaRIIB1 interactions, respectively[J]. J Gen Virol, 2005, 86(Pt 6): 1771-1784. 10.1099/vir.0.80791-0 [DOI] [PubMed] [Google Scholar]
- 24.YI C, LIU X, LIU Y, et al. Hemagglutinin protein of measles virus induces apoptosis of HeLa cells via both extrinsic and intrinsic pathways[J]. Can J Microbiol, 2013, 59(12): 814-824. 10.1139/cjm-2013-0544 [DOI] [PubMed] [Google Scholar]
- 25.ZHANG C D, WANG Y L, ZHOU D M, et al. A recom-binant Chinese measles virus vaccine strain rMV-Hu191 inhibits human colorectal cancer growth through inducing autophagy and apoptosis regulating by PI3K/AKT pathway[J]. Transl Oncol, 2021, 14(7): 101091. 10.1016/j.tranon.2021.101091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.PATEL M R, JACOBSON B A, BELGUM H, et al. Measles vaccine strains for virotherapy of non-small-cell lung carcinoma[J]. J Thorac Oncol, 2014, 9(8): 1101-1110. 10.1097/jto.0000000000000214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LV Y, ZHOU D, HAO X Q, et al. A recombinant measles virus vaccine strain rMV-Hu191 has oncolytic effect against human gastric cancer by inducing apoptotic cell death requiring integrity of lipid raft microdomains[J]. Cancer Lett, 2019, 460: 108-118. 10.1016/j.canlet.2019.06.010 [DOI] [PubMed] [Google Scholar]
- 28.PHUONG L K, ALLEN C, PENG K W, et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel thera-peutic agent against glioblastoma multiforme[J]. Cancer Res, 2003, 63(10): 2462-2469. [PubMed] [Google Scholar]
- 29.ZHANG C D, JIANG L H, ZHOU X, et al. Synergistic antitumor efficacy of rMV-Hu191 and Olaparib in pancreatic cancer by generating oxidative DNA damage and ROS-dependent apoptosis[J]. Transl Oncol, 2024, 39: 101812. 10.1016/j.tranon.2023.101812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WU A, LI Z, WANG Y, et al. Recombinant measles virus vaccine rMV-Hu191 exerts an oncolytic effect on esophageal squamous cell carcinoma via caspase-3/GSDME-mediated pyroptosis[J]. Cell Death Discov, 2023, 9(1): 171. 10.1038/s41420-023-01466-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.ZHU M, WANG Y, QU C, et al. Recombinant Chinese Hu191 measles virus exhibits a significant antitumor activity against nephroblastoma mediated by immuno-genic form of apoptosis[J]. Am J Transl Res, 2021, 13(4): 2077-2093. [PMC free article] [PubMed] [Google Scholar]
- 32.RUDAK P T, YAO T, RICHARDSON C D, et al. Measles virus infects and programs MAIT cells for apoptosis[J]. J Infect Dis, 2021, 223(4): 667-672. 10.1093/infdis/jiaa407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ACHARD C, GUILLERME J B, BRUNI D, et al. Oncolytic measles virus induces tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated cytotoxicity by human myeloid and plasmacytoid dendritic cells[J/OL]. Oncoimmunology, 2016, 6(1): e1261240. 10.1080/2162402x.2016.1261240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.VUORINEN T, PERI P, VAINIONPÄÄ R. Measles virus induces apoptosis in uninfected bystander T cells and leads to granzyme B and caspase activation in peripheral blood mononuclear cell cultures[J]. Eur J Clin Invest, 2003, 33(5): 434-442. 10.1046/j.1365-2362.2003.01164.x [DOI] [PubMed] [Google Scholar]
- 35.ESOLEN L M, PARK S W, HARDWICK J M, et al. Apoptosis as a cause of death in measles virus-infected cells[J]. J Virol, 1995, 69(6): 3955-3958. 10.1128/jvi.69.6.3955-3958.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ITO M, YAMAMOTO T, WATANABE M, et al. Detec-tion of measles virus-induced apoptosis of human monocytic cell line (THP-1) by DNA fragmentation ELISA[J]. FEMS Immunol Med Microbiol, 1996, 15(2-3): 115-122. 10.1111/j.1574-695x.1996.tb00061.x [DOI] [PubMed] [Google Scholar]
