SUMMARY
Nearly 850 million people suffer from kidney disease worldwide. Genome-wide association studies identify genetic variations at more than 800 loci associated with kidney dysfunction; however, the target genes, cell types, and mechanisms remain poorly understood. Here, we show that nucleotide variants on chromosome 15 are not only associated with kidney dysfunction but also regulate the expression of Wasp homolog associated with actin, membranes, and microtubules (WHAMM). WHAMM expression is higher in mice and patients with chronic and acute kidney disease. Mice with genetic deletion of Whamm appear healthy at baseline but develop less injury following cisplatin, folic acid, and unilateral ureteral obstruction. In vitro cell studies indicate that WHAMM controls cell death by regulating actin-mediated cytochrome c release from mitochondria and the formation of ASC speck. Pharmacological inhibition of actin dynamics mitigates kidney disease in experimental models. In summary, our study identifies a key role of WHAMM in the development of kidney disease.
In brief
Mukhi et al. identify WHAMM as a kidney disease risk gene by multi-omics annotation of an eGFR GWAS locus on chromosome 15. WHAMM regulates actin dynamics and cell death pathways in kidney injury. Deletion of WHAMM or pharmacological inhibition of actin dynamics ameliorates kidney damage, highlighting WHAMM’s role in kidney disease.
Graphical abstract

INTRODUCTION
Chronic kidney disease (CKD) ranks as the 10th leading cause of death, affecting over 850 million people worldwide. The prevalence of kidney disease is rapidly increasing, making it one of the fastest rising causes of death.1,2 While kidney function is heritable, environmental factors, including aging, diabetes, toxins, hypertension, and ischemia, also play a crucial role in the development of kidney disease.3,4
Genome-wide association studies (GWASs) have been instrumental in cataloging nucleotide variations in the genome associated with kidney function, such as estimated glomerular filtration rate (eGFR), blood urea nitrogen (BUN), serum creatinine (sCr), cystatin C, and urinary albumin.5,6 Recent, large-scale GWAS7 analyzing 1.5 million individuals identified genetic variations at over 800 loci associated with kidney function (eGFR). However, nearly 90% of these genetic variants are found in the non-coding regions of the genome, complicating the understanding of how they contribute to kidney disease. In recent years, it has been observed that disease-causing variants alter chromatin openness and transcription factor binding strength in specific cell types, leading to quantitative changes in transcript and protein levels.8 Human kidney expression9 and epigenome8 datasets have become essential for annotating GWAS variants, such as expression and methylation quantitative trait (QTL), single-cell expression, and open chromatin information. A variety of statistical tools, including Bayesian colocalization10 and summary Mendelian randomization methods,11 have been developed to statistically prioritize kidney disease-causing genes and mechanisms. However, experimental validation of these signals remains critical to infer causality.
Integration of kidney function GWAS and human kidney molecular QTL data prioritized hundreds of genes associated with kidney function. Genes associated with kidney dysfunction show enrichment for proximal tubule (PT)-specific expression.12 The kidney PT cells are highly metabolically active with the second highest density of mitochondria in the body. Most of the genes identified as playing a role in kidney dysfunction regulate tubular metabolism and mitochondrial function, while also increasing cellular sensitivity to inflammatory cell death pathways, such as ferroptosis and pyroptosis.13–15 The release of intracellular contents during cell death and the expression of cytokines seem to be the key catalysts for the influx of macrophages, the activation of stromal cells, and the development of kidney fibrosis.16,17
Actin nucleation and microtubule organization play pivotal roles in determining cell architecture and functions. Actin nucleation, a fundamental step for the formation of actin filaments and networks, is governed by nucleation-promoting factors such as Wiskott-Aldrich syndrome protein (WASP), WASH1, WAVE, JMY, and WHAMM.18 WHAMM is a vertebrate-specific actin nucleation-promoting factor.19 These factors control the organization of actin bundles and networks, which are crucial for various intracellular trafficking processes, including the formation of endosomes, microsomes, and autophagosomes, as well as cell trafficking.20 The ability of WHAMM to negotiate crosstalk between the actin and microtubule cytoskeleton seems central to its role in influencing membrane dynamics.21 WHAMM has been proposed to play role in determining Golgi structure and anterograde ER-Golgi transport.19,22
Proteins associated with the cytoskeleton and actin nucleation regulate almost all programmed cell death pathways.23 For instance, the WASP family protein WAVE1 and WHAMM may regulate apoptosis by impacting mitochondrial permeabilization.24 The actin machinery is also required for pyroptosis,25,26 a process wherein F-actin plays a crucial role in recruiting apoptosis-associated speck-like (ASC) and pyrin proteins together.27 Disruption of the actin network reduced the secretion of cytokines IL-18 and IL-1B, a well-known indicator of pyroptosis.28 In necroptosis, RIPK3, a key regulatory protein, can promote the oligomerization of the terminal protein MLKL and its translocation to the cell periphery. MLKL ultimately triggers cell rupture.29 However, the role of the actin cytoskeleton in the development of CKD is not fully understood.
Here, by integrating GWAS, eQTL, and single-nucleus ATAC data, we have identified WHAMM as a kidney disease risk gene. Mice with genetic deletion of WHAMM exhibited protection against kidney disease, highlighting its potential role in disease development. In vitro kidney cell culture models further demonstrated the crucial role of WHAMM in regulating changes in the actin cytoskeleton, apoptosis, and pyroptosis during kidney disease. Pharmacological inhibition of actin cytoskeletal regulators rescued acute and CKD, suggesting that targeting WHAMM and cytoskeletal genes could be a viable therapeutic strategy for kidney disease.
RESULTS
GWAS and integrated omics analyses prioritized WHAMM as a kidney risk gene
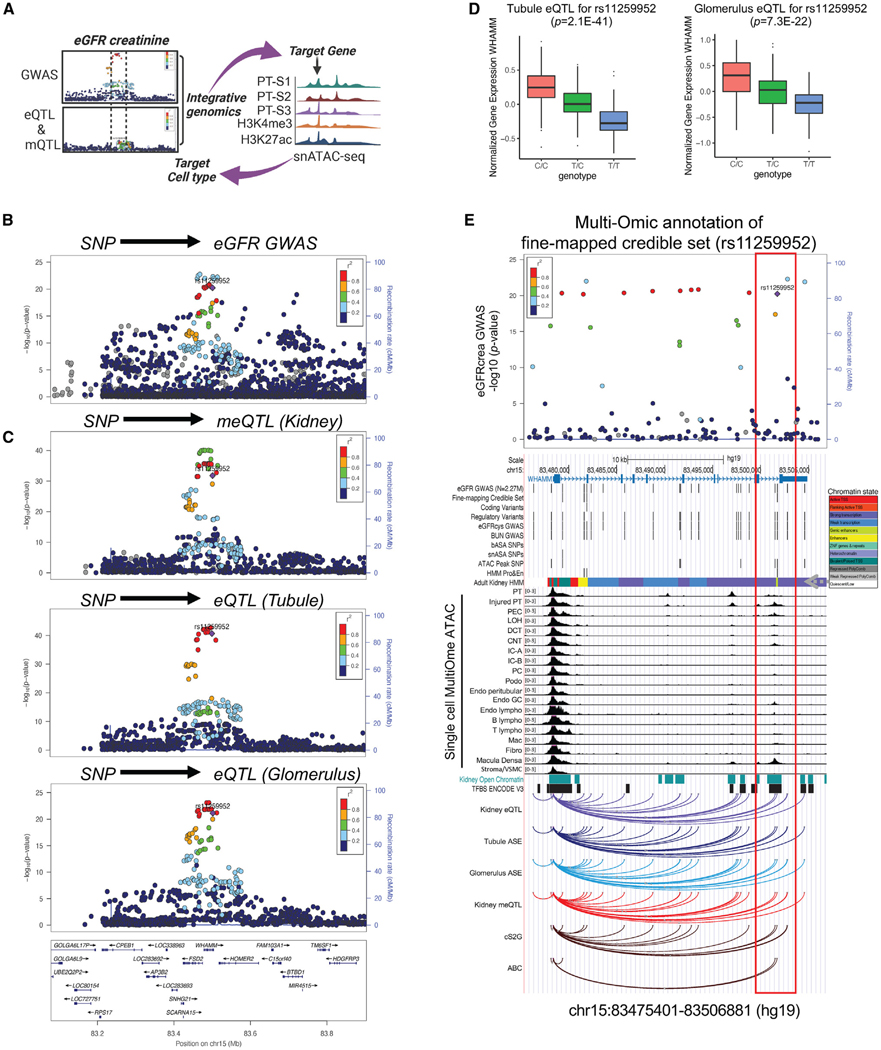
GWAS of kidney function (eGFRcrea) in 2.27 million subjects30 revealed a strong association between variants on chromosome 15 and eGFRcrea (Figures 1A and 1B). The locus has already been identified by a prior kidney function GWAS.7 In this locus, fine mapping analysis identified three independent credible sets, led by eGFRcrea-associated variants rs11259952 (posterior inclusion probability, PIP = 0.9996), rs17507300 (PIP = 0.9998), and rs371891811 (PIP = 0.8425), respectively (Figure S1A). Our gene prioritization strategy for this locus incorporated multiple methods and indicated that two of these GWAS signals target WHAMM (see STAR Methods for details and Table S1). The genetic variant rs11259952 demonstrated a strong association with kidney function (p = 5.8E—21) (Figure 1B), robust association with DNA cytosine methylation (p = 1.6E—32), and the expression of WHAMM in human kidney tubules (p = 2.1E—41) and glomeruli (p = 7.3E—22) (Figures 1C and 1D). Both tubule and glomerular eQTL effect sizes showed a negative association with GWAS effect sizes, suggesting that higher WHAMM expression is linked to lower eGFR (Figure S1B). Bulk RNA-seq data from patients with CKD revealed a negative correlation between WHAMM expression and eGFR and a positive correlation with the degree of kidney fibrosis (Figures S1C and S1D). WHAMM protein levels were observably higher in human CKD samples (Figure S1E). Bayesian colocalization analysis of GWAS and human kidney eQTL and ASE data suggested that the genetic variants driving kidney function and WHAMM expression were shared (PPH4 > 0.8) (Table S1). In a prior GWAS study,7 summary Mendelian randomization analysis revealed that WHAMM expression mediated the genotype effect on kidney function (p = 9.43E—04) (Table S1). Furthermore, we analyzed human kidney single-cell level epigenome (single-cell Multi-Ome ATAC) data and found that the variant rs11259952 overlapped with an open chromatin region and genic enhancer (Figure 1E).
Figure 1. Genetic analysis prioritized WHAMM as a kidney disease gene.
(A) Experimental scheme of gene prioritization.
(B) LocusZoom plot of WHAMM eGFR GWAS locus in 2.27 million individuals of multi-ethnic ancestry. The x axis shows the chromosomal location, and the y axis shows the strength of association (–log(p)) with kidney function. The fine-mapped variant (rs11259952) was used as the index SNP in each panel, with the color representing its squared correlation (r2) with other variants.
(C) Upper: LocusZoom plot of genetic variants associated with kidney methylation (n = 443), (middle) kidney tubule (n = 356), and (lower) glomerulus (n = 303) compartment-specific QTLs. The x axis indicates chromosomal location, and the y axis shows the strength of the association (–log(p)) on chromosome 15.
(D) Human kidney WHAMM expression in tubule (n = 356) and glomerulus (n = 303) compartments. The y axis shows normalized WHAMM expression, and the x axis shows genotype information.
(E) Multi-omic annotation of the fine-mapped credible set (rs11259952). From top to bottom: GWAS LocusZoom, gene structure, annotation of variants, human kidney chromatin states, single-cell MultiOme ATAC, kidney open chromatin, transcription factor binding sites, kidney eQTL, tubule and glomerulus ASE, Cell2 Gene (cS2G), and ABC. See also Figure S1 and Table S1.
In conclusion, our GWAS and genetic analysis identified a genetic signal for kidney function on chromosome 15. Tissue omics annotation indicated that WHAMM is the likely target gene mediating the genotype effect on kidney function.
Cellular expression of WHAMM
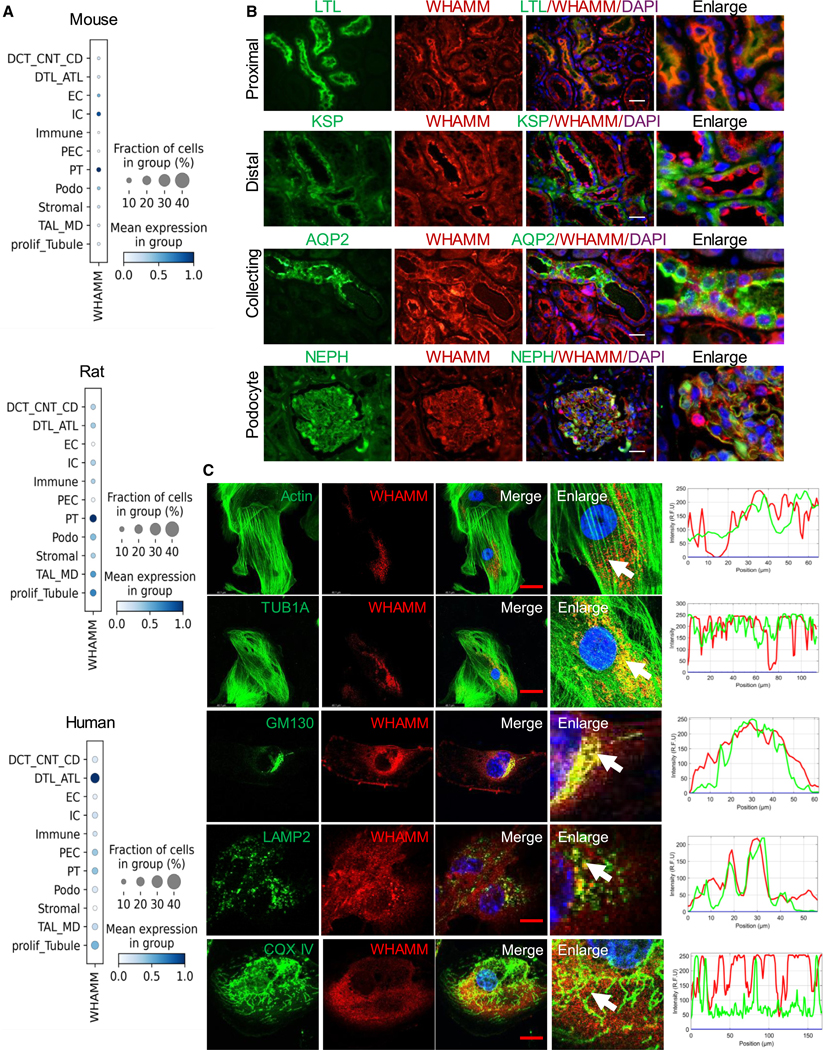
We next investigated WHAMM expression across various kidney cell types using single-cell analysis of mouse,31 rat,32 and human33 samples. We observed WHAMM transcript expression in different kidney epithelial cells, including PT cells (PT), intercalated cells, the distal thin limb, endothelial cells, and podocytes (Figure 2A).
