Abstract
We previously identified five derivatives of Klenow fragment DNA polymerase that have lower fidelity because of amino acid substitutions in the polymerase active site. One of these has alanine substituted for the invariant Glu-710, whose side chain interacts with the deoxyribose of the incoming dNTP. Here we show that the E710A enzyme has reduced fidelity for five of the 12 possible mismatches. All but one of these involve misinsertion of pyrimidines, including two transition mismatches A-dCTP and G-dTTP. In contrast, E710A polymerase error rates for the reciprocal C-dATP and T-dGTP transition mismatches were similar to those of the wild-type enzyme. The kinetics of formation of correct base pairs and transition mismatches by the wild-type and E710A polymerases, combined with information on the structure of the DNA polymerase active site and the asymmetry of wobble base pairs, provides a plausible explanation for the differential effects of the E710A mutation on fidelity. The data suggest that the Glu-710 side chain plays a pivotal role in excluding wobble base pairs between template pyrimidines and purine triphosphates by steric clash. Moreover, this same side chain enhances the stability of incoming correct dNTPs, such that loss of this interaction on removal of the side chain leads to lower selectivity against mismatches involving incoming pyrimidines.
The accuracy or fidelity of DNA polymerases covers a wide range: the “classical” polymerases involved in replication and repair typically make one mistake for every 103 to 105 bases copied (1–3), whereas the recently discovered bypass polymerases (family Y) have much lower fidelity, which may be related to their ability to negotiate template lesions (4–6). To understand the molecular basis for polymerase fidelity, we have used the Klenow fragment of DNA polymerase I, a highly accurate polymerase for which a wealth of structural, biochemical, and genetic data exists (reviewed in ref. 7).
It has long been recognized that the accuracy of copying by DNA polymerases is substantially greater than would be expected merely on the basis of the energetics of complementary vs. noncomplementary base pairing (1). It has been suggested that the additional fidelity could be derived from three sources: exclusion of water from the enzyme's active site, close steric complementarity between the active site and correctly base-paired substrates, and scanning of the minor groove of the DNA duplex to detect mispairs. Polymerase crystal structures support these ideas (8–12). In unliganded polymerases or polymerase–DNA binary complexes, the polymerase domain resembles a half-open right hand in which the palm contains the catalytic center, the thumb binds the primer–template duplex, and the fingers subdomain contains most of the side chains that will bind the incoming nucleotide. When a ternary complex (polymerase-DNA–dNTP) is formed, the hand closes because of a substantial movement of the fingers subdomain, forming a tight binding pocket around the nascent base pair. Additionally, several side chains are hydrogen bonded to the minor groove of the template–primer duplex, so that they could discriminate between correct and incorrect base pairs close to the primer terminus.
In a previous study, we identified five single amino acid changes within the polymerase active site region of Klenow fragment that decreased fidelity, giving a mutator phenotype (13). One of these mutations, E710A, removed a side chain that binds the deoxyribose portion of the incoming dNTP. This same side chain, Glu-710, is invariant in the Pol I family of DNA polymerases and serves as the “steric gate” that prevents rNTP incorporation (14, 15). Our initial studies showed that the E710A polymerase has an unusual error specificity, with the predominant error being incorporation of dCTP opposite A, whereas the wild-type enzyme makes mostly T-dGTP errors. Here we report a detailed analysis of the error specificity and kinetics of the E710A mutant of Klenow fragment, which point to the role of the substrate-binding pocket, and Glu-710 in particular, in preventing specific mispairings.
Experimental Procedures
Materials.
Mutant derivatives of Klenow fragment.
The E710A and E710Q mutant derivatives of Klenow fragment were constructed and purified as described (16, 17). The biochemical properties of these mutant derivatives have been reported previously (14). All polymerases in this study carried the D424A mutation, which eliminates 3′-5′ exonuclease activity (18); thus, the D424A Klenow fragment was used as the “wild-type” control. Protein concentrations were determined by the Bradford colorimetric assay (19).
Oligonucleotides.
DNA oligonucleotides were from Research Genetics (Huntsville, AL) or the Keck Biotechnology Resource Laboratory at Yale Medical School. They were annealed to produce the substrates listed in Table 3; in each case, the primer strand was labeled at the 5′ end with 32P.
Table 3.