- 37.WEI B, CUI Y, HUANG Y, et al. Tom70 mediates Sendai virus-induced apoptosis on mitochondria[J]. J Virol, 2015, 89(7): 3804-3818. 10.1128/jvi.02959-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.CHEN J, HAN H, WANG B, et al. Inactivated Tianjin strain, a novel genotype of Sendai virus, induces apoptosis in HeLa, NCI-H446 and Hep3B cells[J]. Oncol Lett, 2016, 12(1): 49-56. 10.3892/ol.2016.4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.QIAN M, TAN H M, YU N, et al. Inactivated Sendai virus induces ROS-dependent apoptosis and autophagy in human prostate cancer cells[J]. Biomed Environ Sci, 2018, 31(4): 280-289. [DOI] [PubMed] [Google Scholar]
- 40.LIU L W, NISHIKAWA T, KANEDA Y. An RNA molecule derived from Sendai virus DI particles induces antitumor immunity and cancer cell-selective apoptosis[J]. Mol Ther, 2016, 24(1): 135-145. 10.1038/mt.2015.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MATSUSHIMA-MIYAGI T, HATANO K, NOMURA M, et al. TRAIL and Noxa are selectively upregulated in prostate cancer cells downstream of the RIG-I/MAVS signaling pathway by nonreplicating Sendai virus particles[J]. Clin Cancer Res, 2012, 18(22): 6271-6283. 10.1158/1078-0432.ccr-12-1595 [DOI] [PubMed] [Google Scholar]
- 42.LALLEMAND C, BLANCHARD B, PALMIERI M, et al. Single-stranded RNA viruses inactivate the trans-criptional activity of p53 but induce NOXA-dependent apoptosis via post-translational modifications of IRF-1, IRF-3 and CREB[J]. Oncogene, 2007, 26(3): 328-338. 10.1038/sj.onc.1209795 [DOI] [PubMed] [Google Scholar]
- 43.CHEN J, HAN H, CHEN M, et al. Inactivated Sendai virus strain Tianjin induces apoptosis in human breast cancer MDA-MB-231 cells[J]. Asian Pac J Cancer Prev, 2014, 15(12): 5023-5028. 10.7314/apjcp.2014.15.12.5023 [DOI] [PubMed] [Google Scholar]
- 44.ZHANG Q, ZHU H, XU X, et al. Inactivated Sendai virus induces apoptosis and autophagy via the PI3K/Akt/mTOR/p70S6K pathway in human non-small cell lung cancer cells[J]. Biochem Biophys Res Commun, 2015, 465(1): 64-70. 10.1016/j.bbrc.2015.07.130 [DOI] [PubMed] [Google Scholar]
- 45.HAN Z, LI Q, SUN S, et al. Inactivated Sendai virus strain Tianjin induces apoptosis and autophagy through reactive oxygen species production in osteosarcoma MG-63 cells[J]. J Cell Physiol, 2019, 234(4): 4179-4190. 10.1002/jcp.27176 [DOI] [PubMed] [Google Scholar]
- 46.LI Q, MA H, SUN S, et al. Anticancer effect of inac-tivated Sendai virus strain Tianjin on human osteo-sarcoma HOS cells[J]. Gen Physiol Biophys, 2019, 38(4): 335-342. 10.4149/gpb_2019015 [DOI] [PubMed] [Google Scholar]
- 47.SHI L, CHEN J, ZHONG Q, et al. Inactivated Sendai virus strain Tianjin, a novel genotype of Sendai virus, inhibits growth of murine colon carcinoma through inducing immune responses and apoptosis[J]. J Transl Med, 2013, 11: 205. 10.1186/1479-5876-11-205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.HUANG S, QU L K, KOROMILAS A E. Induction of p53-dependent apoptosis in HCT116 tumor cells by RNA viruses and possible implications in virus-mediated oncolysis[J]. Cell Cycle, 2004, 3(8): 1043-1045. 10.4161/cc.3.8.1016 [DOI] [PubMed] [Google Scholar]