Figure 2. Human kidney WHAMM expression and its cellular localization.
(A) WHAMM expression in mouse (left), rat (middle), and human (right) kidney single-nucleus RNA sequencing. The color indicates mean expression, and the size of the bubble indicates the percentage of cells expressing WHAMM.
(B) Double-immunofluorescence of WHAMM with cell-type-specific markers in human kidney samples. Proximal (LTL, lotus tetragonolobus lectin), distal (KSP, kidney-specific cadherin), collecting tubules (AQP2, aquaporin 2), and podocyte (NEPH, nephrin). Scale bars, 20 μm.
(C) Left: confocal imaging for WHAMM (red) with F-actin (phalloidin, green), microtubules (TUB1A, green), cis-Golgi (GM130, green), lysosomes (LAMP2, green), and mitochondria (COX IV, green) in human renal proximal tubule cells. Scale bars 20 μm. Right: arrowheads represent the colocalized region analyzed for profile intensity.
To understand the protein expression level, we performed WHAMM double-immunofluorescence (IF) stains with various nephron segment markers. Consistent with the snRNA-seq data, IF stains demonstrated WHAMM protein expression in almost all kidney tubule cells, including the proximal (PT, labeled by LTL), distal (labeled by KSP), collecting tubules (labeled by AQP2), and in the glomerulus (labeled by nephrin [NEPH]) (Figure 2B).
To understand the intracellular expression of WHAMM we performed double-IF stains in cultured primary mouse kidney tubule cells. WHAMM displayed colocalization with actin filaments stained with FITC-phalloidin, the microtubule marker TUB1A, the cis-Golgi marker GM130, and the lysosomal marker LAMP2, but not with the mitochondrial protein COXIV (Figure 2C). Consistent with prior publication,19 these results indicate a broad intracellular expression of WHAMM in kidney tubule cells.
Characterizing autophagy in WHAMM knockout kidney tubule cells
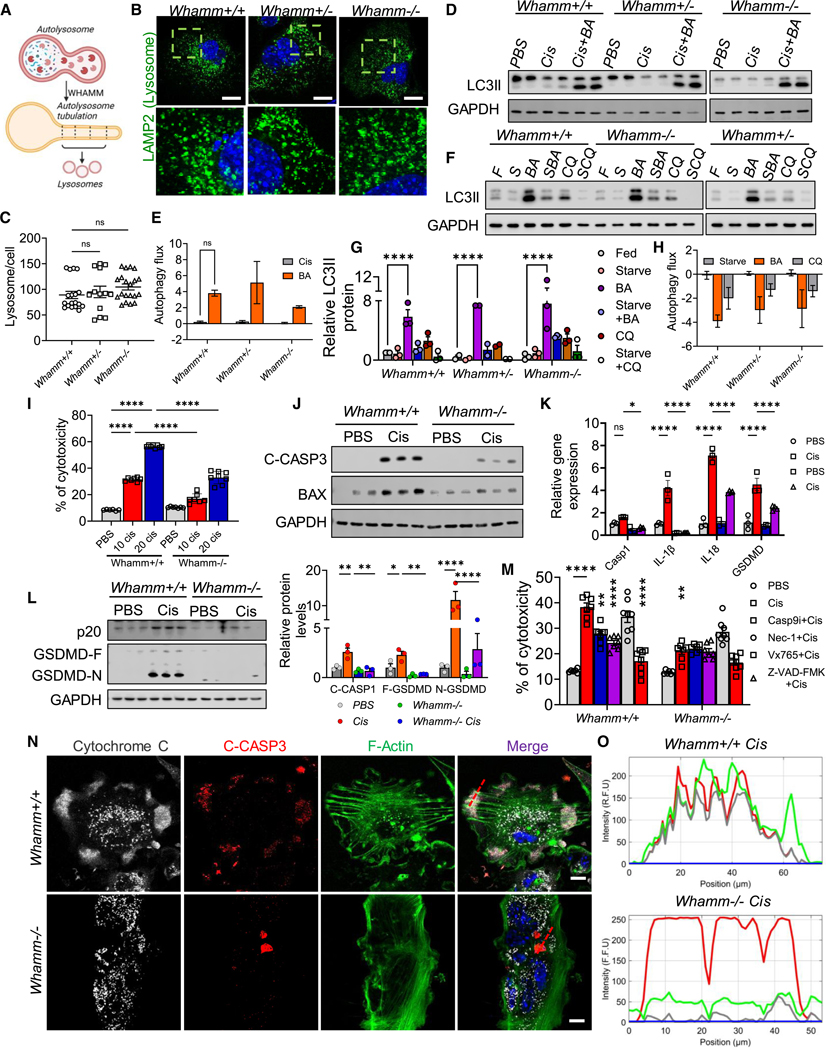
WHAMM has been recognized as an actin nucleation-promoting factor and has been postulated to have a role in autophagy, including autophagosome biogenesis34 and tubulation of autolysosomes, which is critical for lysosome reformation35 (Figure 3A). Defects in this process could alter lysosomal number. Therefore, we first analyzed lysosomal numbers in Whamm+/+, Whamm+/−, and Whamm−/− primary tubule cells. We did not find observable differences in lysosomal number in cells lacking WHAMM (Figures 3B and 3C).
Figure 3. Characterizing autophagy and cell death in WHAMM knockout primary tubule cells.
(A) The process of autolysosome reformation.
(B) Confocal imaging of LAMP2 (green) and DAPI (blue) in Whamm+/+, Whamm+/−, and Whamm−/− primary tubule cells (TECs). Scale bars, 20 μm.
(C) Quantification of the number of lysosomes per cell.
(D) Immunoblots of LC3II and GAPDH in TECs treated with 20 μM cisplatin (Cis) for 3 h, with or without bafilomycin A (Cis+BA).
(E) Autophagy flux in TECs treated with cisplatin for 3 h.
(F) Immunoblots of LC3II and GAPDH in TECs under serum starvation for 10 h with various autophagy inhibitors: fed (F), starved (S), BA in the fed state (BA), BA with starvation (SBA), chloroquine in the fed state (CQ), and chloroquine with starvation (SCQ).
(G) Quantification of LC3II protein levels in TECs treated with various autophagy inducers and blockers.
(H) Autophagy flux was measured in TECs under serum starvation for 10 h.
(I) Percent of cytotoxicity in TECs treated with PBS or 20 μM cisplatin.
(J) Immunoblots of cleaved caspase-3 (C-CASP3) and BAX in TECs treated with PBS or cisplatin.
(K) Transcript levels of Caspase 1, IL-1β, IL-18, and gasdermin d (Gsdmd) in TECs treated with PBS or cisplatin.
(L) Left: immunoblots of cleaved caspase-1 (CASP1-p20), gasdermin D full-length (GSDMD-F), cleaved (GSDMD-N) protein, and GAPDH in TECs treated with PBS or cisplatin. Right: quantification of immunoblots normalized to relative GAPDH level.
(M) Cell death analysis in TECs treated with PBS or cisplatin, in the presence of inhibitors for caspase-9 (Casp9i), pan-apoptosis (Z-VAD-FMK), necrosis (Nec-1), and pyroptosis (Vx 765).
(N) Confocal imaging of F-actin (green), cleaved-CASP3 (red), and cytochrome c (white) in TECs treated with cisplatin. Scale bars, 20 μm.
(O) Profile intensity plots of a colocalized region (red dotted lines).
Data are presented mean ± SEM. p values were determined by one-way ANOVA in GraphPad Prism 10 software. *p < 0.05, ***p < 0.001, ****p < 0.0001. See also Figures S2 and S3.
Next, we analyzed autophagy in cultured Whamm+/+, Whamm+/−, and Whamm−/− kidney tubule cells. Autophagy, being a dynamic process, it can be monitored through the levels of LC3 and lipidated-LC3 (LC3II) both at baseline and following autophagy induction by treating cells with cisplatin (20 mM for 3 h) and bafilomycin A (BA), which impedes lysosome acidification and lysosomal fusion with autophagosomes.
Cisplatin treatment induced LC3 lipidation in Whamm+/+, Whamm+/−, and Whamm−/− primary tubule cells (Figure 3D). We did not observe a difference in autophagy flux between Whamm+/+ and Whamm+/−, or Whamm−/− primary cells, following BA treatment (Figure 3E). To understand autolysosome tubulation event and autolysosome recycling in Whamm−/− cells, we analyzed these cells after 10 h of starvation. We found that lipidated LC3 levels following BA treatment (in fed state) were still not different in Whamm+/− or Whamm−/− cells compared with Whamm+/+ cells (Figures 3F and 3G). Quantification of the autophagy flux indicated similar inhibition in Whamm+/+, Whamm+/−, and Whamm−/− cells when treated with BA or chloroquine (Figure 3H).
WHAMM knockout primary tubule cells are resistant to cell death
WHAMM has also been proposed to play a role in regulating cell death.24 We did not observe differences in lactate dehydrogenase (LDH) release in cultured primary Whamm+/+ and Whamm−/− kidney tubule cells at baseline (Figure 3I). The role of WHAMM has been specifically proposed in DNA damage-induced p53-dependent and ARP2/3-mediated cell death.36 Therefore, we next treated cells with cisplatin, an agent known to induce DNA damage and kidney disease. Cisplatin-treated Whamm−/− cells exhibited lower cell death when compared with Whamm+/+ cisplatin-treated cells, as indicated by lower LDH release at all tested cisplatin concentrations (Figure 3I).
To analyze which cell death pathway might be influenced by WHAMM, we examined the effects of cisplatin treatment on necroptosis. Cisplatin treatment increased tubule cell necroptosis, as indicated by the expression of necroptosis markers MLKL and RIPK (at both RNA and protein levels), but we observed no differences between Whamm+/+ and Whamm−/− cells (Figures S2A–S2C). Similarly, gene and protein expression levels of ferroptosis markers, including ACSL4 and GPX4, were not different between Whamm+/+ and Whamm−/− cells treated with cisplatin (Figures S2D–S2F). Lipid peroxidation, an indicator of ferroptosis, estimated by BODIPY C11 staining, revealed no differences between Whamm+/+ and Whamm−/− cells (Figures S2G and S2H). Furthermore, cGAS-STING-mediated type I interferon signaling genes37 showed no observable differences between Whamm+/+ and Whamm−/− cells (Figure S2I).
On the other hand, markers of apoptosis, including Bax and Caspase3 (Casp3) were expressed at a higher level in Whamm+/+ cells treated with cisplatin, while their levels were lower in Whamm−/− cells treated with cisplatin (Figure S2J). Protein analysis further confirmed that BAX and cleaved CASP3 were markedly lower in Whamm−/− cells (Figure 3J). Immunocytochemistry of cleaved CASP3 corroborated these findings, indicating a lower rate of cell death in Whamm−/− cells compared with Whamm+/+ cells treated with cisplatin (Figure S2K).
We also investigated markers of pyroptosis, including Cas-pase1 (Casp1), gasdermin D (Gsdmd), IL-1b, and IL-18. These markers were expressed at lower levels in Whamm−/− cells (Figure 3K). Protein levels of full-length GSDMD (GSDMD-F), cleaved N-terminal GSDMD (GSDMD-N), and cleaved-CASP1 were higher in Whamm+/+ cells treated with cisplatin but were lower in Whamm−/− cells (Figure 3L). To confirm the contributions of pyroptosis and apoptosis to cisplatin-induced cell death, we treated cells with apoptosis inhibitors, including a CASP9 inhibitor (Z-LEHD-FMK), a pan-apoptosis inhibitor (Z-VAD-FMK), and a CASP1 inhibitor (VX-765). These inhibitors prevented cisplatin-induced cell death in Whamm+/+ kidney tubule cells but had no additional effect in Whamm−/− cells (Figure 3M).
These results suggest that the genetic deletion of Whamm protects cells from cisplatin-induced apoptosis and pyroptosis without notable effects on necroptosis or ferroptosis.
The role of WHAMM and actin cytoskeleton reorganization in tubular cell death
Actin exists as globular G-actin or filamentous F-actin within the cell. F-Actin can form straight filaments or branched filaments, with the latter supported by the ARP2/3 complex. WHAMM has been shown to play a role in actin nucleation and branching by binding ARP2/3 and simultaneously interacting with microtubules via its coiled-coil domain.19,21
To investigate whether the genetic deletion of WHAMM alters actin and tubulin organization in kidney tubule cells, we analyzed microtubule staining and distribution. No significant differences were observed between Whamm+/+ and Whamm−/− cells (Figures S2L and S2M). However, changes in F-actin density, the number of branched F-actin filaments, and branch features (e.g., branch length, triple points, and quadruple points) were detected in Whamm−/− cells compared with Whamm+/+ cells (Figures S2L, S3B, and S3A).
WHAMM has been implicated in cytochrome c release and apoptosome formation through its role in F-actin polymerization.36 Our primary tubule cell experiments revealed differences in both F-actin staining and cell viability. To assess the role of actin in cisplatin-induced cell death, we performed immunocytochemical analyses of cytochrome c, CASP3, and F-actin in cisplatin-treated cells. Cytochrome c, cleaved-CASP3, and F-actin colocalized in Whamm+/+ cells treated with cisplatin but not in Whamm−/− cells (Figures 3N and 3O). The percentage of cells with triple-positive puncta was higher in Whamm+/+ cells compared with Whamm−/− cells (Figure S3C).