Insertion kinetics for wild-type and E710A Klenow fragment
| DNA substrate | Enzyme* | Kd, μM | kpol, s−1 | kpol/Kd, s−1/M | Kd, μM | kpol, s−1 | kpol/Kd, s−1/M | Selectivity |
|---|---|---|---|---|---|---|---|---|
| A⋅dTTP | A⋅dCTP | |||||||
| 5′-TGGGTAACGCCAGGGTTTTC | WT | 9.5 | 18 | 1.9 × 106 | 250 | 1.9 × 10−2 | 77 | 2.5 × 104 |
| 3′-ACCCATTGCGGTCCCAAAAGAGTCAGTGCTGCA | E710A | 99 | 0.13 | 1.3 × 103 | 120 | 1.2 × 10−3 | 9.7 | 130 |
| 5′-GCTTCGCGACGGCATCAACA | WT† | 24 | 170 | 7.1 × 106 | 570 | 5.4 × 10−2 | 95 | 7.5 × 104 |
| 3′-CGAAGCGCTGCCGTAGTTGTACTAGAAATACT | E710A† | 58 | 12 | 2.0 × 105 | 53 | 1.6 × 10−2 | 310 | 670 |
| G⋅dCTP | G⋅dTTP | |||||||
| 5′-GGGTAACGCCAGGGTTTTCT | WT | 8.6 | 130 | 1.5 × 107 | 180 | 3.0 × 10−2 | 160 | 9.4 × 104 |
| 3′-ACCCATTGCGGTCCCAAAAGAGTCAGTGCTGCA | E710A | 35 | 6.4 | 1.8 × 105 | 33 | 5.2 × 10−3 | 160 | 1.1 × 103 |
| T⋅dATP | T⋅dGTP | |||||||
| 5′-GGTAACGCCAGGGTTTTCTC | WT | 9.1 | 144 | 1.6 × 107 | 48 | 0.16 | 3.4 × 103 | 4.7 × 103 |
| 3′-ACCCATTGCGGTCCCAAAAGAGTCAGTGCTGCA | E710A | 55 | 6.3 | 1.1 × 105 | 7.9 | 2.2 × 10−4 | 29 | 3.8 × 103 |
Both proteins also have the D424A (3′–5′ exonuclease-deficient) mutation. WT, wild type.
Single determinations. All other data were the average of two or more determinations.
Methods
Gap-Filling Synthesis and Forward Mutation Assays.
The sources and description of strains and reagents, the preparation of gapped DNA substrate, and the basic methodology were all as described previously (20). Reactions (25 μl) contained 1.4 nM M13mp2 DNA with a 407-nt gap (from nucleotides −216 through +191 of the lacZ gene), 20 mM Tris⋅HCl, pH 7.5, 10 mM MgCl2, 10 mM DTT, and 1 mM dNTPs. Synthesis was initiated by adding either 25 pmol E710A or 8.6 pmol E710Q Klenow fragment; these amounts were determined empirically to be necessary for complete gap filling. Reactions were incubated at 37°C for 10 min and terminated by adding EDTA to 15 mM. Agarose gel electrophoresis revealed that the gap was filled to apparent completion in all reactions whose fidelity was analyzed here. DNA products of the reactions were assayed for the frequency of lacZ mutants. DNA from independent lacZ mutant phage was sequenced to identify the errors made during gap-filling synthesis, and error rates were calculated.
Kinetic Measurements.
Single-turnover measurements of nucleotide incorporation by wild-type and mutant Klenow fragment derivatives were carried out as described (13, 21).
Molecular Graphics.
Coordinates were taken from Protein Data Bank files 3KTQ and 1QSY, corresponding to ternary complexes of Klentaq (the Klenow fragment-like portion of Taq DNA polymerase I) in which the nascent base pairs were G-dCTP and T-dATP, respectively (12, 22). The coordinates for a T-G mispair within an A-DNA helix were taken from the file ADH019 (23); an A-DNA coordinate set was chosen because the DNA within the polymerase active site in the ternary complex crystal structures is A-form (10, 12). The mispair was superimposed on the nascent base pair by using spock (24), both for the superposition and to generate figures.
Results
Error Specificity in an in Vitro Gap-Filling Forward Mutation Assay.
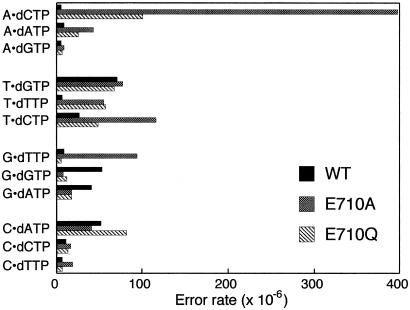
In our previous study, the E710A mutant showed lower fidelity than wild-type Klenow fragment in gap-filling synthesis on an M13 substrate in which base substitution errors were detected by reversion of an opal codon (13). Over 90% of the revertants produced by the E710A polymerase corresponded to errors in which dCTP had been inserted opposite template A, contrasting with wild-type Klenow fragment where the error rate for A-dCTP misinsertion was about 70-fold lower and T-dGTP was the most common error. Because the earlier study used reversion substrates, it focused on only a few errors within a defined DNA sequence, limiting the deductions that could be made from the information obtained. In particular, G-dTTP errors, which are likely to be structurally the most similar to A-dCTP, are not detected in the opal codon reversion assay. We therefore carried out a more detailed analysis of the errors produced by the E710A mutant protein, and by the more conservative E710Q derivative, by using a forward mutation assay capable of detecting base substitution errors at 125 different sites and frameshift errors at 199 sites within the target sequence (20). Gap-filling synthesis was carried out in vitro on an M13mp2 gapped substrate containing the lacZα complementation sequence, and DNA was sequenced from 135 independent lacZ mutants generated by E710A and 96 mutants generated by E710Q. Table 1 and Fig. 1 compare the results obtained from E710A and E710Q with the previously reported error specificity of wild-type Klenow fragment (25), all three proteins being proofreading-defective.