- 49.BITZER M, PRINZ F, BAUER M, et al. Sendai virus infection induces apoptosis through activation of caspase-8 (FLICE) and caspase-3 (CPP32)[J]. J Virol, 1999, 73(1): 702-708. 10.1128/jvi.73.1.702-708.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.TANAKA M, SHIMBO T, KIKUCHI Y, et al. Sterile alpha motif containing domain 9 is involved in death signaling of malignant glioma treated with inactivated Sendai virus particle (HVJ-E) or type Ⅰ interferon[J]. Int J Cancer, 2010, 126(8): 1982-1991. 10.1002/ijc.24965 [DOI] [PubMed] [Google Scholar]
- 51.TROPEA F, TROIANO L, MONTI D, et al. Sendai virus and herpes virus type 1 induce apoptosis in human peripheral blood mononuclear cells[J]. Exp Cell Res, 1995, 218(1): 63-70. 10.1006/excr.1995.1131 [DOI] [PubMed] [Google Scholar]
- 52.ZHANG L F, TAN D Q, JEYASEKHARAN A D, et al. Combination of vaccine-strain measles and mumps virus synergistically kills a wide range of human hemato-logical cancer cells: special focus on acute myeloid leukemia[J]. Cancer Lett, 2014, 354(2): 272-280. 10.1016/j.canlet.2014.08.034 [DOI] [PubMed] [Google Scholar]
- 53.TAKIKITA S, TAKANO T, NARITA T, et al. Neuronal apoptosis mediated by IL-1 beta expression in viral encephalitis caused by a neuroadapted strain of the mumps virus (Kilham Strain) in hamsters[J]. Exp Neurol, 2001, 172(1): 47-59. 10.1006/exnr.2001.7773 [DOI] [PubMed] [Google Scholar]
- 54.WANG Q, WU H, CHENG L, et al. Mumps virus induces innate immune responses in mouse ovarian granulosa cells through the activation of Toll-like receptor 2 and retinoic acid-inducible gene Ⅰ[J]. Mol Cell Endocrinol, 2016, 436: 183-194. 10.1016/j.mce.2016.07.033 [DOI] [PubMed] [Google Scholar]
- 55.MOROVATI S, MOHAMMADI A, MASOUDI R, et al. The power of mumps virus: matrix protein activates apoptotic pathways in human colorectal cell lines[J/OL]. PLoS One, 2023, 18(12): e0295819. 10.1371/journal.pone.0295819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.JIANG Q, WANG F, SHI L, et al. Correction: C-X-C motif chemokine ligand 10 produced by mouse Sertoli cells in response to mumps virus infection induces male germ cell apoptosis[J]. Cell Death Dis, 2018, 9: 789. 10.1038/s41419-018-0834-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.SIMPSON J, LOH Z, ULLAH M A, et al. Respiratory syncytial virus infection promotes necroptosis and HMGB1 release by airway epithelial cells[J]. Am J Respir Crit Care Med, 2020, 201(11): 1358-1371. 10.1164/rccm.201906-1149oc [DOI] [PubMed] [Google Scholar]
- 58.ECKARDT-MICHEL J, LOREK M, BAXMANN D, et al. The fusion protein of respiratory syncytial virus triggers p53-dependent apoptosis[J]. J Virol, 2008, 82(7): 3236-3249. 10.1128/jvi.01887-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.BEM R A, BOS A P, WÖSTEN-VAN ASPEREN R M, et al. Potential role of soluble TRAIL in epithelial injury in children with severe RSV infection[J]. Am J Respir Cell Mol Biol, 2010, 42(6): 697-705. 10.1165/rcmb.2009-0100oc [DOI] [PubMed] [Google Scholar]
- 60.KOTELKIN A, PRIKHOD’KO E A, COHEN J I, et al. Respiratory syncytial virus infection sensitizes cells to apoptosis mediated by tumor necrosis factor-related apoptosis-inducing ligand[J]. J Virol, 2003, 77(17): 9156-9172. 10.1128/jvi.77.17.9156-9172.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.HUANG Y, JIANG C, LIU X, et al. Melatonin suppresses TLR4-mediated RSV infection in the central nervous cells by inhibiting NLRP3 inflammasome formation and autophagy[J/OL]. J Cell Mol Med, 2024, 28(9): e18338. 10.1111/jcmm.18338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.CHOI S H, PARK B K, LEE K W, et al. Effect of respira-tory syncytial virus on the growth of hepatocellular carcinoma cell-lines[J]. BMB Rep, 2015, 48(10): 565-570. 