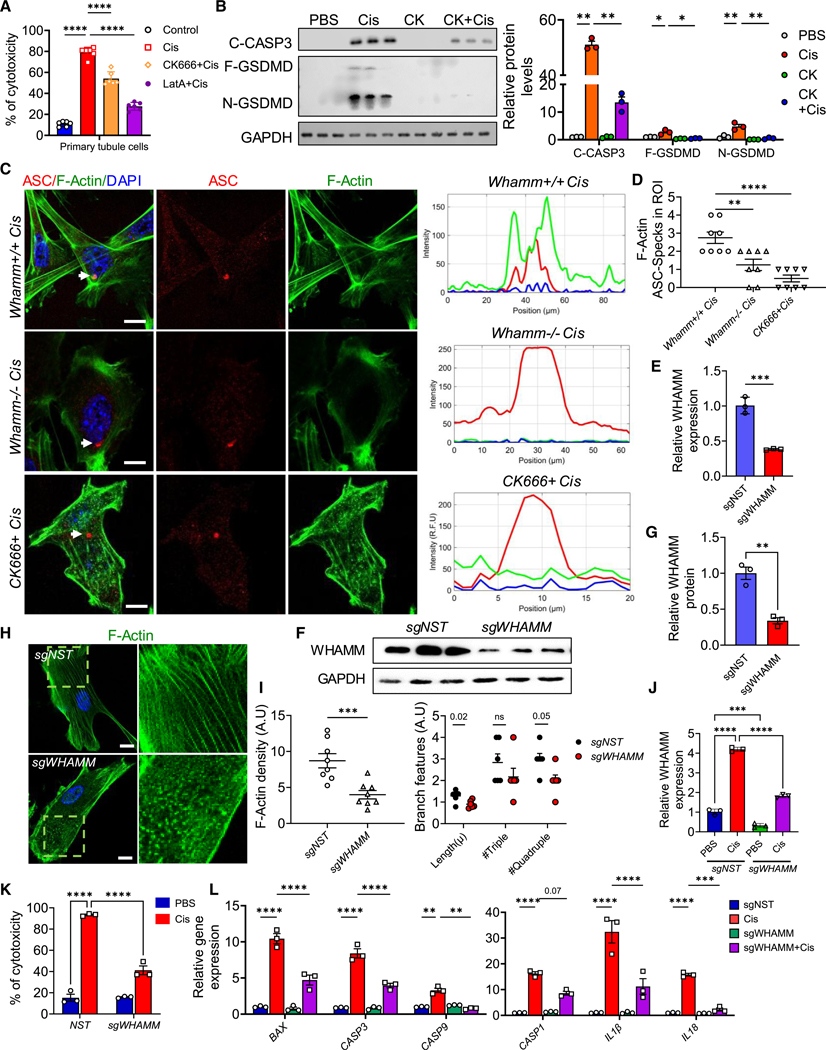
To understand the role of WHAMM’s actin nucleation activity in apoptosis, we treated primary tubule cells with CK666 (an ARP2/3 inhibitor) and latrunculin A (LatA, which prevents G-actin polymerization into F-actin). Both treatments induced F-actin depolymerization without increasing baseline cell death (Figures S3D–S3F). Immunocytochemical analyses showed that CK666 and LatA disrupted the colocalization of cytochrome c and cleaved CASP3 with F-actin (Figures S3G and S3H). Furthermore, CK666 and LatA reduced cisplatin-induced cell death (Figures 4A and S3H), with markedly lower cleaved CASP3 protein levels (Figures 4B, S3I, and S3J). These findings suggest that WHAMM facilitates F-actin polymerization and promotes cytochrome c release.
Figure 4. Role of actin polymerization during apoptosis and pyroptosis.
(A) Percent of cytotoxicity of primary tubule cells (TECs) treated with PBS or cisplatin in the presence or absence of CK666 (CK) or latrunculin A (LatA).
(B) Left: immunoblots of cleaved caspase-3 (C-CASP3), full-length gasdemrin D (F-GSDMD), cleaved GSDMD (N-GSDMD) protein, and GAPDH in TECs treated with PBS, cisplatin, or CK666 plus cisplatin. Right: quantification of immunoblots normalized to relative GAPDH level.
(C) Left: confocal imaging of F-actin and ASC in Whamm+/+ and Whamm−/− TECs treated with cisplatin, and in Whamm+/+ TECs treated with CK666 plus cisplatin. Scale bars, 20 μm. Right: ImageJ analysis for the colocalized region (arrow): (upper) Whamm+/+, (middle) Whamm−/−, (lower) Whamm+/+ with CK666.
(D) Quantification of ASC specks in TECs treated with cisplatin or CK666 plus cisplatin per region of interest (ROI).
(E) WHAMM transcript levels in human renal proximal tubular epithelial cells (RPTECs) transduced with sgWHAMM or non-target (sgNST) virus. (F) Immunoblot of WHAMM in RPTECs after CRISPRi-mediated WHAMM silencing.
(G) Quantification of WHAMM immunoblot normalized to GAPDH level.
(H) F-Actin staining in RPTECs after CRISPRi-mediated silencing of WHAMM. Scale bar, 20 μm. Box indicates zoomed area.
(I) ImageJ analysis of F-actin density and actin branch features.
(J) WHAMM transcript levels in RPTECs following CRISPRi-mediated WHAMM silencing and treated with 40 μM of cisplatin for 24 h.
(K) Percent of cytotoxicity in RPTECs after sgWHAMM or sgNST infection and treated with cisplatin for 24 h.
(L) Transcript levels of BAX, CASPASE3 (CASP3), CASP9, and CASP1, interleukin 1β (IL-1B), and IL-18 in RPTECs after sgWHAMM or sgNST infection and treated with cisplatin for 24 h. Data are presented mean ± SEM. p values were determined by one-way ANOVA or unpaired t test in GraphPad Prism 10 software.
*p < 0.05, ***p < 0.001, ****p < 0.0001. See also Figure S3.
To explore the role of F-actin in NLRP3-dependent inflammasome activation, we treated cells with CK666. CK666 inhibited the conversion of full-length GSDMD (F-GSDMD) into its active form (N-GSDMD) during cisplatin treatment, highlighting the involvement of actin regulators in pyroptosis (Figure 4B). NLRP3 inflammasome activation requires ASC speck formation,38 which localizes to regions enriched in polymerizing actin.27,28 Immunostaining revealed that ASC specks colocalized with F-actin in Whamm+/+ cells but not in Whamm−/− cells (Figure 4C). ASC speck numbers were also reduced in Whamm−/− cells compared with Whamm+/+ cells (Figure 4D). CK666 treatment efficiently inhibited ASC speck formation and its colocalization with F-actin (Figures 4C and 4D), indicating that WHAMM-dependent actin nucleation is essential for ASC speck formation during pyroptosis.
To corroborate these findings, we generated human renal proximal tubule cells (RPTECs) expressing dCas9 and transduced them with viral guide RNAs to repress WHAMM expression. Four days post-transduction, these cells exhibited reduced WHAMM RNA and protein levels, confirming successful CRISPR-mediated knockdown (Figures 4E–4G). WHAMM knockdown resulted in reduced F-actin density and branch features compared with control cells (Figures 4H and 4I). Upon cisplatin treatment, WHAMM expression was higher in control RPTECs than in sgWHAMM cells (Figure 4J), and cell death was lower in sgWHAMM cells (Figure 4K). The expression of cell death markers, including CASP3, CASP9, IL-1B, IL-18, and GSDMD, was also reduced in WHAMM knockdown cells (Figure 4L).
In summary, our results demonstrate that WHAMM-dependent actin polymerization facilitates cisplatin-induced apoptosis and pyroptosis in kidney tubule cells.
Whamm knockout mice are protected from acute kidney injury
To investigate the role of WHAMM in kidney disease development, we obtained Whamm knockout mice (B6N(Cg)-Whammtm1b(KOMP)Wtsi/3J;Whamm−/−). Transcript and protein levels of WHAMM confirmed the genetic deletion of Whamm in kidney (Figures S4A–S4C). No major differences were observed between Whamm+/+ and Whamm−/− mice under baseline conditions.39 Total L-amino acid levels in urine samples were comparable between Whamm−/− and Whamm+/+ mice (Figure S4D). Urinary KIM1 and albumin levels were similar in both groups (Figures S4E and S4F). BUN levels remained steady in both groups (Figure S4G). Kidney histological analysis using PAS staining showed no observable signs of kidney damage (Figure S4H). Gene expression and protein levels of major solute carriers and angiotensin-converting enzyme II (ACE2) were also similar between Whamm−/− mice and Whamm+/+ mice (Figures S4I and S4J). Immunostaining for ACE2 and wheat germ agglutinin revealed no notable differences between the groups (Figures 4K and S4L). However, immunoblotting for LC3 protein demonstrated increased levels of LC3I and LC3II in Whamm−/− mice compared with Whamm+/+ mice, consistent with prior findings39 (Figures S4M and S4N).
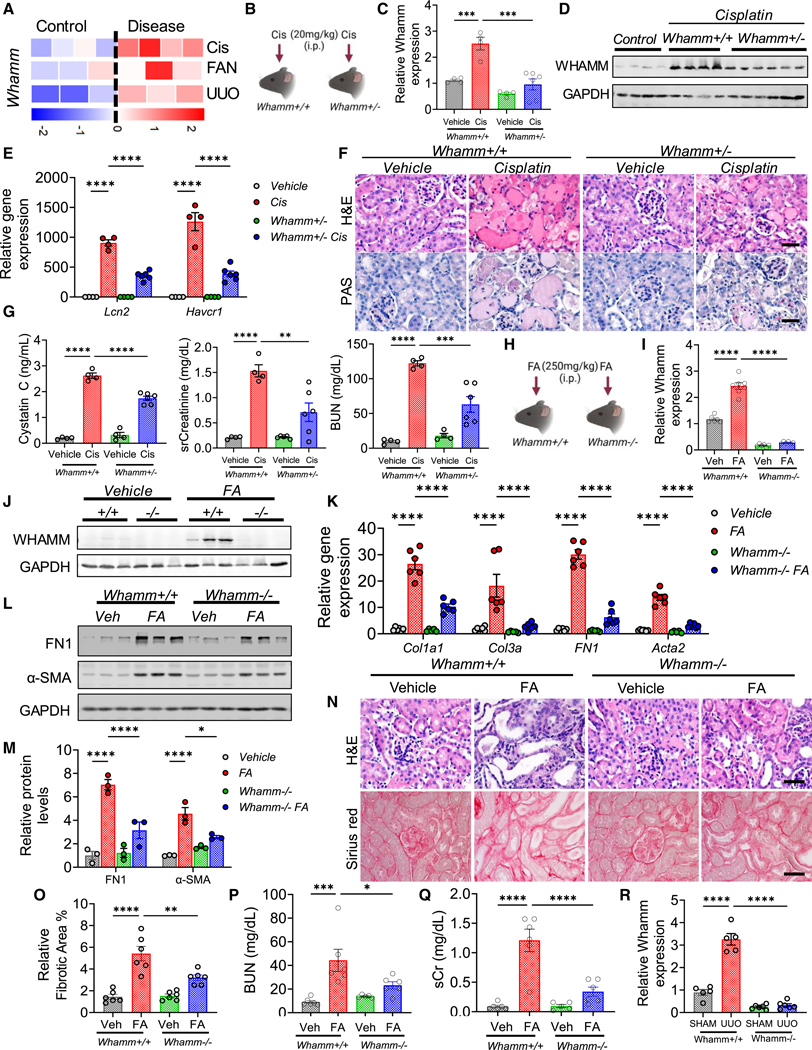
We next hypothesized that Whamm knockout mice might exhibit altered sensitivity to kidney injury (Figures 5A and 5B). To test this, we injected mice with cisplatin and analyzed their kidneys 3 days later (Figure 5B). Whamm expression was elevated following cisplatin treatment in Whamm+/+ mice but was lower in the kidneys of Whamm+/− and Whamm−/− mice (Figures 5C, 5D, and S4O–S4Q). Gene expression of acute kidney injury (AKI) markers, including Lcn2 and Havcr1, was higher in Whamm+/+ mice injected with cisplatin (Figure 5E). In contrast, Whamm+/− and Whamm−/− mice treated with cisplatin showed lower expression of these AKI markers (Figures 5E and S4R).
Figure 5. Genetic deletion of Whamm protects from cisplatin-AKI and FA-induced nephropathy.
(A) Whamm gene expression in RNA-seq from experimental mouse kidney diseases (n= 3–4 per group). Each square is one kidney sample. The rows represent the disease model, cisplatin (Cis), FAN (folic acid), UUO (unilateral-ureteral-obstruction). Colors indicate relative gene expression (blue, low; red, high).
(B) Experimental scheme of the cisplatin disease model.
(C) Whamm transcript levels in kidneys of Whamm+/+ (n = 4) and Whamm+/− mice (n = 6) injected with saline (vehicle) or cisplatin (Cis).
(D) Immunoblots of WHAMM and GAPDH in kidney lysates of vehicle- or cisplatin-treated mice.
(E) Kidney transcript levels of Lcn2 and Havcr1 in cisplatin-injected mice.
(F) H&E and PAS kidney sections from cisplatin-injected mice.
(G) Cystatin C, serum creatinine (sCr), and blood urea nitrogen (BUN) in serum samples of cisplatin-injected mice.
(H) Experimental scheme of the folic acid (FA) disease model.
(I) Transcript levels of Whamm in Whamm+/+ (n = 6) and Whamm−/− mice (n = 6) injected with FA or vehicle (Veh).
(J) Immunoblots of WHAMM and GAPDH in kidney lysates of mice injected with FA.
(K) Transcript levels of collagen type 1 (Col1a1), type 3a (Col3a), fibronectin (FN1), and alpha smooth muscle actin (Acta2) in mice injected with FA.
(L) Immunoblots of FN1, α-SMA, and GAPDH in kidney lysates of mice injected with FA.
(M) Quantification of FN1 and α-SMA normalized to relative GAPDH level.
(N) H&E and Sirius red staining in kidney sections of mice injected with FA. Scale bars, 20 μm.
(O) Relative percentage of kidney fibrosis in mice injected with FA.
(P) BUN in the FA model.