Table 1.
Errors made by wild-type and E710A Klenow fragment
| Error | WT*†
|
E710A*
|
Ratio E710A/WT ¶ | ||
|---|---|---|---|---|---|
| No.‡ | ER§ (×10−6) | No. | ER§ (×10−6) | ||
| Mispair: | |||||
| A⋅dCTP | 1 | 5.3 | 51 | 400 | 75 |
| A⋅dATP | 2 | 8.4 | 7 | 43 | 5.1 |
| A⋅dGTP | 0 | ≤5.9 | 0 | ≤8.7 | |
| T⋅dGTP | 19 | 71 | 14 | 77 | 1.1 |
| T⋅dTTP | 1 | 6.3 | 6 | 56 | 8.9 |
| T⋅dCTP | 6 | 26 | 18 | 120 | 4.6 |
| G⋅dTTP | 2 | 9.2 | 14 | 94 | 10 |
| G⋅dGTP | 10 | 53 | 1 | 7.8 | 0.15 |
| G⋅dATP | 10 | 40 | 3 | 18 | 0.45 |
| C⋅dATP | 13 | 53 | 7 | 41 | 0.77 |
| C⋅dCTP | 0 | ≤11 | 0 | ≤16 | |
| C⋅dTTP | 0 | ≤6.3 | 2 | 19 | |
| Frameshift: | |||||
| −1 run | 8 | 18 | 7 | 24 | 1.3 |
| +1 run | 1 | 2.3 | 0 | ≤3.4 | |
| −1 non-run | 7 | 4.6 | 8 | 7.7 | 1.7 |
| −2 deletions | 6 | 3.1 | 0 | ≤0.74 | |
| Other | 8 | 13 | |||
| None | 0 | 1 | |||
| Total‖ | 94 | 135 | |||
| MF (×10−4) | 57 | 120 | |||
Deficient in 3′–5′ exonuclease activity because of the D424A mutation.
Data for the wild-type Klenow fragment (WT) were taken from ref. 25.
Number of errors observed in each category.
Error rates (ER), defined as errors per detectable nucleotide incorporated, were calculated by multiplying the mutant frequency (MF) by the proportion of mutants for each class. This value was divided by 0.6, to correct for expression of errors on transfection, and by the number of known detectable sites for each class of mutation.
For each mispair, the E710A error rate is compared to that for wild-type Klenow fragment. Where no value is shown, the number of mutants observed was insufficient to define this ratio with confidence.
The total number of mutants from which DNA was isolated for sequencing. Some of these mutants contained multiple base substitution mutations, which were treated as independent events in the calculations of individual base substitution error rates.
Figure 1.
Graphical representation of the error rates for formation of the 12 possible mispairs, by wild-type Klenow fragment and the E710A and E710Q mutant derivatives. Data for the wild-type and E710A enzymes are taken from Table 1. The E710Q error specificity data are shown in Table 4, which is published as supporting information on the PNAS web site (www.pnas.org).
The E710A enzyme gave a 2-fold increase in the overall forward mutant frequency relative to wild-type Klenow fragment, whereas no significant overall increase was observed for the E710Q derivative. As with the wild-type enzyme, base substitutions were the predominant errors by the E710A mutant. Base substitutions accounted completely for the higher overall error frequency of the E710A polymerase, with an increase of 2- to 3-fold relative to the wild-type level; by contrast, E710A showed no increase over the wild-type level of addition or deletion errors.
More dramatic changes were apparent when comparing individual base substitution mutations generated by the wild-type and E710A Klenow fragment derivatives (Table 1 and Fig. 1). The most common errors of wild-type Klenow fragment involved misinsertion of purines: G opposite template T; G or A opposite G; and A opposite C (25). By contrast, the majority of the mutations generated by the E710A derivative resulted from misinsertion of pyrimidines. The largest increase in error frequency was observed for dCTP insertion opposite a template A (75-fold increase relative to wild-type Klenow fragment), consistent with our earlier reversion assays (13). Substantial increases relative to wild-type were also seen for misinsertions of T opposite G (10-fold) and T or C opposite T (9- and 5-fold, respectively). There may also have been an increase in misinsertion of T opposite C, but the number of occurrences of such changes was too low to allow us to say this with certainty. Insertion of A opposite A was the only example of an error involving purine misinsertion that occurred more frequently (by 5-fold) with E710A than with wild-type Klenow fragment; by contrast, insertions of G or A opposite G were less frequent with the mutant protein. The effect of the E710A polymerase on transition mispairs was asymmetrical; those errors resulting from misinsertion of a pyrimidine (A-dCTP and G-dTTP) were significantly increased, whereas the error rates for T-dGTP and C-dATP, requiring misinsertion of a purine, were similar to those obtained with the wild-type enzyme.