10.5483/bmbrep.2015.48.10.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.ROE M E, BLOXHAM D M, WHITE D K, et al. Lym-phocyte apoptosis in acute respiratory syncytial virus bronchiolitis[J]. Clin Exp Immunol, 2004, 137(1): 139-145. 10.1111/j.1365-2249.2004.02512.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.WANG S Z, SMITH P K, LOVEJOY M, et al. The apoptosis of neutrophils is accelerated in respiratory syncytial virus (RSV)-induced bronchiolitis[J]. Clin Exp Immunol, 1998, 114(1): 49-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.CHEN Y, ZHU S, LIAO T, et al. The HN protein of Newcastle disease virus induces cell apoptosis through the induction of lysosomal membrane permeabilization[J/OL]. PLoS Pathog, 2024, 20(2): e1011981. 10.1371/journal.ppat.1011981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.ELANKUMARAN S, ROCKEMANN D, SAMAL S K. Newcastle disease virus exerts on colysis by both intrinsic and extrinsic caspase-dependent pathways of cell death[J]. J Virol, 2006, 80(15): 7522-7534. 10.1128/jvi.00241-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.MOLOUKI A, HSU Y T, JAHANSHIRI F, et al. New-castle disease virus infection promotes Bax redistri-bution to mitochondria and cell death in HeLa cells[J]. Intervirology, 2010, 53(2): 87-94. 10.1159/000264198 [DOI] [PubMed] [Google Scholar]
- 68.MOZAFFARI NEJAD A S, FOTOUHI F, MEHRBOD P, et al. Oncolytic effects of Hitchner B1 strain of Newcastle disease virus against cervical cancer cell proliferation is mediated by the increased expression of cytochrome C, autophagy and apoptotic pathways[J]. Microb Pathog, 2020, 147: 104438. 10.1016/j.micpath.2020.104438 [DOI] [PubMed] [Google Scholar]
- 69.AHMAD U, AHMED I, KEONG Y Y, et al. Inhibitory and apoptosis-inducing effects of Newcastle disease virus strain AF2240 on mammary carcinoma cell line[J]. Biomed Res Int, 2015, 2015: 127828. 10.1155/2015/127828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.BIAN J, WANG K, KONG X, et al. Caspase- and p38-MAPK-dependent induction of apoptosis in A549 lung cancer cells by Newcastle disease virus[J]. Arch Virol, 2011, 156(8): 1335-1344. 10.1007/s00705-011-0987-y [DOI] [PubMed] [Google Scholar]
- 71.GHRICI M, ZOWALATY M EL, OMAR A R, et al. Newcastle disease virus Malaysian strain AF2240 induces apoptosis in MCF-7 human breast carcinoma cells at an early stage of the virus life cycle[J]. Int J Mol Med, 2013, 31(3): 525-532. 10.3892/ijmm.2013.1244 [DOI] [PubMed] [Google Scholar]
- 72.YAN Y, LIU S, LI M, et al. Recombinant Newcastle disease virus expressing human IFN-λ1 (rL-hIFN-λ1)-induced apoptosis of A549 cells is connected to endo-plasmic reticulum stress pathways[J]. Thorac Cancer, 2018, 9(11): 1437-1452. 10.1111/1759-7714.12857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.BU X, ZHAO Y, ZHANG Z, et al. Recombinant New-castle disease virus (rL-RVG) triggers autophagy and apoptosis in gastric carcinoma cells by inducing ER stress[J]. Am J Cancer Res, 2016, 6(5): 924-936. [PMC free article] [PubMed] [Google Scholar]
- 74.LI Y, JIANG W, NIU Q, et al. eIF2α-CHOP-BCl-2/JNK and IRE1α-XBP1/JNK signaling promote apoptosis and inflammation and support the proliferation of Newcastle disease virus[J]. Cell Death Dis, 2019, 10(12): 891. 10.1038/s41419-019-2128-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.ALI R, ALABSI A M, ALI A M, et al. Cytolytic effects and apoptosis induction of Newcastle disease virus strain AF2240 on anaplastic astrocytoma brain tumor cell line[J]. Neurochem Res, 2011, 36(11): 2051-2062. 10.1007/s11064-011-0529-8 [DOI] [PubMed] [Google Scholar]