(Q) sCr in the FA model. R. Whamm transcript levels in Whamm+/+ (n = 5) and Whamm−/− mice (n = 6) in SHAM and UUO operated kidneys. Data are presented mean ± SEM. p values were determined by one-way ANOVA in GraphPad Prism 10 software. *p < 0.05, ***p < 0.001, ****p < 0.0001. See also Figures S4 and S5.
Histological analysis revealed less tubular injury in Whamm−/− mice compared with Whamm+/+ mice treated with cisplatin (Figures 5F and S4S). Indicators of kidney function, including cystatin C, sCr, and BUN levels, were lower in Whamm+/− and Whamm−/− mice compared with Whamm+/+ mice following cisplatin treatment (Figures 5G and S4T). These results suggest that Whamm genetic deletion ameliorates cisplatin-induced AKI.
WHAMM knockout mice are protected from the development of fibroinflammation
To understand WHAMM’s role in kidney fibrosis development, we injected mice with 250 mg/kg of folic acid (FA) and analyzed the outcomes on day 7 (Figure 5H). Both gene expression and protein levels of WHAMM were higher in Whamm+/+ mice injected with FA compared with the vehicle controls (Figures 5I, 5J, S5A, and S5B). Gene expression analysis of fibrosis markers such as collagen type 1a1 (Col1a1), collagen type 3a (Col3a), fibronectin 1 (Fn1), and alpha-smooth muscle actin (Acta2) were in line with kidney fibrosis observed in Whamm+/+ mice injected with FA (Figure 5K). Gene markers of fibrosis were lower in Whamm+/− or Whamm−/− mice injected with FA (Figures 5K and S5C). Western blotting of FN1 and α-SMA showed that their protein levels were higher in Whamm+/+ mice than in Whamm+/− or Whamm−/− mice (Figures 5L, 5M, and S5D). Histopathological damage and kidney function parameters, including BUN and sCr levels, were higher in Whamm+/+ mice injected with FA, and were improved in Whamm+/− or Whamm−/− mice (Figures 5N–5Q, S5E, and S5F), indicating that the genetic deletion of WHAMM mitigates kidney injury.
We next analyzed Whamm mice in a unilateral ureteral obstruction (UUO)-induced kidney fibrosis model. WHAMM expression at both RNA and protein levels was higher in UUO model of kidney fibrosis (Figures 5R and S5G). Gene expression analysis of fibrosis markers such as Col1a1, Col3a, Fn1, Acta2, vimentin, and Tgfβ1 was consistently lower in Whamm+/− or Whamm−/− mice compared with Whamm+/+ mice with UUO injury (Figures S5H and S5I). Protein levels of FN1 and α-SMA were lower in Whamm+/− or Whamm−/− mice (Figures S5J–S5M). Histological analysis revealed that kidney injury and fibrosis were less severe in Whamm+/− or Whamm−/− mice compared with Whamm+/+ mice with UUO (Figure S5N).
These results suggest that WHAMM plays a role in kidney fibrosis development. Genetic deletion of WHAMM protects mice from AKI and fibrosis.
Whamm deletion is associated with lower pyroptosis, and apoptosis in vivo
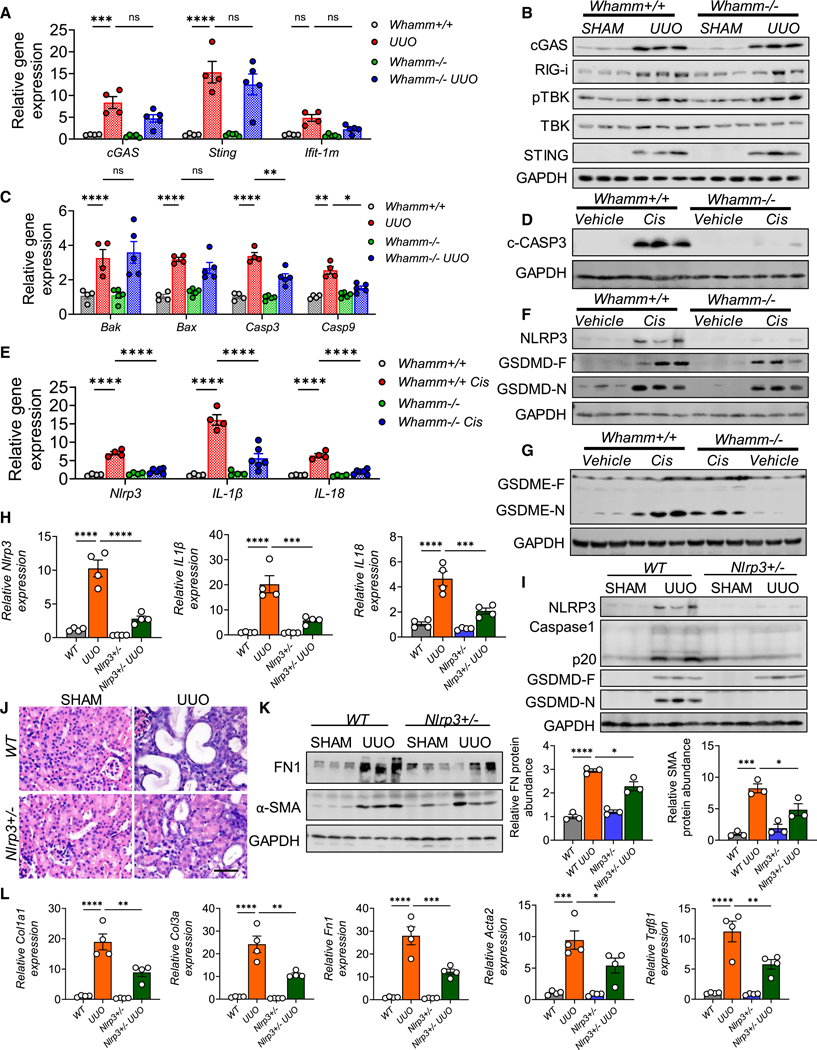
To examine cell death pathways in Whamm+/+ and Whamm−/− kidneys, we analyzed baseline and post-injury conditions. Gene expression levels of cGAS, Sting, and Ifit1m were similar in Whamm−/− and Whamm+/+ mice subjected to UUO (Figure 6A). Similarly, protein levels of cGAS, STING, RIG-I, pTBK, and TBK were comparably elevated in Whamm−/− and Whamm+/+ UUO mice (Figure 6B). Ferroptosis-associated genes, including Acsl4 and Gpx4, exhibited similar changes in Whamm+/+, Whamm+/−, and Whamm−/− mice following cisplatin or UUO treatment (Figures S6A and S6B).
Figure 6. Lower apoptosis and NLRP3 inflammasome activation in Whamm−/− mice.
(A) Transcript levels of cGAS, Sting, and Ifit-1m in wild-type (Whamm+/+) (n = 4) and Whamm knockout (Whamm−/−) mice (n = 6) following UUO or SHAM operation.
(B) Immunoblots of cGAS, RIG-i, pTBK, TBK, STING, and GAPDH in the UUO model.
(C) Transcript levels of Bak, Bax, caspase-3 (Casp3), and Casp9 SHAM or UUO operated kidneys in Whamm+/+ (n = 4) and Whamm−/− mice (n = 5).
(D) Immunoblots of cleaved caspase-3 (C-CASP3), and GAPDH in the cisplatin model.
(E) Transcript levels of Nlrp3, IL-1β, and IL-18 in Whamm+/+ (n = 4) and Whamm−/− mice (n = 6) injected with vehicle or cisplatin.
(F) Immunoblots of NLRP3, gasdermin D full-length (GSDMD-F), cleaved (GSDMD-N) forms, and GAPDH in the cisplatin model.
(G) Immunoblots of GSDME-F, GSDME-N, and GAPDH cisplatin models.
(H) Transcript levels of Nlrp3, IL-1β, and IL-18 in WT (n = 4) and Nlrp3+/− (n = 4) subjected to SHAM or UUO injury.
(I) NLRP3, caspase-1, p20 (cleaved caspase-1), GSDMD-F, and GSDMD-N immunoblots in the UUO model.
(J) H&E-stained kidney images. Insets are zoomed regions. Scale bar, 20 μm.
(K) Left: immunoblots of FN1, α-SMA, and GAPDH in UUO kidneys. Right: quantification of FN1 and α-SMA normalized to the relative GAPDH level.
(L) Transcript levels of Col1a1, Col3a, Fn1, Acta2Tgfβ1 in UUO kidneys of WT and Nlrp3+/− mice (n = 4). Data are presented mean ± SEM. p values were determined by one-way ANOVA in GraphPad Prism 10 software. *p < 0.05, ***p < 0.001, ****p < 0.0001. See also Figure S6.
In contrast, Casp3 and Casp9, markers of apoptosis showed marked differences between Whamm+/+ and Whamm−/− UUO mice (Figure 6C). Protein levels of cleaved-CASP9 and CASP3 were higher in Whamm+/+ kidneys subjected to UUO, cisplatin, and FA injury but were lower in Whamm−/− kidneys (Figures 6D, S6B, and S6C). BAX protein levels were elevated in both Whamm+/+ and Whamm−/− UUO kidneys (Figure S6B).
We then assessed NLRP3 inflammasome activation in the cisplatin-induced AKI model. Cisplatin treatment resulted in increased expression of Nlrp3, IL-1b, and IL-18 in Whamm+/+ mice (Figure 6E), while Whamm+/− and Whamm−/− mice displayed reduced expression of these markers (Figures 6E and S6D). Protein levels of NLRP3, GSDMD, cleaved GSDMD (N-GSDMD), and cleaved GSDME were higher in cisplatin-treated Whamm+/+ mice but not in Whamm−/− mice (Figures 6F, 6G, and S6E). These results highlight reduced NLRP3 inflammasome activation in the absence of WHAMM.
We further analyzed kidney fibrosis models. Whamm+/+ mice exhibited higher expression of Nlrp3, IL-1β, and IL-18 (Figures S6F–S6H). In contrast, Whamm+/− and Whamm−/− kidneys showed reduced expression of these genes following UUO or FA injection (Figures S6F–S6H). Protein levels of NLRP3, F-GSDMD, N-GSDMD, and cleaved GSDME were also lower in Whamm+/− and Whamm−/− kidneys compared with Whamm+/+ kidneys subjected to UUO or FA injury (Figures S6I–S6L).
Given the role of WHAMM in NLRP3 inflammasome activation, we next analyzed Nlrp3 heterozygous (Nlrp3+/−) mice following UUO surgery. Wild-type (WT) UUO mice showed elevated expression of Nlrp3, IL-1β, and IL-18 (Figure 6H), while Nlrp3+/− mice displayed reduced RNA and protein levels of NLRP3, CASP1, GSDMD, and N-GSDMD (Figures 6H and 6I). Histological analysis demonstrated less tubular injury in Nlrp3+/− mice compared with WT mice (Figure 6J). Profibrotic markers such as FN1 and α-SMA, along with profibrotic gene expression (Cola1, Col3a, Fn1, Acta2, and Tgfβ1), were lower in Nlrp3+/− UUO kidneys compared with WT UUO kidneys (Figures 6K and 6L).
In conclusion, our findings indicate that WHAMM plays a critical role in NLRP3 inflammasome activation and fibrosis development. WHAMM knockout mice are protected against kidney injury, likely due to reduced apoptosis and attenuated NLRP3 inflammasome activation.
CK666 attenuates cisplatin-induced AKI and adenine CKD
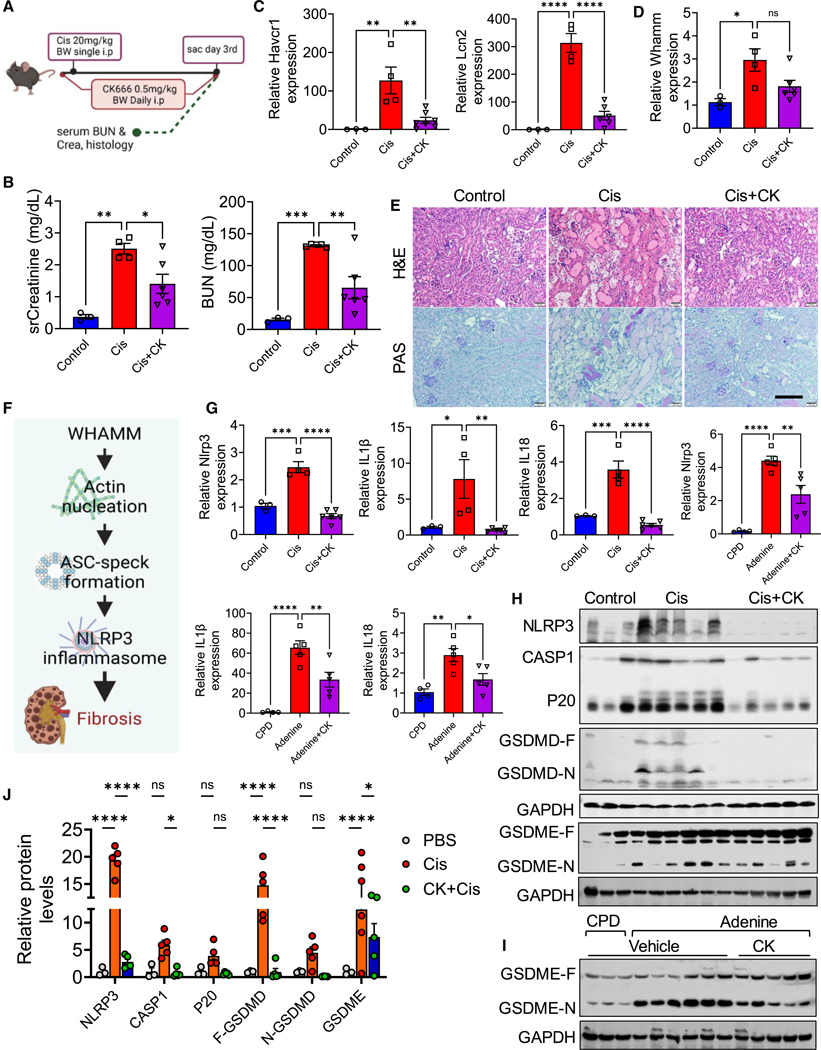
Our studies demonstrated the role of WHAMM and the cellular actin network in mediating pyroptosis and kidney disease development. To explore whether pharmacological targeting of these pathways could protect mice from kidney disease, we investigated the effect of CK666, an inhibitor of ARP2/3-mediated actin polymerization—a step that requires WHAMM (Figure 7A). sCr and BUN levels were lower in mice injected with CK666 compared with those treated with cisplatin alone (Figure 7B). Kidney injury markers, such as Havcr1 and Lcn2, showed reduced transcript levels in CK666-treated mice compared with cisplatin-only mice (Figure 7C). Whamm expression was higher in the kidneys of mice treated with CK666 and cisplatin (Figure 7D). Histological analysis revealed that CK666 treatment protected mice from cisplatin-induced AKI (Figure 7E).