The conservative E710Q mutation had a more modest effect compared with E710A (Fig. 1). The overall forward mutant frequency was lower than that of E710A and was not significantly different from the wild-type level. As with E710A, larger changes were apparent when examining the error rates for individual mispairs, with the majority of these error rates between the wild-type and E710A values; for example, A-dCTP mispairs were increased only 18-fold over the wild-type level, compared with 75-fold for E710A. Curiously, E710Q showed no increase in error rate for the G-dTTP mispair, relative to the wild-type polymerase.
Reaction Kinetics.
To understand the role of Glu-710 in polymerase fidelity, we needed to explain not only the higher error frequency of the E710A polymerase, but also why E710A is a mutator for some base substitutions but not others. The increased rates for specific base substitutions could be due either to an increase in misinsertion or to an increased likelihood of extending the resulting mispair. From our previous work, it seemed unlikely that the mutator phenotype of E710A was caused by increased extension of mispairs, because E710A showed greater discrimination than wild type against extending a (template)T-(primer)G mispair (13). However, the data did not exclude a selective favoring of a subset of mispairs, including the abundant (template)A-(primer)C error. We therefore measured the rates of extension of A-C and A-A mispairs by wild type and E710A Klenow fragment under conditions similar to those used for primed synthesis in the M13 mutational assays (Table 2). Although the difference between wild type and mutant enzymes in discrimination between correct and incorrect primer termini varied from mispair to mispair, E710A was more discriminating than wild type for each of the substrates tested.
Table 2.
Extension of mismatches by wild-type and E710A Klenow fragment
| Mismatch† | Wild-type*
|
E710A*
|
|||||
|---|---|---|---|---|---|---|---|
| kmismatch, s−1 | kcorrect, s−1 | kcorrect/kmismatch | kmismatch, s−1 | kcorrect, s−1 | kcorrect/kmismatch | E710A/wild type‡ | |
| T⋅G at T87§ | 0.29 | 130 | 450 | 2 × 10−4 | 3.4 | 17,000 | 38 |
| A⋅C at A89 | 0.32 | 190 | 590 | 0.0016 | 7.6 | 4,800 | 8 |
| A⋅C at A−33 | 1.0 | 130 | 130 | 0.010 | 5.7 | 570 | 4 |
| A⋅A at A89 | 0.0053 | 190 | 36,000 | 1.9 × 10−5 | 7.6 | 390,000 | 11 |
| A⋅A at A−33 | 0.013 | 130 | 10,000 | ||||
Single-turnover rate constants were measured at 1 mM dNTP as described (13).
Both proteins also have the D424A (3′–5′ exonuclease-deficient) mutation.
The template base is listed first. Oligonucleotide substrates with mispaired primer termini were designed to place the mispair at the indicated position within a DNA sequence corresponding to a portion of the lacZα complementation target (20). Positions T87 to A89 correspond to the opal codon used in the reversion assay. A−33 is the site of several errors caused by A-C mispairings in the E710A mutational spectrum (D.T.M. and T.A.K., unpublished data; available on request).
The ratio of kcorrect/kmismatch for E710A divided by the ratio for wild-type Klenow fragment on the same substrate.
Data taken from ref. 13.
Having ruled out selective mispair extension as a likely source of the mutator phenotype of the E710A mutant protein, we focused on the misinsertion reaction. We measured single-turnover kinetics for several correct and incorrect dNTP insertions by wild-type and E710A Klenow fragment using the oligonucleotide substrates listed in Table 3. The substrates used in the majority of these experiments correspond to the DNA sequence surrounding the opal codon (underlined in Table 3) in the template used in the reversion assays. Using a high concentration (1 μM) of polymerase to ensure that all of the DNA substrate was enzyme-bound, we measured the rate of incorporation as a function of dNTP concentration to determine kpol for the polymerase reaction and Kd for dNTP binding (Table 3). The quantity kpol/Kd gives the efficiency of the reaction, and the ratio (kpol/Kd)correct/(kpol/Kd)incorrect gives the selectivity, a measure of the ability of the polymerase to discriminate between the relevant correct and incorrect nucleotides.
The overall selectivity values calculated from the kinetic data correspond precisely to the forward mutation data. Thus, the selectivity values show that wild-type Klenow fragment discriminates more against A-dCTP and G-dTTP misinsertions than against T-dGTP misinsertions. For E710A, the discrimination against A-dCTP and G-dTTP misinsertions is about 100-fold lower than for wild-type Klenow fragment, whereas the discrimination against T-dGTP misinsertions is similar to wild type. A detailed breakdown of the kinetic constants indicates that the high selectivity of wild-type Klenow fragment against Pu-dPyTP mispairs results from a Kd increase of ≈25-fold and a kpol decrease of at least 103-fold, when comparing correct with incorrect dNTP insertions. With E710A, the Kd values are essentially the same for correct and incorrect nucleotides, and the kpol discrimination is 3- to 10-fold less than with the wild-type polymerase. Very similar results were obtained for A-dCTP insertion in two different sequence contexts, suggesting that the results obtained can be generalized to other sequences. This was despite the unusually low kpol value seen at the template A position of the opal codon, which we suspect may be related to the run of Ts close to the primer terminus.