- 76.BAI Y, CHEN Y, HONG X, et al. Newcastle disease virus enhances the growth-inhibiting and proapoptotic effects of temozolomide on glioblastoma cells in vitro and in vivo [J]. Sci Rep, 2018, 8(1): 11470. 10.1038/s41598-018-29929-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.JUNG B K, AN Y H, JANG S H, et al. The tumor suppressive effect and apoptotic mechanism of TRAIL gene-containing recombinant NDV in TRAIL-resistant colorectal cancer HT-29 cells and TRAIL-nonresistant HCT116 cells, with each cell bearing a mouse model[J]. Cancer Med, 2023, 12(20): 20380-20395. 10.1002/cam4.6622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.ALABSI A M, BAKAR S A, ALI R, et al. Effects of Newcastle disease virus strains AF2240 and V4-UPM on cytolysis and apoptosis of leukemia cell lines[J]. Int J Mol Sci, 2011, 12(12): 8645-8660. 10.3390/ijms12128645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.KOKS C A, GARG A D, EHRHARDT M, et al. New-castle disease virotherapy induces long-term survival and tumor-specific immune memory in orthotopic glioma through the induction of immunogenic cell death[J/OL]. Int J Cancer, 2015, 136(5): E313-E325. 10.1002/ijc.29202 [DOI] [PubMed] [Google Scholar]
- 80.LAM K M. Apoptosis in chicken embryo fibroblasts caused by Newcastle disease virus[J]. Vet Microbiol, 1995, 47(3-4): 357-363. 10.1016/0378-1135(95)00111-5 [DOI] [PubMed] [Google Scholar]
- 81.LAM K M, VASCONCELOS A C, BICKFORD A A. Apoptosis as a cause of death in chicken embryos inoculated with Newcastle disease virus[J]. Microb Pathog, 1995, 19(3): 169-174. 10.1006/mpat.1995.0055 [DOI] [PubMed] [Google Scholar]
- 82.WASHBURN B, WEIGAND M A, GROSSE-WILDE A, et al. TNF-related apoptosis-inducing ligand mediates tumoricidal activity of human monocytes stimulated by Newcastle disease virus[J]. J Immunol, 2003, 170(4): 1814-1821. 10.4049/jimmunol.170.4.1814 [DOI] [PubMed] [Google Scholar]
- 83.BEDIENT L, POKHAREL S M, CHIOK K R, et al. Lytic cell death mechanisms in human respiratory syncytial virus-infected macrophages: roles of pyroptosis and necroptosis[J]. Viruses, 2020, 12(9): 932. 10.3390/v12090932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.SCHOCK S N, CHANDRA N V, SUN Y, et al. Induction of necroptotic cell death by viral activation of the RIG-I or STING pathway[J]. Cell Death Differ, 2017, 24(4): 615-625. 10.1038/cdd.2016.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.LIAO Y, WANG H X, MAO X, et al. RIP1 is a central signaling protein in regulation of TNF-α/TRAIL mediated apoptosis and necroptosis during Newcastle disease virus infection[J]. Oncotarget, 2017, 8(26): 43201-43217. 10.18632/oncotarget.17970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.DONNELLY O G, ERRINGTON-MAIS F, STEELE L, et al. Measles virus causes immunogenic cell death in human melanoma[J]. Gene Ther, 2013, 20(1): 7-15. 10.1038/gt.2011.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.YE T, JIANG K, WEI L, et al. Oncolytic Newcastle disease virus induces autophagy-dependent immunogenic cell death in lung cancer cells[J]. Am J Cancer Res, 2018, 8(8): 1514-1527. [PMC free article] [PubMed] [Google Scholar]