Figure 7. Pharmacological disruption of actin filament branching and network formation in AKI and CKD.
(A) Experimental scheme of generating the CK666 (CK)-cisplatin model (CK-Cis).
(B) Serum creatinine (sCr) and blood urea nitrogen (BUN) in the CK-Cis model (n = 3–6 mice per group).
(C) Transcript levels of Havcr1, and Lcn2 in the CK-Cis model.
(D) Whamm transcript level in the CK-Cis model.
(E) H&E and PAS staining in kidney sections from the CK-Cis model. Scale bar, 50 μm.
(F) The proposed mechanism for WHAMM in kidney disease.
(G) Transcript levels of Nlrp3, IL-1β, and IL-18 in the CK-Cis model or mice fed on adenine or control diet (CPD) (n = 4–5 mice per group).
(H) Immunoblots of NLRP3, CASP1, cleaved CASP1 (P20), GSDMD-F, GSDMD-N, and GSDME in the CK-Cis model.
(I) Immunoblots for cleaved GSDME and GAPDH in kidneys of control (n = 3) or adenine-fed mice (n = 6) injected with CK666 (n = 5).
(J) Quantification of NLRP3, CASP1, cleaved CASP1 (P20), GSDMD-F, GSDMD-N, and cleaved GSDME immunoblots in the CK-Cis model. Data are presented mean ± SEM. p values were determined by one-way ANOVA in GraphPad Prism 10 software. *p < 0.05, ***p < 0.001, ****p < 0.0001. See also Figure S7.
Next, we tested CK666 in an adenine-induced CKD mouse model (Figure S7A). Mice treated with CK666 exhibited lower sCr and BUN levels compared with vehicle-treated mice on an adenine diet (Figure S7B). Whamm expression was lower in the kidneys of CK666-treated mice compared with adenine-fed, vehicle-treated controls (Figure S7C). Histological analysis using H&E and Sirius red staining indicated that CK666 effectively prevented adenine-induced CKD (Figure S7D). Furthermore, fibrosis marker genes, including Acta2 (αSMA), Fn1 (FN1), Col1a1, Col3a, Tgfβ1, and Pai1, were expressed at lower levels in CK666-treated mice compared with vehicle-treated mice (Figure S7E).
Mechanistic studies in cells indicated that WHAMM-mediated actin nucleation facilitates ASC speck formation, driving NLRP3 inflammasome activation and contributing to kidney fibrosis (Figure 7F). Therefore, we analyzed apoptosis and NLRP3 inflammasome markers in the cisplatin-AKI and adenine-CKD models treated with CK666. Gene expression of Casp3, Bax, and Casp9 was elevated in mice treated with cisplatin or fed on adenine diet, but their expression was abrogated in CK666-treated mice (Figures S7F and S7G). Protein levels of cleaved CASP3 were reduced in CK666-treated mice compared with cisplatin-only mice (Figure S7H).
Similarly, gene expression of Nlrp3, IL-1β, and IL-18 was elevated in mice treated with cisplatin or fed an adenine diet, but was lower following CK666 treatment (Figure 7G). Protein levels of NLRP3, CASP1, cleaved CASP1 (P20), GSDMD-F, GSDMD-N, and cleaved GSDME were lower in CK666-treated mice compared with mice treated with cisplatin or fed an adenine diet (Figures 7H–7J and S7I).
These findings highlight the critical role of the actin network in both AKI and CKD. They also suggest the potential therapeutic use of small-molecule actin network disruptors, such as CK666, for the treatment of kidney diseases.
DISCUSSION
In this study, we identified WHAMM as a novel gene implicated in kidney disease. By integrating diverse approaches from human genetics, genomics, epigenetics, and single-cell analyses, we highlighted WHAMM on chromosome 15 as a causative gene for kidney dysfunction. Using a combination of in vivo and in vitro models, as well as small-molecule inhibitors, we elucidated the role of WHAMM in kidney disease development. Elevated expression of WHAMM was observed in both human patients and animal models of kidney disease. Mice lacking WHAMM were resistant to AKI and fibrosis.
Furthermore, our in vitro studies demonstrated the involvement of cytoskeletal proteins in cell death processes, including apoptosis and pyroptosis, thereby establishing a mechanistic link between genetic mapping and kidney disease pathogenesis. These findings suggest that targeting regulators of the actin cytoskeleton could present promising therapeutic strategies for combating kidney disease.
To identify promising drug targets, we analyzed the heritability of kidney function in over 2.27 million individuals,30 uncovering a link between kidney dysfunction and nucleotide variants. Subsequent eQTL analysis revealed that risk genotypes are associated with increased expression of WHAMM. Our previous work, employing TWAS40 and SMR41 analyses, also demonstrated a correlation between risk variants and elevated WHAMM expression, which was further associated with decreased eGFR in GWAS samples. While computational tools are invaluable for initial gene prioritization, animal and cell systems remain essential to establish the causal role of specific genes in disease development.
WHAMM has been demonstrated to play a role in the organization of actin, microtubules, and membranes.19 Previous studies have suggested that WHAMM is involved in autophagosome biogenesis,34 and autolysosome reformation.35 However, our comprehensive analyses of kidney tubule cells, using western blotting and immunocytochemistry, did not reveal consistent WHAMM-associated changes in autophagy initiation, flux, or lysosome number. We discovered that WHAMM promotes actin polymerization following injury to kidney tubule cells. WHAMM deletion was associated with a decrease in both apoptosis and NLRP3 inflammasome activation. Notably, apoptosis was diminished in primary tubule cells derived from WHAMM knockout mice, likely due to a delayed release of cytochrome c, which relies on WHAMM-dependent, ARP2/3-mediated actin nucleation.24,36 In addition, WHAMM loss reduced the formation of ASC specks and their localization with F-actin. These findings suggest that WHAMM-dependent actin nucleation is essential for ASC speck formation during pyroptosis.
In our study, we employed WHAMM knockout mice. These mice exhibited no baseline differences but demonstrated marked resistance to the onset of kidney disease, including acute injury induced by cisplatin and CKD and fibrosis caused by UUO. WHAMM deletion was associated with reduced apoptosis and NLRP3 inflammasome activation.
Importantly, treatment with CK666, an inhibitor of ARP2/3-mediated actin polymerization—a process requiring WHAMM— mitigated both cisplatin-induced AKI and adenine-induced CKD. Notably, CK666 has also been shown to reduce idiopathic pulmonary fibrosis42 in mouse models, suggesting a broader therapeutic potential for CK666 in chronic fibrotic diseases.
In conclusion, our study identifies WHAMM, a regulator of the actin and microtubule network, as a critical kidney disease gene. Our findings reveal that WHAMM plays a pivotal role in ARP2/3-mediated actin polymerization by regulating apoptosis and pyroptosis, thereby contributing to kidney disease. This work not only uncovers a novel mechanism underlying kidney disease but also highlights small-molecule therapeutics as a promising approach to treating this debilitating condition.
Limitations of the study
First, the analyzed GWAS locus contains multiple signals and numerous genes. While we specifically focused on WHAMM at this locus, other genes at this locus may also be kidney disease risk genes. Second, although we extensively investigated the role of WHAMM in relation to its actin nucleation function, we did not explore its involvement in anterograde transport, another process in which WHAMM has been implicated. Third, while our data indicate that WHAMM contributes to kidney tubule cell death through NLRP3 inflammasome activation, the precise involvement of actin and molecules directly interacting with WHAMM in these processes remains to be fully elucidated. One potential explanation is that ARP2/3-bound actin may recruit pyrin and ASC to these pathways.27 In addition, although a previous study39 reported abnormalities in the proximal tubule cells of Whamm−/− mice, we were unable to replicate these findings, apart from observing some changes in the expression of autophagy-related proteins.
To address these limitations, future investigations should employ advanced cell and molecular biology tools, such as fusion constructs, mito-QC, and knockin and knockout mice, to provide a more comprehensive understanding of the molecular interactions underlying kidney disease.
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Katalin Susztak (ksusztak@pennmedicine.upenn.edu).
Materials availability
All commercial materials used in this study are reported in the key resources table. Unique materials will be shared by the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
|
| ||
| Antibodies | ||
|
| ||
| Rabbit WHAMM | Abcam | Cat#ab122572; RRID: AB_11128128 |
| Rabbit WHAMM | Millipore | Cat#ABT60; RRID: AB_11204195 |
| Rabbit cleaved CASP3 | Cell Signaling Technology | Cat#9664S; RRID: AB_2070042 |
| Rabbit RIPK1 | ThermoFisher Scientific | Cat#PA5–20811; RRID: AB_11154790 |
| Rabbit RIPK3 | Millipore | Cat#PRS2283; RRID: AB_1856303 |
| Rabbit ACLS4 | Abcam | Cat#ab155282; RRID: AB_2714020 |
| Rabbit GPX4 | ThermoFisher Scientific | Cat#MA5–35089; RRID: AB_2848994 |
| Mouse NLRP3 | Adipogen | Cat#AG-20B-0014; RRID: AB_2885199 |
| Mouse CASP1 | Adipogen | Cat#AG-20B-0042; RRID: AB_2755041 |
| Rabbit cleaved GSDMD | Cell Signaling Technology | Cat#36425S; RRID: AB_ 2799099 |
| Rabbit GSDME | Protein Tech | Cat#13075–1-AP; RRID: AB_2093053 |
| Rabbit FN1 | Abcam | Cat#2413; RRID: AB_2262874 |
| Mouse aSMA | ThermoFisher Scientific | Cat#MA1–06110; RRID: AB_557419 |
| Rabbit CASP9 | Abcam | Cat#ab202068; RRID: AB_2889070 |
| Rabbit BAX | Cell Signaling Technology | Cat#2772S; RRID: AB_10695870 |
| Rabbit LC3 | Cell Signaling Technology | Cat#2557S; RRID: AB_915950 |
| Rabbit cGAS | Cell Signaling Technology | Cat#31659S; RRID: AB_2799008 |
| Rabbit TBK | Cell Signaling Technology | Cat#38066S; RRID: AB_2827657 |
| Rabbit phospho TBK | Cell Signaling Technology | Cat#5483S; RRID: AB_10693472 |
| Rabit Rig-i | Cell Signaling Technology | Cat#3743S; RRID: AB_2269233 |
| Rabbit STING | Cell Signaling Technology | Cat#13647S; RRID: AB_2732796 |
| Mouse Rabbit cytochrome c | Cell Signaling Technology | Cat#12963S; RRID: AB_2637072 |
| Mouse KSP | Santacruz Biotechnologies | Cat#sc-393153; RRID: AB_3665346 |
| Goat AQP2 | Santacruz Biotechnologies | Cat#sc-9882; RRID: AB_2289903 |
| Mouse NPHS1 | Santacruz Biotechnologies | Cat#sc-377246; RRID: AB_3665345 |
| Goat ACE2 | ThermoFisher Scientific | Cat#PA5–47488; RRID: AB_2606505 |
| Rabbit ACE2 | ThermoFisher Scientific | Cat#MA5–32307; RRID: AB_2809589 |
| Rabbit SLC6A13 | ThermoFisher Scientific | Cat#PA5–68331; RRID: AB_2691971 |
| Rabbit ASC | ThermoFisher Scientific | Cat#PA5–50915; RRID: AB_2636363 |
| LTL Fluorescein | Vector labs | Cat#FL-1321; RRID: AB_2336558 |
| Wheat Germ Agglutinin Alexa 488 | ThermoFisher Scientific | Cat#W11261, RRID: AB_3665707 |
| FITC-Phalloidin | Invitrogen | Cat#F432; RRID: AB_3665343 |
| Mouse COX IV | Cell Signaling Technology | Cat#11967S; RRID: AB_2797784 |
| Mouse GM130 | ThermoFisher Scientific | Cat#MA5–47668; RRID: AB_2942649 |
| Rat LAMP2 | DHSB | Cat#GL2A7; RRID: AB_2314736 |
| Mouse TUB1A | Invitrogen | Cat#A11126; RRID: AB_2534135 |
| Mouse GAPDH | Invitrogen | Cat#MA1–16757; RRID: AB_568547 |
| Anti rabbit IgG Dy Light 800 | Cell Signaling Technology | Cat#5151S; RRID: AB_10697505 |
| Anti mouse IgG Dy Light 680 | Cell Signaling Technology | Cat#5470S; RRID: AB_10696895 |
| Anti rabbit IgG HRP conjugate | Cell Signaling Technology | Cat#7074S; RRID: AB_2099233 |
| Anti mouse IgG HRP conjugate | Cell Signaling Technology | Cat#7076S; RRID: AB_330924 |
|
| ||
| Bacterial and virus strains | ||
|
| ||
| Stbl3 E. coli | ThermoFisher Scientific | Cat#C737303 |
| Human WHAMM virus | This paper | N/A |
|
| ||
| Biological samples | ||
|
| ||
| Mouse primary cells (WHAMM KO) | This paper | N/A |
| Mouse kidney tissues | This paper | N/A |
| Mouse kidney sections | N/A | |
| Human RPTECs (WHAMM KO) | This paper | N/A |
| Human kidney sections | This paper | N/A |
|
| ||
| Chemicals, peptides, and recombinant proteins | ||
|
| ||
| Folic acid | ACROS Organics | Cat#216632500 |
| NAHCO3 | USB Chemicals | Cat#21602 |
| Cisplatin | Cayman | Cat#13119 |
| T4 Polynucleotide Kinase | New England Biolabs | Cat#M0201S |