The situation for T-dGTP mispairs was surprising. Even though the overall selectivity values were very similar for wild type and E710A Klenow fragment, this was the result of large but compensatory changes in the kinetic constants of the mutant protein. Discrimination by wild-type Klenow fragment against T-dGTP misinsertion was the combination of a small increase (5-fold) in Kd for the incorrect nucleotide and a large (103-fold) decrease in kpol. For E710A, the Kd was 10-fold more favorable for the T-dGTP mispair than for T-dATP, but the kpol was more than 104-fold lower for misinsertion than for addition of the correct nucleotide.
Discussion
This study addresses two facts about DNA replication fidelity that have long been known (reviewed in ref. 26) but are not yet well understood. The first is why different DNA polymerases can have very different error rates, even for the same error in the same local sequence context. The results in Table 1 illustrate that a single amino acid difference not only can reduce replication fidelity but can do so in a highly selective manner, affecting some substitutions but not others. In this regard, the E710A mutant Klenow polymerase is similar to the R283K mutant of DNA polymerase β, which has elevated error rates for some mismatches and wild-type rates for others (27). In both instances, these selective influences on error rates are inferred to reflect altered interactions in the binding pocket for the nascent base pair that involve the mutated amino acid side chains and the DNA minor groove.
The second issue is why rates for a mismatch composed of the same two bases can vary depending on orientation (e.g., T-dGTP vs. G-dTTP). The unusual error specificity of the E710A mutant Klenow fragment polymerase (13) (Table 1) provides an opportunity to investigate this. As explained above, the overall selectivity values calculated from our kinetic data (Table 3) account for the error specificity of the E710A mutant protein. Moreover, when considered in conjunction with the ternary complex structures of Pol I family polymerases, the changes in individual kinetic constants suggest a plausible model for the role of Glu-710 in polymerase fidelity. In developing this model, we started with the assumption that the Pu-Py mispairs, A-C and G-T, will adopt the wobble-pair structures seen by NMR and crystallography (28–31). As we and others have argued elsewhere, this scenario seems more likely than that Pu-Py mispairs involve rare tautomers in a Watson–Crick geometry (29, 32–34). Although the involvement of disfavored tautomers cannot formally be eliminated, it is harder to rationalize the influence of protein mutations on misinsertion frequency if one postulates that the nascent mispairs are virtually indistinguishable from their correctly paired counterparts.
Structural Interpretation.
Because no ternary complex structure exists for Klenow fragment, we illustrate our model by using the ternary complex of Klentaq, the polymerase portion of Taq DNA polymerase (12). The polymerase domains of the Taq and Escherichia coli enzymes are highly homologous, with almost identical three-dimensional structures, and the majority of the side chains that are in close proximity to the substrates at the active site are identical in both proteins. To visualize the constraints that come into play when a nascent mispair is present in the active site, we started with Klentaq coordinate sets having either G-dCTP or T-dATP as the nascent base pair within the polymerase active site (12, 22). Because the DNA duplex within the polymerase active site region in the ternary complex is A form, we took the coordinates for a G-T mispair structure within an A-DNA helix. [Both Pu-Py mispairs adopt very similar wobble pair structures (29), and so the conclusions presented here for the G-T mispair can equally well be applied to the A-C mispair.] To superimpose the mispair onto the nascent base pair, we assumed that the template base, being constrained as part of a DNA strand, would have much less freedom of movement than the incoming dNTP. We therefore superimposed the template base of the mispair onto the position occupied by the template base of the nascent base pair and determined where, as a consequence, the incoming incorrect dNTP would be located. Clearly, this modeling does not take into account the structural adjustments that would almost certainly take place to accommodate a nascent mispair in the polymerase active site. Nevertheless, we feel it serves to identify the interactions that are likely to be problematic when binding an incorrect incoming dNTP and can therefore provide an acceptable framework for interpreting fidelity data until such time as ternary complex crystal structures with mispaired bases are solved.