- 88.SHAO X, WANG X, GUO X, et al. STAT3 contributes to oncolytic Newcastle disease virus-induced immuno-genic cell death in melanoma cells[J]. Front Oncol, 2019, 9: 436. 10.3389/fonc.2019.00436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.YU Z, CHEN Y, CHEN S, et al. Oligoadenylate synthe-tase-like aggravated Newcastle disease virus-induced necroptosis in glioma cells[J]. Front Oncol, 2025, 15: 1574214. 10.3389/fonc.2025.1574214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.KAN X, YIN Y, SONG C, et al. Newcastle-disease-virus-induced ferroptosis through nutrient deprivation and ferritinophagy in tumor cells[J]. iScience, 2021, 24(8): 102837. 10.1016/j.isci.2021.102837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.SUN Y, TANG L, KAN X, et al. Oncolytic Newcastle disease virus induced degradation of YAP through E3 ubiquitin ligase PRKN to exacerbate ferroptosis in tumor cells[J/OL]. J Virol, 2024, 98(3): e0189723. 10.1128/jvi.01897-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.UPTON J W, CHAN F K. Staying alive: cell death in antiviral immunity[J]. Mol Cell, 2014, 54(2): 273-280. 10.1016/j.molcel.2014.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.VAN DEN BERG E, VAN WOENSEL J B M, BEM R A. Apoptosis in pneumovirus infection[J]. Viruses, 2013, 5(1): 406-422. 10.3390/v5010406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.FANG Y, PENG K. Regulation of innate immune responses by cell death-associated caspases during virus infection[J]. FEBS J, 2022, 289(14): 4098-4111. 10.1111/febs.16051 [DOI] [PubMed] [Google Scholar]
- 95.DANTHI P, PRUIJSSERS A J, BERGER A K, et al. Bid regulates the pathogenesis of neurotropic reovirus[J/OL]. PLoS Pathog, 2010, 6(7): e1000980. 10.1371/journal.ppat.1000980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.ABDULLAH H, BRANKIN B, BRADY C, et al. Wild-type measles virus infection upregulates poliovirus receptor-related 4 and causes apoptosis in brain endothelial cells by induction of tumor necrosis factor-related apoptosis-inducing ligand[J]. J Neuropathol Exp Neurol, 2013, 72(7): 681-696. 10.1097/nen.0b013e31829a26b6 [DOI] [PubMed] [Google Scholar]
- 97.SERVET-DELPRAT C, VIDALAIN P O, AZOCAR O, et al. Consequences of Fas-mediated human dendritic cell apoptosis induced by measles virus[J]. J Virol, 2000, 74(9): 4387-4393. 10.1128/jvi.74.9.4387-4393.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.KRUMOVA S, ANDONOVA I, STEFANOVA R, et al. Measles virus and subacute sclerosing panencephalitis[J]. Clin Lab, 2022, 68(9): 1850-1855. 10.7754/clin.lab.2021.211147 [DOI] [PubMed] [Google Scholar]
- 99.JORGENSEN I, MIAO E A. Pyroptotic cell death defends against intracellular pathogens[J]. Immunol Rev, 2015, 265(1): 130-142. 10.1111/imr.12287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.XIAO C, CAO S, LI Y, et al. Pyroptosis in microbial infectious diseases[J]. Mol Biol Rep, 2023, 51(1): 42. 10.1007/s11033-023-09078-w [DOI] [PubMed] [Google Scholar]
- 101.SHIL N K, POKHAREL S M, BANERJEE A K, et al. Inflammasome antagonism by human parainfluenza virus type 3 C protein[J/OL]. J Virol, 2018, 92(4): e01776-17. 10.1128/jvi.01776-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.LÊ V B, DUBOIS J, COUTURE C, et al. Human metap-neumovirus activates NOD-like receptor protein 3 inflammasome via its small hydrophobic protein which plays a detrimental role during infection in mice[J/OL]. PLoS Pathog, 2019, 15(4): e1007689. 10.1371/journal.ppat.1007689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.RAO Z, ZHU Y, YANG P, et al. Pyroptosis in inflam-matory diseases and cancer[J]. Theranostics, 2022, 12(9): 4310-4329. 10.7150/thno.71086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.TANG Z, MAO Y, RUAN P, et al. Drugs targeting CMPK2 inhibit pyroptosis to alleviate severe pneumonia caused by multiple respiratory viruses[J/OL]. J Med Virol, 2024, 96(5): e29643. 10.1002/jmv.29643 [DOI] [PubMed] [Google Scholar]