| T4 DNA ligase | New England Biolabs | Cat#B0202S |
| Bsmb I | New England Biolabs | Cat#R0739L |
| Polyethyleneimine | Tocris | Cat#7854 |
| Polybrene | Santa cruz Biotechnologies | Cat#sc-134220 |
| Collagenase IV | Worthington Biochemical | Cat#LS004197 |
| Z-LEHD-FMK | MedChem Express | Cat#HY-P1010 |
| Vx-765 | Cayman | Cat#28825 |
| Nec-1 | Cayman | Cat#11658 |
| Z-VAD(OMe)-FMK | Cayman | Cat#11658 |
| CK666 | Cayman | Cat#29038 |
| Cytochalasin D | Cayman | Cat#11330 |
| Latrunculin A | Cayman | Cat#10010630 |
|
| ||
| Critical commercial assays | ||
|
| ||
| Blood Urea Nitrogen | Pointe Scientific | Cat#B7552150 |
| Serum Creatinine | Diazyme | Cat#DZ072B-KY1 |
| Cystatin C ELISA | ThermoFisher scientific | Cat#EMCST3 |
| High-capacity cDNA Reverse Transcription | Applied Biosystems | Cat#4368813 |
| Hoechst 33342 | ThermoFisher Scientific | Cat#62249 |
| BODIPY™ 581/591 C11 | ThermoFisher Scientific | Cat#D3861 |
| hRPTECs media supplements | ATCC | Cat#ACS-4007 |
| RPMI media | Corning | Cat#MT10–040-CM |
| L-amino acid quantitation kit | Sigma-Aldrich | Cat#MAK002 |
| SDS Blue loading buffer pack | Cell Signaling Technology | Cat#7722 |
| Mouse KIM-1 ELISA | ThermoFisher Scientific | Cat#EMHAVCR1 |
| Mouse albumin ELISA | ThermoFisher Scientific | Cat#EEL119 |
| Non-radioactive cytotoxicity assay | Promega | Cat#G1780 |
|
| ||
| Deposited data | ||
|
| ||
| Human kidney micro dissected eQTLs | Qiu et al.9; Sheng et al.43; Susztak lab | http://susztaklab.com/eQTLci/index.php |
| Kidney Meta GWAS | Liu et al.30; Susztak lab | http://www.susztaklab.com/GWAS/GWAS.php |
| Human kidney methylation QTL | Liu et al.7; Susztak lab | http://www.susztaklab.com/Kidney_meQTL/index.php |
| Human kidney RNA-seq, scRNA-seq, snRNA-seq, snATAC-seq, and single cell Multi-Ome | Liu et al.30; CMDA.org | CMDGA: https://cmdga.org/search/?type=Experiment&searchTerm=FNIH0000000 |
| Multi species integrated single cell atlas | Susztak lab | https://susztaklab.com/SISKA/ |
| Human kidney H3K4me3, and H3K27ac seq | GEO website | GEO: GSM621648; GEO: GSM1112806 |
| Mice kidney bulk RNA seq | Doke et al.16; Dhillon et al.44; GEO website | GEO: GSE207587; GEO: GSE156686 |
| Raw western blots | This paper; Mendeley Data | Mendeley Data: https://data.mendeley.com/preview/yyjfghps97?a=53c81718-9cc1-4c9f-a255-5cad98580b83 |
|
| ||
| Experimental models: Cell lines | ||
|
| ||
| pLXSN-hTERT (human RPTECs) | ATCC | Cat#CRL-4031 |
| HEK293T | ATCC | Cat#CRL-3126 |
|
| ||
| Experimental models: Organisms/strains | ||
|
| ||
| B6N(Cg)-Whammtm1b(KOMP)Wtsi/3J | Jackson Laboratory | Stock#027472 |
| Nlrp3 / mice | Jackson Laboratory | Stock#21302 |
|
| ||
| Oligonucleotides | ||
|
| ||
| WHAMM-sg1-oligo 1 CACCGTCGGACTGTCCCGTGACAGG |
This paper | N/A |
| WHAMM-sg1-oligo 2 AAACCCTGTCACGGGACAGTC CGAC |
This paper | N/A |
| WHAMM-sg2-oligo 1 CACCGACGACCGTACCGCGCA GCAG |
This paper | N/A |
| WHAMM-sg2-oligo 2 AAACCTGCTGCGCGGTACGGT CGTC |
This paper | N/A |
| Mouse and human qRT PCR primers, see Table S2 | This paper | N/A |
|
| ||
| Recombinant DNA | ||
|
| ||
| LRG2.1 | Addgene | Cat#108098 |
| psPAX2 | Addgene | Cat#12260 |
| pMD2.G | Addgene | Cat#12259 |
| dCas9 (pHR-UCOE-SFFV-dCas9-mCherry-ZIM3-KRAB) | Addgene | Cat#154473 |
|
| ||
| Software and algorithms | ||
|
| ||
| Fiji ImageJ | NIH | https://imagej.net/software/fiji/ |
| ImageJ | NIH | https://imagej.nih.gov/ij |
| Image Studio lite Version 6.0 | Li-COR Bio | https://www.licor.com/bio/image-studio-lite/ |
| QuantStudio 5 | ThermoFisher Scientific | https://www.thermofisher.com |
| Leica Microsystems Acquisition and image processing | Leia Microsystems | Stellaris 5.0; RRID: SCR_022373 |
| Cell sense software | Evident Scientific | https://evidentscientific.com/en/software/cellsens |
| Biotek Gen 5 software | Agilent | https://www.agilent.com/imager-reader-control-analysis-software |
| R studio 4.4.2 | Posit | https://posit.co/ |
| Prism 10 | GraphPad | https://www.graphpad.com |
|
| ||
| Other | ||
|
| ||
| 0.2% adenine diet | Inotiv, USA | Cat#TD.160020 |
| Control diet (casein calcium phosphate adjusted diet) | Inotiv, USA | Cat#TD.150303 |
| Standard Chow diet | LabDiet | Cat#5010 |
| Omni Homogenizer | OMNI-GLH | Cat#LR60902 |
| Quant Studio 5 Realtime quantitative PCR | Applied Biosystems | Cat#A34322 |
| Leica Stellaris Confocal Microscope | Leica Microsystems | Cat#SCR-02273 |
| Olympus Fluorescence Microscope with DP73 camera | Olympus | Cat#Olympus BX43 Model U-LHLEDC |
| X-cite series fluorescence light source for Olympus microscope | Lumen Dynamics | Cat#XI120-Q model |
| Li-COR Imager | L-COR Bio | Cat#Odyssey-Fc Model 2800 |
Data and code availability
This study does not report or use any custom computer code. The GWAS, eQTL, and multi-species snRNA-seq data are available to the public at https://susztaklab.com/. This work did not generate a new sequencing data or computational code. However, the work utilized previously published single nuclear ATAC-seq, single nuclear Multi-Ome, and single-cell and nuclear RNA-seq datasets that were deposited with the Common Metabolic Diseases Genome Atlas (CMDGA: https://cmdga.org/search/?type=Experiment&searchTerm=FNIH0000000). Kidney H3K4me3 and H3K27ac data are available with accession numbers GEO: GSM621648 and GEO: GSM1112806 at the GEO website. Mice kidney RNA-seq raw data from injury models have been previously published with accession numbers GEO: GSE207587 and GEO: GSE156686. All other data needed to evaluate the conclusions are presented in the paper and the supplemental information. Original immunoblots have been deposited at Mendeley (Mendeley Data: https://doi.org/10.17632/yyjfghps97.1) and are publicly available as of the date of publication. Microscopy data reported in this paper will be shared by the lead contact upon request.
STAR★METHODS
EXPERIMENTAL MODEL AND STUDY PARTICIPANT DETAILS
Human kidney sections acquisition
The human kidney sample collection protocol was approved by the Institutional Review Board (IRB) of the University of Pennsylvania. Tumor-adjacent nephrectomies, were de-identified, and clinical data were collected by an external honest broker to ensure patient confidentiality. This study was classified as IRB-exempt (Exemption Category IV) since no personal identifying information was collected. Pathological scoring was performed by a local nephropathologist.
Animal studies
The Institutional Animal Care and Use Committee (IACUC) at the University of Pennsylvania approved all animal related protocols. The Whamm knockout mice were procured from Jackson labs (#027472, Jackson). Mice were housed in a temperature- and humidity-controlled pathogen-free animal facility with a 12-h day and night cycle. They were provided with a standard chow diet (#5010, LabDiet) and water ad libitum.
Seven-week-old male Whamm+/+ and Whamm−/− mice were randomly assigned to control and experimental groups (unilateral ureteral obstruction (UUO), folic acid nephropathy (FA), and cisplatin-induced AKI). For FA nephropathy, folic acid (#216632500, ACROS Organics) was dissolved in 300 mM sodium bicarbonate (NaHCO3) (#21602, USB chemicals) and injected intraperitoneally (i.p.) at a dose of 250 mg/kg body weight into male mice only. To induce UUO kidney fibrosis, the left kidney ureter was ligated with a nylon string. The right kidney was collected as a sham control. For cisplatin-induced AKI, cisplatin (#13119, Cayman) was dissolved in a 0.9% NaCl solution and injected at a dose of 20 mg/kg body weight via i.p. All mice were monitored according to the post-operative procedures according to the IACUC guidelines. UUO and FA mice were sacrificed on day 7 of the injury. While the cisplatin mice were sacrificed on day 3 of cisplatin injection. Kidney tissues were collected for histology and molecular studies.
Urine was collected from Whamm+/+ and Whamm−/− male littermates at 20, 21, and 22 weeks and mice were sacrificed at 22 weeks. Kidneys were collected for histological and biochemical assay and serum was collected for BUN estimation (#B7552150, Pointe Scientific).
CK666 inhibitor studies on cisplatin-induced AKI and Adenine-CKD
For inhibitor studies, 7–8 weeks-old littermate male mice were selected for cisplatin and CK666 injections. Mice were injected with CK666 0.5 mg/kg body weight the day before the cisplatin injection. Mice were injected with CK666 at 0.5 mg/kg (ip) body weight, prior to a single dose of cisplatin 20 mg/kg body weight (ip) in saline. CK666 (#29038, Cayman) injections were continued for the rest of the two days of cisplatin-AKI and sacrificed on day 3rd. The control group and cisplatin-alone groups received equivalent amounts of PBS.
For Adenine-induced CKD, 7–8 weeks old wild-type male littermates were randomly assigned to experimental groups such as adenine, and adenine plus CK666. Five male mice were injected with CK666 at above mentioned dosage one day before feeding 0.2% adenine diet45 (#TD.160020, Inotiv, USA). Drug injections were continued throughout adenine chow for up to 14 days. Four age matched male littermates fed on control diet (casein calcium phosphate adjusted diet; CPD) (#TD.150303, Inotiv, USA) were served as controls throughout this study. Animals were sacrificed, and blood samples and tissues were harvested for biochemical analysis as mentioned above.
Preparation of primary kidney tubular epithelial cells
Primary kidney tubular epithelial cells were isolated from Whamm+/+, Whamm+/−, and Whamm−/− pups (2.5–3wk old). Kidneys were minced in RPMI media (#MT10–040-CM, Corning). Kidney tissue was digested with 100ug/ml collagenase IV (#LS004197, Worthington Biochemical) at 37°C for 30min. Tissue homogenate was then passed through 100μm, 70μm, and finally 40μm cell strainers. The flow through was centrifuged at 1000rpm for 5 min at 4°C. The cell pellet was resuspended in 1 mL of RBC lysis buffer (Hy-Clone) for lysis of red blood cells. Cell lysis was inactivated by ice-cold PBS and centrifuged at 1000rpm for 10 min at 4°C. Finally, the cell pellet was resuspended in RPMI complete media (10% FBS with antibiotics 1X ITS and 50 ng/ml human EGF) and plated in 10cm dishes. Cells were grown in the incubator at 5% CO2 at 37°C.
Culturing human renal proximal tubule epithelial cells
Immortalized human proximal tubule epithelial cells (pLXSN-hTERT) were procured from ATCC (#CRL-4031, ATCC). The cells were cultured in base medium DMEM F12 (#30–2006, ATCC) supplemented with hTERT RPTEC Growth kit (#ACS-4007, ATCC), and 0.5% fetal bovine serum at 37°C with 5% CO2, and 95% Air in incubator. Cells were subculture when reached ~90% confluency by trypsinizing with 2mL of Trypsin-EDTA (#PCS-999-003, ATCC) for 5–8 min at 37°C and neutralized with trypsin-neutralizing solution (Soybean trypsin inhibitor).
METHOD DETAILS
Gene prioritization strategy
The original eGFR GWAS analyzed 2.27 million multi-ethnic subjects.30 Fine mapping analysis was performed to identify the potential causal variants within the WHAMM locus using CARMA.46 Gene prioritization analysis was performed to identify the target gene of this locus by integrating 32 types of functional datasets, including coding variants, methylation, and expression quantitative trait data (meQTL and eQTL),7 allele-specific expression in (ASE) tubule and glomerulus, fine-mapping credible sets, Bayesian colocalization, and summary-based Mendelian randomization (Table S1). Human kidney single cell MultiOme ATAC-sequencing data is publicly available at Kidney Biobak at the Susztak lab.com (www.susztaklab.com).
Kidney function measurements
Spot urine was collected from 20 weeks old male mice and immediately assayed for L-amino acids using Sigma kit (#MAK002, Sigma), urine creatinine (#DZ072B-KY1, Diazyme), and urine kidney injury molecule 1 (KIM-1) by ELISA (#EMHAVCR1, ThermoFisher Scientific). The assays were repeated at 21 and 22 weeks to ensure reproducible measurements. Albumin ELISA was performed in urine samples collected from 22 weeks old mice (#EEL119, ThermoFisher Scientific) and normalized to urine creatinine.
Serum creatinine (#DZ072B-KY1, Diazyme), blood urea nitrogen (#B7552150, Pointe Scientific), and cystatin C levels were tested by the commercially available kits. Cystatin C was measured by ELISA kit (#EMCST3, ThermoFisher Scientific) according to the manufacturer’s protocol.