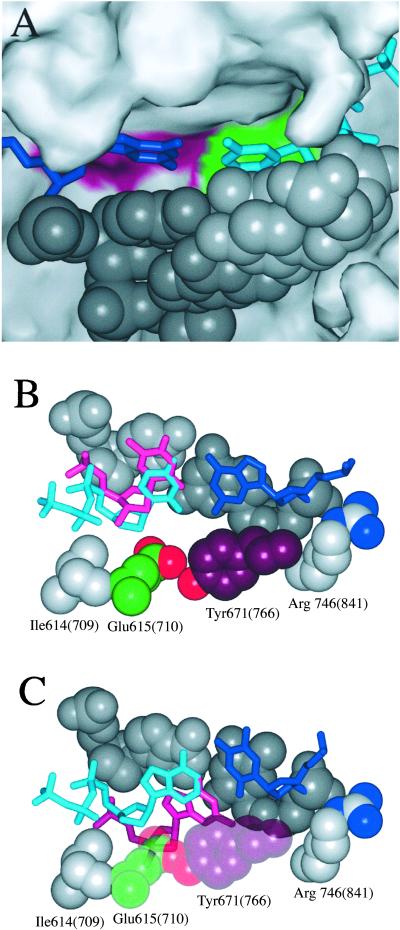
In the Klentaq ternary complex, the nascent base pair fits into a snug binding pocket, sandwiched between the primer-terminal base pair on one side and the O-helix of the fingers subdomain on the other side (12) (Fig. 2A). Several important, and highly conserved, side chains line the bottom of the binding pocket (Fig. 2 B and C). One of these is the invariant Glu-615 (Glu-710 in Klenow fragment), whose position beneath the sugar of the incoming nucleotide prevents incorporation of ribonucleotides (14). Additionally, Glu-615, together with Asn-750 and Gln-754, coordinates a water molecule that interacts with the minor groove side of the nascent base pair and can thus be thought of as part of the binding pocket surface (35). Because Pu-Py mispairs are much less symmetric than correct Watson–Crick base pairs (29), the Pu-dPyTP and Py-dPuTP combinations will interact differently with the binding pocket, which may account for the different behavior of the two classes of mispair. In a wobble mispair, the pyrimidine partner is shifted toward the major groove, and the purine partner is shifted toward the minor groove, relative to a correct base pair. The consequences for the polymerase active site are illustrated in Fig. 2 B and C. Fig. 2B compares the binding of a nascent G-dCTP base pair with the situation when the incoming nucleotide is an incorrect T. Displacement of the T toward the major groove side of the template–primer duplex has the consequence of moving it out of the binding pocket, away from the Glu-615 side chain. Fig. 2C compares a nascent T-dATP base pair with an incorrect incoming G. In this case, the incoming purine would be displaced toward the minor groove, moving it down into the binding pocket and resulting in unfavorable steric clashes of the sugar moiety with the side chains that form the lower surface of the binding pocket.
Figure 2.
Comparison of the binding of correct and mispaired purine–pyrimidine pairs in the nascent base pair binding pocket of Klentaq DNA polymerase. (A) Surface representation, demonstrating the snug fit of the binding pocket around the nascent base pair. The binding pocket, viewed from above, is defined on one side by the duplex primer terminus, of which two base pairs are shown (in gray, with the template strand darker than the primer), and on the other side by the fingers subdomain of the polymerase. To avoid obscuring the top of the binding pocket, most of the thumb subdomain of the polymerase is not shown. The incoming dCTP nucleotide is shown in cyan, with the opposing template G residue in blue. The portions of the binding pocket surface contributed by Glu-615 and Tyr-671 are colored green and purple, respectively. (B) Comparison of the position of a correct G-dCTP nascent base pair, with the proposed position of a G-dTTP mispair, viewed from the fingers side of the binding pocket. The template base occupies the same position in both cases and is shown in blue. The correctly paired incoming dCTP is shown in cyan and the mispaired dTTP in magenta. The binding pocket is represented by the primer-terminal base pair (colored as above), which interacts with one face of the nascent base pair, and by the four side chains illustrated that form much of the “floor” of the binding pocket on the minor groove side of the base pair. The residue numbers correpond to the Klentaq structure; residue numbers for the corresponding side chains in Klenow fragment are in parentheses. (C) An analogous comparison of a template T (blue)–dATP (cyan) correct pair, with a mispaired incoming dGTP (magenta). The Glu-615 and Tyr-671 side chains are shown partly transparent to illustrate the potential steric clash with the incoming mispaired nucleotide. For clarity, the water molecule coordinated by Glu-615 in four Klentaq ternary complex structures with correct nascent base pairs (35) is not shown. No information exists currently as to whether this water molecule would be present in complexes with mispaired dNTPs or with mutant proteins lacking the Glu side chain. Although the presence or absence of this water molecule does not affect the overall features of our model, a change in solvent interactions in the active site is one factor that may account for the observed differences in binding of incorrect pyrimidine nucleotides by wild type and the E710A mutant (Table 3; see also ref. 42).
Kinetic Evidence.
Strong support for this structural model comes from our kinetic data, which are entirely consistent with the interactions inferred from Fig. 2. When misinsertion involves an incorrect pyrimidine opposite a template purine, the situation in Fig. 2B suggests that the incoming dNTP will make fewer interactions with the binding pocket into which the nascent base pair normally fits. The loss of interactions accounts for the much higher Kd value when wild-type Klenow fragment inserts dCTP opposite A or dTTP opposite G. The lower kpol may result from suboptimal geometry for the phosphoryl transfer reaction because of the displacement of the incoming pyrimidine away from the active site residues. The absence of the Glu-710 side chain in E710A decreases the binding affinity of the correct dNTP and also eliminates the difference in Kd between correctly and incorrectly paired pyrimidines. We therefore conclude that interactions with the side chain of Glu-710 and/or its coordinated water molecule stabilize a correctly paired dNTP within the active site, and that these interactions (which cannot take place with the more distantly located mispaired pyrimidine) are critical for discrimination.