- 105.NEWTON K, STRASSER A, KAYAGAKI N, et al. Cell death[J]. Cell, 2024, 187(2): 235-256. 10.1016/j.cell.2023.11.044 [DOI] [PubMed] [Google Scholar]
- 106.KROEMER G, GALASSI C, ZITVOGEL L, et al. Immu-nogenic cell stress and death[J]. Nat Immunol, 2022, 23(4): 487-500. 10.1038/s41590-022-01132-2 [DOI] [PubMed] [Google Scholar]
- 107.LI C, WU K, YANG R, et al. Comprehensive analysis of immunogenic cell death-related gene and construction of prediction model based on WGCNA and multiple machine learning in severe COVID-19[J]. Sci Rep, 2024, 14(1): 8450. 10.1038/s41598-024-59117-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.SHALHOUT S Z, MILLER D M, EMERICK K S, et al. Therapy with oncolytic viruses: progress and challenges[J]. Nat Rev Clin Oncol, 2023, 20(3): 160-177. 10.1038/s41571-022-00719-w [DOI] [PubMed] [Google Scholar]
- 109.XU X Q, XU T, JI W, et al. Herpes simplex virus 1-induced ferroptosis contributes to viral encephalitis[J/OL]. mBio, 2023, 14(1): e0237022. 10.1128/mbio.02370-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.WANG J, ZHU J, REN S, et al. The role of ferroptosis in virus infections[J]. Front Microbiol, 2023, 14: 1279655. 10.3389/fmicb.2023.1279655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.CHANDRASEKAR A P, BADLEY A D. Prime, shock and kill: BCL-2 inhibition for HIV cure[J]. Front Immunol, 2022, 13: 1033609. 10.3389/fimmu.2022.1033609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.SUEHIRO Y, TSUGE M, KURIHARA M, et al. Hepa-titis B virus (HBV) upregulates TRAIL-R3 expression in hepatocytes resulting in escape from both cell apoptosis and suppression of HBV replication by TRAIL[J]. J Infect Dis, 2023, 227(5): 686-695. 10.1093/infdis/jiac044 [DOI] [PubMed] [Google Scholar]
- 113.GAUTAM A, BOYD D F, NIKHAR S, et al. Necroptosis blockade prevents lung injury in severe influenza[J]. Nature, 2024, 628(8009): 835-843. 10.1038/s41586-024-07265-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.POTERE N, GARRAD E, KANTHI Y, et al. NLRP3 inflammasome and interleukin-1 contributions to COVID-19-associated coagulopathy and immunothrom-bosis[J]. Cardiovasc Res, 2023, 119(11): 2046-2060. 10.1093/cvr/cvad084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.DIDIERLAURENT A M, LAUPÈZE B, DI PASQUALE A, et al. Adjuvant system AS01: helping to overcome the challenges of modern vaccines[J]. Expert Rev Vaccines, 2017, 16(1): 55-63. 10.1080/14760584.2016.1213632 [DOI] [PubMed] [Google Scholar]
- 116.ROMAN F, BURNY W, CEREGIDO M A, et al. Adju-vant system AS01: from mode of action to effective vaccines[J]. Expert Rev Vaccines, 2024, 23(1): 715-729. 10.1080/14760584.2024.2382725 [DOI] [PubMed] [Google Scholar]
- 117.EWER K J, BARRETT J R, BELIJ-RAMMERSTORFER S, et al. T cell and antibody responses induced by a single dose of ChAdOx1 nCoV-19 (AZD1222) vaccine in a phase 1/2 clinical trial[J]. Nat Med, 2021, 27(2): 270-278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
本文附加文件见电子版。
Data Availability Statement
This study did not generate any new datasets, and all data analyzed are from publicly available sources, as cited in the manuscript