Gene expression analysis
A total of 15–20 mg of kidney tissue was subjected to total RNA isolation by Trizol (Ambion) method. Briefly, kidney tissue was homogenized in 1mL of Trizol in the QiaTissue Lyzer II. For separation of RNA, 200μL of chloroform was added directly to the tissue lysate, followed by vertexing for 10 s and incubation for 3 min at room temperature. Subsequently, the samples were centrifuged, and the top clear layer was collected and transferred to a new tube. An equal volume of 100% isopropanol was added to the collected top layer, and the tubes were centrifuged at 12,500 rpm at 4°C for 15 min. The resulting RNA pellet was washed in 70% ethanol, dried, and resuspended in clean RNase-free and DNase-free water. Before to cDNA conversion, the RNA was treated with DNase I.
For cDNA synthesis, 2μg of total RNA was converted into cDNA using the High-Capacity cDNA Reverse Transcription Kit (#4368813, Applied Biosystems). Real-time PCR analysis was performed in Applied Biosystems Quant Studio 5 system and quantified the relative fold change by the 2^-ΔΔCT method. To isolate RNA from primary cells, we followed the same procedure as described above. qPCR primer sequences were listed in Table S2.
Western blotting
Approximately 15 mg of kidney tissue was used to prepare protein sample. Briefly, tissue was homogenized (#LR60902, OMNI-GLH) in SDS-blue loading buffer (#7722, CST) with 42 mM DTT. The proteins were then separated on SDS-PAGE gels and transferred onto a PVDF membrane at 100 V for an hour on ice. Then, the membranes were blocked in 3% non-fat dry milk powder in tris-buffer saline containing 0.1% Tween 20 (TBST) solution for 30 min at room temperature. Next, the blots were incubated with primary antibodies overnight at 4°C. All primary antibodies diluted at 1:1000 dilution in TBST solution. After the primary antibody incubation, the blots were washed three times with TBST and probed with IRdye-conjugated or HRP-conjugated secondary antibodies for an hour at room temperature. All secondary antibodies diluted at 1:10,000 dilution in 3% skimmed-milk powder dissolved in TBST solution. Finally, the blots were scanned at excitation wavelengths of 600, 700, and 800 or chemiluminescence using a Li-COR imager (Odyssey Fc) and analyzed using Image Studio software. Blot quantifications were performed in ImageJ software and normalized to relative GAPDH level. Autophagy flux was calculated as described.47
H&E and sirius red staining
Briefly, mouse tissues were fixed in formalin and then dehydrated using an ethanol gradient, starting from 30% and progressing to 50%, 75%, 95%, and finally 100% ethanol. The processed tissues were embedded and cut in a microtome at the IDOM Histology Core. Subsequently, staining procedures including H&E (at IDOM core), Sirius red, and Periodic acid Schiff’s (PAS) staining were performed. Images were captured in Olympus 5000 microscope equipped with Cell Sense software at 60X (scale bar = 20μm) or 20X (scale bar = 50μm) magnification. Fibrosis percentage was analyzed in ImageJ software.
CRISPRi-mediated gene silencing
Single guide RNAs were designed for the human WHAMM gene in the GPPP web portal (Broad Institute). Phosphorylated (#M0201S T4PNK, NEB) and PCR annealed guide RNAs were ligated (#B0202S T4 DNA ligase, NEB) to a Lenti viral vector (Addgene #108098, LRG2.1) at the Bsmb1 site (#R0739L, NEB). Transformed into a competent bacterial cell (#C737303, ThermoFisher Scientific). Guide RNA cloning was confirmed by sanger sequencing. Viral particles carrying sgRNA were generated by transfecting LRG2.1 with Lenti vial packaging vectors (psPAX2 (#12260, Addgene), and pMD2.G (#12259, Addgene) at 1:1:0.5 ratio (LRG2.1, psPAX2, pMD2.G) into HEK293T (#CRL-3126, ATCC) cells. Transfection was performed using polyethyleneimine (#7854, Tocris). After three days of transfection, viral media was harvested by centrifugation at 3000rpm/15 min at 4°C.
dCas9 (Addgene #154473, pHR-UCOE-SFFV-dCas9-mCherry-ZIM3-KRAB) expressing human renal proximal tubule cells were generated and cultured as described.48 After reaching 80% confluence, the culture media was replaced with virus loaded media with 5μg of polybrene (#sc-134220, Santa Cruz Biotechnology). Six-8 h after transduction the viral loaded media was replaced with DMEM F12 culture media. Three days after transduction cells were collected for gene expression, western blotting, and analyzed to evaluate CRISPRi mediated gene silencing.
In vitro cell death measurements
Primary cells were cultured overnight in 96-well plates. Cells were treated with cisplatin (10μM or 20μM) for 24h. At the same time cells were assayed for cell death in various cell death inhibitors including Z-VAD(OMe)-FMK (20μM) (#11658, Cayman), Casp9i (Z-LEHD-FMK) (20μM) (#HY-P1010, MedChem Express), Vx-765 (10μM) (#28825, Cayman), and Nec-1 (20μM). Cell death was assayed based on LDH-release compared to maximal cell lysis (#G1780, Promega). Cells were treated with Latrunculin A (0.1μM) (#10010630, Cayman), cytochalasin D (0.25μM) (#11330, Cayman), and CK666 (10μM) (#HY-16926, MedChem Express) overnight. LDH assays were also performed in RPTECs using the same kit. To induce cell death in RPTECs, we used 40μM of cisplatin for 24h.
Live cell BODYPI C11 staining
Primary tubule cells were cultured on cover glass overnight. Cells were treated with cisplatin 20 μM overnight. Lipid peroxidation was estimated by incubating cells in RPMI containing 1.5μM BODIPY 581/591 C11 (#D3861, ThermoFisher Scientific) for 30 min at 37°C in a 5% CO2 incubator. After two washes with warm PBS, cells were then incubated with Hoechst 33342 (#62249, ThermoFisher Scientific) at a final concentration of 1 μg/mL in PBS for 20min. Finally, cells were washed in PBS two times and then imaged with an Olympus DP73 microscope.
For BODIPY 581/591 C11 quantification, cells were cultured in 96-well plates and treated with cisplatin overnight. Following BODIPY 581/591 C11 staining, the plates were washed three times with PBS and measured fluorescence at 581/591 and 488/510 in a spectrophotometer (BioTek). The data was normalized to the mean fluorescence of the Hoechst 33342 dye from each well.
Immunofluorescence staining
5μm thin formalin-fixed paraffin-embedded kidney sections were deparaffinized and rehydrated using ethanol gradients from 100% to 70%. Slides were preheated in 10mM citrate buffer to retrieve the target antigen and blocked with PBS supplemented with 10% BSA and 0.1% Tween for 1h at RT. Slides were incubated with the following primary antibodies in PBS overnight at 4°C. Anti-WHAMM at 1:50 dilution (#ABT60, Millipore), LTL Fluorescein at 1:200 (#FL-1321, Vector labs), anti-KSP at 1:50 dilution (#sc-393153, Santa Cruz Biotechnologies), anti-AQP2 at 1:50 dilution (#sc-9882, Santa Cruz Biotechnologies), anti-NPHS1 (#sc-377246, Santa Cruz Biotechnologies), anti-ACE2 (#PA5–47488, ThermoFisher Scientific) and WGA Alexa flour 488 (W11261, ThermoFisher Scientific). Next, slides were incubated at 37C with anti-rabbit Alexa Fluor 488 (#A21206, Invitrogen), anti-goat Alexa Fluor 488 (#A11078, Invitrogen), anti-goat Alexa Fluor 555 (#A21432, Invitrogen), and anti-mouse Alexa Fluor 488 (#A21202, Invitrogen) at 1:400 dilution. Sections were stained with DAPI containing anti-fade mounting media (#P36941, Invitrogen). Tissue immunofluorescence images were captured in Olympus 5000 microscope equipped with Cell Sense software at 60X magnification (scale bar = 20μm).
Double immunostaining of WHAMM with cell organelle markers was similarly performed on fixed cells as described.49 Briefly, cells were cultured on a sterile cover glass overnight as described above. Cells were fixed in ice-cold acetone: methanol (1:1 ratio) or 4% paraformaldehyde (#J19943, ThermoFisher Scientific), or 10% PBS-buffered formalin for 10 min at RT, and permeabilized with 0.1% Triton X-100 for 10 min at RT. Cells were incubated in 5% BSA-PBS solution for 1h at RT and then incubated with primary antibodies against WHAMM at 1:50 dilution (#ABT60, Millipore), COX IV at 1:100 (#11967, CST), GM130 at 1:100 (#MA5–47668, ThermoFisher Scientific), LAMP2 at 1:100 (#GL2A7, DSHB), Phalloidin-FITC at 30μM (#F432, Invitrogen), TUB1A at 1:100 (#A11126, Invitrogen) for overnight at 4°C. Fluorescent tagged-secondary anti-rabbit Alexa Fluor 555 (#A31572, Invitrogen), anti-rat Alexa Fluor 488 (#A11006, Invitrogen), and anti-mouse Alexa Fluor 564 (#A10036, Invitrogen) or anti-mouse Alexa Fluor 488 (#A21202, Invitrogen) were mixed in BSA containing PBS (1:400) solution and incubated for another hour and processed for imaging.
F-actin, cleaved CASP3, and cytochrome c double immunostaining was performed as described above. Briefly, after BSA blocking, cells were incubated with F-actin, primary antibodies against cleaved CASP3 at 1:100 (#9664, CST), and cytochrome c at 1:100 dilution (#12963, CST) for overnight at 4°C. Then cells incubated with fluorescent tagged-secondary anti-rabbit Alexa Fluor 555 (#A31572, Invitrogen), and anti-mouse Alexa Fluor 635 (#A31575, Invitrogen) were mixed in BSA containing PBS at 1:400 dilution for an hour at RT. F-actin and ASC co-immunostaining was performed as described above. Anti-ASC (#PA5–50915, Invitrogen) antibody was diluted at 1:100 in FITC-Phalloidin containing blocking solution. Images were acquired in a Leica (Stellaris 5) confocal microscope at 60X magnification (scale bar = 20μm) at the Cell and Developmental Biology core (RRID: SCR_022373) at the University of Pennsylvania.
ImageJ quantification
Profile intensity plots of WHAMM colocalization with cellular organelles such as GM130, F-actin, TUB1A, and COX IV. F-actin, cytochrome c, and cleaved CASP3 colocalization was analyzed using the RGB profile intensity plot plugin. The percentage of cells positive for F-actin, CASP3, and cytochrome c colocalization was manually counted. For, tubulin density, actin density, filament number, filament length, and branch points such as triple and quadruples were analyzed using skeletonize, tubeness, and particle analyzer plugins.50 Actin fluorescence intensity was measured manually with a free hand loop. ASC specks were manually counted in a region of interest (ROI) and its colocalization with F-actin was analyzed using RGB-profile intensity plot plugin. LAMP2 positive lysosomes were analyzed using a particle analyzer plugin. ACE2 immunostaining area percentage was calculated manually using RGB stacks, make montage and threshold options on 4X images of an entire kidney. Statistical differences and graphs were prepared in GraphPad prism 10.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistics and reproducibility
Statistical significance was tested in GraphPad Prism 10 software. Immunoblot of a protein in a sample was normalized to a total level of a housekeeping protein, such as GAPDH. One-way ANOVA was performed to calculate the statistical significance between three or more groups followed by Tukey’s post-hoc test was applied. Unpaired two-tailed t-tests were applied to calculate the statistical significance between two groups. No outliers were excluded from the analysis unless indicated in the figure legend. The sample size per group was not determined through sample size estimation but rather based on the number of animals available in the colony of a specific age and gender. The figures and/or figure legends provide the information about the number of replicates, and sample size in each animal experiment. p values of <0.05 were considered statistically significant. The level of significance presented in the figures as follows: p < 0.05 (), p < 0.01 (), p < 0.001 (), and p < 0.0001 (****).
ADDITIONAL RESOURCES
Human single cell, nuclear RNA transcriptomics, and single cell MultiOme ATAC-seq data are publicly available at the Susztak lab Kidney Biobank at https://susztaklab.com/. Integrated multi-species transcriptomic data is available at the Susztalab Kidney Biobank at https://susztaklab.com/SISKA/mouse. Genome wide association summary data, kidney compartment specific eQTL, and mQTL data is available at the Susztak lab Kidney Biobank at https://susztaklab.com/Kidney_eQTL/index.php, https://susztaklab.com/Kidney_meQTL/index.php.
Supplementary Material
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2025.115462.
Highlights.