Even more compelling are the data for T-dGTP misinsertion. Here the increased Kd and the decreased kpol for the wild-type polymerase presumably reflect the steric clash that would occur on binding the mispaired purine (Fig. 2C), and the subsequent rearrangement of active site residues that must be necessary for stable binding. As above, binding of the correct incoming dNTP to the E710A mutant protein is weaker than binding to wild-type Klenow fragment, because a favorable interaction has been eliminated. However, the incorrect dGTP binds more strongly to the E710A mutant protein than to wild-type Klenow fragment, supporting the idea that removal of the Glu-710 side chain eliminates a steric clash within the binding pocket. Moreover, dGTP binds more strongly than dATP to the E710A mutant protein, presumably because a nucleotide positioned lower within the binding pocket is able to interact more effectively with the smaller Ala side chain. Why, then, is E710A not a mutator for T-dGTP misinsertion? As noted above (Table 3), the apparently wild-type phenotype of E710A with respect to T-dGTP errors results from opposing effects: more favorable binding of the incorrect nucleotide coupled with a much slower reaction rate. Misincorporation of G opposite template T is remarkable in that kpol for the mutant protein is 103-fold lower than for wild-type; for the A-dCTP and G-dTTP misinsertions, the difference is typically around 10-fold. We suggest that the absence of the Glu-710 side chain permits binding of the mispaired dGTP in a position that deviates substantially from the geometry appropriate for efficient phosphoryl transfer; in the wild-type enzyme, steric hindrance between Glu-710 and the mispaired dGTP weakens binding but forces the nucleotide to adopt a more appropriate geometry.
Other Mutations.
The behavior of the E710Q mutant is intermediate between that of wild-type and the more disruptive E710A mutant, suggesting that, even though Glu and Gln are isosteric, they have different effects on the dNTP-binding pocket. This could be solely because of the loss of negative charge caused by the E710Q mutation or because, in addition, the Gln side chain is differently oriented in the active site. In ternary complex crystal structures from the Pol I family, the residue equivalent to Glu-710 is seen to be hydrogen bonded to the invariant tyrosine at the C terminus of the O-helix (Tyr-766 in Klenow fragment) (10, 12). This hydrogen bond, which fixes the position of the Glu-710 side chain, would be expected to be either weaker or absent in the E710Q mutant, depending on the precise positioning of the Gln side chain. Simple modeling of a Gln side chain in place of Glu-615 of Klentaq, by using the swiss-pdb viewer (36), suggests that the preferred Gln rotamer would not occupy the same position as Glu in the wild-type protein (unpublished observations). Consistent with this idea, the phenotype of E710Q is also intermediate between wild-type and E710A in rNTP discrimination (14), which likewise depends on the positioning of the 710 side chain in the binding pocket.
The phenotype of mutator mutations at Tyr-766 of Klenow fragment is quite complex (37, 38), but it is worth noting that the largest fold increases, relative to wild-type Klenow fragment, were for the Pu-dPyTP errors (A-dCTP and G-dTTP), just as for E710A. This subset of errors could therefore be attributed to repositioning of the Glu-710 side chain as a consequence of the absence of Tyr-766, whereas others may result from structural rearrangements on the template side of the nascent base pair-binding pocket (see Fig. 2).
Recent work by Loeb and colleagues has demonstrated that the Ile side chain adjacent to Glu-710 or its homolog (Ile-709 in Klenow fragment, Ile-614 in Taq DNA polymerase; see Fig. 2) plays an important role both in fidelity and in excluding ribonucleotide residues from the polymerase active site (39, 40). Although the errors produced by the resulting mutator polymerases were not characterized in detail, it would not be surprising to find that these mutators share some specificity with the E710A polymerase.
Conclusion
Of the three mechanisms proposed to account for DNA polymerase fidelity, our data demonstrate the importance of the close steric complementarity between the nascent base pair-binding pocket and a correctly paired incoming dNTP in excluding purine–pyrimidine mispairs. We suggest that the invariant Glu-710 side chain selects by direct steric hindrance against those mispairs (such as T-dGTP) with an incoming purine nucleotide. We further suggest that this side chain provides an interaction that normally stabilizes correctly paired dNTPs but not mispaired incoming dPyTPs that are displaced toward the major groove and therefore have reduced interaction with the floor of the binding pocket. These ideas are entirely consistent with the unique dPyTP misincorporation specificity of the E710A/Q mutator polymerases (Table 1 and Fig. 1) and the kinetic data (Table 3). In combination with earlier studies (13, 14), our data indicate a dual role for Glu-710 in determining DNA polymerase specificity: both excluding ribonucleotides and preventing Pu-Py mispairs. In other “classical” polymerase families where ternary complex structures are available, the snug fit of the nascent base pair binding pocket suggests that similar mechanisms may operate to prevent dNTP misinsertions. Conversely, the lower fidelity of certain bypass DNA polymerases may result from their binding pockets having different, perhaps more open, conformations to catalyze efficient synthesis past template lesions that distort normal Watson–Crick base-pairing geometry, a prediction that is supported by recent structural data (41).