Integrative genomics identified WHAMM as a novel kidney disease risk gene
Higher WHAMM expression negatively associated with eGFR
Genetic deletion of WHAMM attenuates kidney disease in mouse models
WHAMM plays role in actin dynamics and activation of cell death pathways
ACKNOWLEDGMENTS
This research is supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases grants R01DK076077, R01 DK087635, R01DK132630, and R01DK105821 to K.S.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Evans M, Lewis RD, Morgan AR, Whyte MB, Hanif W, Bain SC, Davies S, Dashora U, Yousef Z, Patel DC, and Strain WD (2022). A Narrative Review of Chronic Kidney Disease in Clinical Practice: Current Challenges and Future Perspectives. Adv. Ther 39, 33–43. 10.1007/s12325-021-01927-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zoccali C, Vanholder R, Massy ZA, Ortiz A, Sarafidis P, Dekker FW, Fliser D, Fouque D, Heine GH, Jager KJ, et al. (2017). The systemic nature of CKD. Nat. Rev. Nephrol 13, 344–358. 10.1038/nrneph.2017.52. [DOI] [PubMed] [Google Scholar]
- 3.Tuttle KR, Alicic RZ, Duru OK, Jones CR, Daratha KB, Nicholas SB, McPherson SM, Neumiller JJ, Bell DS, Mangione CM, and Norris KC (2019). Clinical Characteristics of and Risk Factors for Chronic Kidney Disease Among Adults and Children: An Analysis of the CURECKD Registry. JAMA Netw. Open 2, e1918169. 10.1001/jamanetworkopen.2019.18169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mukhi D, and Susztak K. (2020). The transcriptomic signature of the aging podocyte. Kidney Int. 98, 1079–1081. 10.1016/j.kint.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tin A, and Köttgen A. (2020). Genome-Wide Association Studies of CKD and Related Traits. Clin. J. Am. Soc. Nephrol 15, 1643–1656. 10.2215/CJN.00020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayanja R, Machipisa T, Soremekun O, Kamiza AB, Kintu C, Kalungi A, Kalyesubula R, Sande OJ, Jjingo D, Fabian J, et al. (2023). Genome-wide association analysis of cystatin-C kidney function in continental Africa. EBioMedicine 95, 104775. 10.1016/j.ebiom.2023.104775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H, Doke T, Guo D, Sheng X, Ma Z, Park J, Vy HMT, Nadkarni GN, Abedini A, Miao Z, et al. (2022). Epigenomic and transcriptomic analyses define core cell types, genes and targetable mechanisms for kidney disease. Nat. Genet 54, 950–962. 10.1038/s41588-022-01097-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claussnitzer M, and Susztak K. (2021). Gaining insight into metabolic diseases from human genetic discoveries. Trends Genet. 37, 1081–1094. 10.1016/j.tig.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu C, Huang S, Park J, Park Y, Ko YA, Seasock MJ, Bryer JS, Xu XX, Song WC, Palmer M, et al. (2018). Renal compartment-specific genetic variation analyses identify new pathways in chronic kidney disease. Nat. Med 24, 1721–1731. 10.1038/s41591-018-0194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giambartolomei C, Vukcevic D, Schadt EE, Franke L, Hingorani AD, Wallace C, and Plagnol V. (2014). Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 10, e1004383. 10.1371/journal.pgen.1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu Z, Zhang F, Hu H, Bakshi A, Robinson MR, Powell JE, Montgomery GW, Goddard ME, Wray NR, Visscher PM, and Yang J. (2016). Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet 48, 481–487. 10.1038/ng.3538. [DOI] [PubMed] [Google Scholar]
- 12.Yan Y, Liu H, Abedini A, Sheng X, Palmer M, Li H, and Susztak K. (2024). Unraveling the epigenetic code: human kidney DNA methylation and chromatin dynamics in renal disease development. Nat. Commun 15, 873. 10.1038/s41467-024-45295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukhi D, Li L, Liu H, Doke T, Kolligundla LP, Ha E, Kloetzer K, Abedini A, Mukherjee S, Wu J, et al. (2023). ACSS2 gene variants determine kidney disease risk by controlling de novo lipogenesis in kidney tubules. J. Clin. Investig 134, e172963. 10.1172/JCI172963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, Ma Z, Raman A, Beckerman P, Dhillon P, Mukhi D, Palmer M, Chen HC, Cohen CR, Dunn T, et al. (2021). APOL1 risk variants in individuals of African genetic ancestry drive endothelial cell defects that exacerbate sepsis. Immunity 54, 2632–2649. 10.1016/j.immuni.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balzer MS, Doke T, Yang YW, Aldridge DL, Hu H, Mai H, Mukhi D, Ma Z, Shrestha R, Palmer MB, et al. (2022). Single-cell analysis highlights differences in druggable pathways underlying adaptive or fibrotic kidney regeneration. Nat. Commun 13, 4018. 10.1038/s41467-022-31772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doke T, Mukherjee S, Mukhi D, Dhillon P, Abedini A, Davis JG, Chellappa K, Chen B, Baur JA, and Susztak K. (2023). NAD(+) precursor supplementation prevents mtRNA/RIG-I-dependent inflammation during kidney injury. Nat. Metab 5, 414–430. 10.1038/s42255-023-00761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohandes S, Doke T, Hu H, Mukhi D, Dhillon P, and Susztak K. (2023). Molecular pathways that drive diabetic kidney disease. J. Clin. Investig 133, e165654. 10.1172/JCI165654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rottner K, Hänisch J, and Campellone KG (2010). WASH, WHAMM and JMY: regulation of Arp2/3 complex and beyond. Trends Cell Biol. 20, 650–661. 10.1016/j.tcb.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Campellone KG, Webb NJ, Znameroski EA, and Welch MD (2008). WHAMM is an Arp2/3 complex activator that binds microtubules and functions in ER to Golgi transport. Cell 134, 148–161. 10.1016/j.cell.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer DA, Piper HK, and Chen B. (2022). WASP family proteins: Molecular mechanisms and implications in human disease. Eur. J. Cell Biol 101, 151244. 10.1016/j.ejcb.2022.151244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen QT, Hsiue PP, Sindelar CV, Welch MD, Campellone KG, and Wang HW (2012). Structural insights into WHAMM-mediated cytoskeletal coordination during membrane remodeling. J. Cell Biol 199, 111–124. 10.1083/jcb.201204010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russo AJ, Mathiowetz AJ, Hong S, Welch MD, and Campellone KG (2016). Rab1 recruits WHAMM during membrane remodeling but limits actin nucleation. Mol. Biol. Cell 27, 967–978. 10.1091/mbc.E15-07-0508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desouza M, Gunning PW, and Stehn JR (2012). The actin cytoskeleton as a sensor and mediator of apoptosis. BioArchitecture 2, 75–87. 10.4161/bioa.20975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.King VL, Leclair NK, Coulter AM, and Campellone KG (2021). The actin nucleation factors JMY and WHAMM enable a rapid Arp2/3 complex-mediated intrinsic pathway of apoptosis. PLoS Genet. 17, e1009512. 10.1371/journal.pgen.1009512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burger D, Fickentscher C, de Moerloose P, and Brandt KJ (2016). F-actin dampens NLRP3 inflammasome activity via Flightless-I and LRRFIP2. Sci. Rep 6, 29834. 10.1038/srep29834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren W, Zhao W, Cao L, and Huang J. (2020). Involvement of the Actin Machinery in Programmed Cell Death. Front. Cell Dev. Biol 8, 634849. 10.3389/fcell.2020.634849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waite AL, Schaner P, Hu C, Richards N, Balci-Peynircioglu B, Hong A, Fox M, and Gumucio DL (2009). Pyrin and ASC co-localize to cellular sites that are rich in polymerizing actin. Exp. Biol. Med 234, 40–52. 10.3181/0806-RM-184. [DOI] [PubMed] [Google Scholar]
- 28.Kim ML, Chae JJ, Park YH, De Nardo D, Stirzaker RA, Ko HJ, Tye H, Cengia L, DiRago L, Metcalf D, et al. (2015). Aberrant actin depolymerization triggers the pyrin inflammasome and autoinflammatory disease that is dependent on IL-18, not IL-1beta. J. Exp. Med 212, 927–938. 10.1084/jem.20142384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan MJ, and Kim YS (2022). Roles of RIPK3 in necroptosis, cell signaling, and disease. Exp. Mol. Med 54, 1695–1704. 10.1038/s12276-022-00868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H, Abedini A, Ha E, Ma Z, Sheng X, Dumoulin B, Qiu C, Aranyi T, Li S, Dittrich N, et al. (2025). Kidney multiome-based genetic scorecard reveals convergent coding and regulatory variants. Science 387, eadp4753. 10.1126/science.adp4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J, Abedini A, Balzer MS, Shrestha R, Dhillon P, Liu H, Hu H, and Susztak K. (2023). Unified Mouse and Human Kidney Single-Cell Expression Atlas Reveal Commonalities and Differences in Disease States. J. Am. Soc. Nephrol 34, 1843–1862. 10.1681/ASN.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abedini A, Sanchez-Navaro A, Wu J, Klotzer KA, Ma Z, Poudel B, Doke T, Balzer MS, Frederick J, Cernecka H, et al. (2024). Single-cell transcriptomics and chromatin accessibility profiling elucidate the kidney-protective mechanism of mineralocorticoid receptor antagonists. J. Clin. Investig 134, 157165. 10.1172/JCI157165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abedini A, Levinsohn J, Klötzer KA, Dumoulin B, Ma Z, Frederick J, Dhillon P, Balzer MS, Shrestha R, Liu H, et al. (2024). Single-cell multi-omic and spatial profiling of human kidneys implicates the fibrotic microenvironment in kidney disease progression. Nat. Genet 56, 1712–1724. 10.1038/s41588-024-01802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kast DJ, Zajac AL, Holzbaur ELF, Ostap EM, and Dominguez R. (2015). WHAMM Directs the Arp2/3 Complex to the ER for Autophagosome Biogenesis through an Actin Comet Tail Mechanism. Curr. Biol 25, 1791–1797. 10.1016/j.cub.2015.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai A, Yu L, and Wang HW (2019). WHAMM initiates autolysosome tubulation by promoting actin polymerization on autolysosomes. Nat. Commun 10, 3699. 10.1038/s41467-019-11694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King VL, and Campellone KG (2023). F-actin-rich territories coordinate apoptosome assembly and caspase activation during DNA damage-induced intrinsic apoptosis. Mol. Biol. Cell 34, ar41. 10.1091/mbc.E22-04-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrade-Silva M, Dhillon P, Sanchez-Navarro A, Mukhi D, Hu H, Kolligundla LP, Bergeson A, Abedini A, Levinsohn J, Dumoulin B, et al. (2025). The critical role of endoplasmic reticulum stress and the stimulator of interferon genes (STING) pathway in kidney fibrosis. Kidney Int. 107, 302–316. 10.1016/j.kint.2024.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stutz A, Horvath GL, Monks BG, and Latz E. (2013). ASC speck formation as a readout for inflammasome activation. Methods Mol. Biol 1040, 91–101. 10.1007/978-1-62703-523-1_8. [DOI] [PubMed] [Google Scholar]
- 39.Coulter AM, Cortes V, Theodore CJ, Cianciolo RE, Korstanje R, and Campellone KG (2024). WHAMM functions in kidney reabsorption and polymerizes actin to promote autophagosomal membrane closure and cargo sequestration. Mol. Biol. Cell 35, ar80. 10.1091/mbc.E24-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doke T, Huang S, Qiu C, Liu H, Guan Y, Hu H, Ma Z, Wu J, Miao Z, Sheng X, et al. (2021). Transcriptome-wide association analysis identifies DACH1 as a kidney disease risk gene that contributes to fibrosis. J. Clin. Investig 131, e141801. 10.1172/JCI141801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ko YA, Yi H, Qiu C, Huang S, Park J, Ledo N, Köttgen A, Li H, Rader DJ, Pack MA, et al. (2017). Genetic-Variation-Driven Gene-Expression Changes Highlight Genes with Important Functions for Kidney Disease. Am. J. Hum. Genet 100, 940–953. 10.1016/j.ajhg.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mergault C, Lisée F, Tiroille V, Magnien M, Parent C, Lenga Mabonda W, Sizaret D, Jaillet M, Crestani B, Marchand-Adam S, and Plantier L. (2022). Inhibition of the Arp2/3 complex represses human lung myofibroblast differentiation and attenuates bleomycin-induced pulmonary fibrosis. Br. J. Pharmacol 179, 125–140. 10.1111/bph.15675. [DOI] [PubMed] [Google Scholar]
- 43.Sheng X, Guan Y, Ma Z, Wu J, Liu H, Qiu C, Vitale S, Miao Z, Seasock MJ, Palmer M, et al. (2021). Mapping the genetic architecture of human traits to cell types in the kidney identifies mechanisms of disease and potential treatments. Nat. Genet 53, 1322–1333. 10.1038/s41588-021-00909-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dhillon P, Park J, Hurtado Del Pozo C, Li L, Doke T, Huang S, Zhao J, Kang HM, Shrestra R, Balzer MS, et al. (2021). The Nuclear Receptor ESRRA Protects from Kidney Disease by Coupling Metabolism and Differentiation. Cell Metab. 33, 379–394. 10.1016/j.cmet.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clinkenbeard EL, Noonan ML, Thomas JC, Ni P, Hum JM, Aref M, Swallow EA, Moe SM, Allen MR, and White KE (2019). Increased FGF23 protects against detrimental cardio-renal consequences during elevated blood phosphate in CKD. JCI Insight 4, e123817. 10.1172/jci.insight.123817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Z, Wang C, Liu L, Khan A, Lee A, Vardarajan B, Mayeux R, Kiryluk K, and Ionita-Laza I. (2023). CARMA is a new Bayesian model for fine-mapping in genome-wide association meta-analyses. Nat. Genet 55, 1057–1065. 10.1038/s41588-023-01392-0. [DOI] [PubMed] [Google Scholar]
- 47.Yoshii SR, and Mizushima N. (2017). Monitoring and Measuring Autophagy. Int. J. Mol. Sci 18, 1865. 10.3390/ijms18091865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang X, Liu H, Hu H, Ha E, Zhou J, Abedini A, Sanchez-Navarro A, Klötzer KA, and Susztak K. (2024). TET2 germline variants promote kidney disease by impairing DNA repair and activating cytosolic nucleotide sensors. Nat. Commun 15, 9621. 10.1038/s41467-024-53798-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mukhi D, Kolligundla LP, Maruvada S, Nishad R, and Pasupulati AK (2023). Growth hormone induces transforming growth factor-beta1 in podocytes: Implications in podocytopathy and proteinuria. Biochim. Biophys. Acta. Mol. Cell Res 1870, 119391. 10.1016/j.bbamcr.2022.119391. [DOI] [PubMed] [Google Scholar]
- 50.Sato Y, Nakajima S, Shiraga N, Atsumi H, Yoshida S, Koller T, Gerig G, and Kikinis R. (1998). Three-dimensional multi-scale line filter for segmentation and visualization of curvilinear structures in medical images. Med. Image Anal 2, 143–168. 10.1016/s1361-8415(98)80009-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study does not report or use any custom computer code. The GWAS, eQTL, and multi-species snRNA-seq data are available to the public at https://susztaklab.com/. This work did not generate a new sequencing data or computational code. However, the work utilized previously published single nuclear ATAC-seq, single nuclear Multi-Ome, and single-cell and nuclear RNA-seq datasets that were deposited with the Common Metabolic Diseases Genome Atlas (CMDGA: https://cmdga.org/search/?type=Experiment&searchTerm=FNIH0000000). Kidney H3K4me3 and H3K27ac data are available with accession numbers GEO: GSM621648 and GEO: GSM1112806 at the GEO website. Mice kidney RNA-seq raw data from injury models have been previously published with accession numbers GEO: GSE207587 and GEO: GSE156686. All other data needed to evaluate the conclusions are presented in the paper and the supplemental information. Original immunoblots have been deposited at Mendeley (Mendeley Data: https://doi.org/10.17632/yyjfghps97.1) and are publicly available as of the date of publication. Microscopy data reported in this paper will be shared by the lead contact upon request.