Supplementary Material
Acknowledgments
We thank Katarzyna Bebenek and Joann Sweasy for their comments on the manuscript. This work was partly supported by funds from the National Institutes of Health (NIH) AIDS Targeted Antiviral Program (D.T.M. and T.A.K.) and by NIH Grant GM-28550 (L.L., N.D.F.G., and C.M.J.).
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Loeb L A, Kunkel T A. Annu Rev Biochem. 1982;52:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- 2.Echols H, Goodman M F. Annu Rev Biochem. 1991;60:477–511. doi: 10.1146/annurev.bi.60.070191.002401. [DOI] [PubMed] [Google Scholar]
- 3.Kunkel T A, Bebenek K. Annu Rev Biochem. 2000;69:497–529. doi: 10.1146/annurev.biochem.69.1.497. [DOI] [PubMed] [Google Scholar]
- 4.Friedberg E C, Feaver W J, Gerlach V L. Proc Natl Acad Sci, USA. 2000;97:5681–5683. doi: 10.1073/pnas.120152397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman M F, Tippin B. Curr Opin Genet Dev. 2000;10:162–168. doi: 10.1016/s0959-437x(00)00057-5. [DOI] [PubMed] [Google Scholar]
- 6.Ohmori H, Friedberg E C, Fuchs R P P, Goodman M F, Hanaoka F, Hinkle D, Kunkel T A, Lawrence C W, Livneh Z, Nohmi T, et al. Mol Cell. 2001;8:7–8. doi: 10.1016/s1097-2765(01)00278-7. [DOI] [PubMed] [Google Scholar]
- 7.Joyce C M, Steitz T A. Annu Rev Biochem. 1994;63:777–822. doi: 10.1146/annurev.bi.63.070194.004021. [DOI] [PubMed] [Google Scholar]
- 8.Brautigam C A, Steitz T A. Curr Opin Struct Biol. 1998;8:54–63. doi: 10.1016/s0959-440x(98)80010-9. [DOI] [PubMed] [Google Scholar]
- 9.Doublié S, Sawaya M R, Ellenberger T. Structure (London) 1999;7:R31–R35. doi: 10.1016/S0969-2126(99)80017-3. [DOI] [PubMed] [Google Scholar]
- 10.Doublié S, Tabor S, Long A, Richardson C C, Ellenberger T. Nature (London) 1998;391:251–258. doi: 10.1038/34593. [DOI] [PubMed] [Google Scholar]
- 11.Kiefer J R, Mao C, Braman J C, Beese L S. Nature (London) 1998;391:304–307. doi: 10.1038/34693. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Korolev S, Waksman G. EMBO J. 1998;17:7514–7525. doi: 10.1093/emboj/17.24.7514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minnick D T, Bebenek K, Osheroff W P, Turner R M, Jr, Astatke M, Liu L, Kunkel T A, Joyce C M. J Biol Chem. 1999;274:3067–3075. doi: 10.1074/jbc.274.5.3067. [DOI] [PubMed] [Google Scholar]
- 14.Astatke M, Ng K, Grindley N D F, Joyce C M. Proc Natl Acad Sci USA. 1998;95:3402–3407. doi: 10.1073/pnas.95.7.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joyce C M. Proc Natl Acad Sci USA. 1997;94:1619–1622. doi: 10.1073/pnas.94.5.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polesky A H, Steitz T A, Grindley N D F, Joyce C M. J Biol Chem. 1990;265:14579–14591. [PubMed] [Google Scholar]
- 17.Joyce C M, Derbyshire V. Methods Enzymol. 1995;262:3–13. doi: 10.1016/0076-6879(95)62003-6. [DOI] [PubMed] [Google Scholar]
- 18.Derbyshire V, Freemont P S, Sanderson M R, Beese L, Friedman J M, Joyce C M, Steitz T A. Science. 1988;240:199–201. doi: 10.1126/science.2832946. [DOI] [PubMed] [Google Scholar]
- 19.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 20.Bebenek K, Kunkel T A. Methods Enzymol. 1995;262:217–232. doi: 10.1016/0076-6879(95)62020-6. [DOI] [PubMed] [Google Scholar]
- 21.Astatke M, Grindley N D F, Joyce C M. J Mol Biol. 1998;278:147–165. doi: 10.1006/jmbi.1998.1672. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Mitaxov V, Waksman G. Proc Natl Acad Sci USA. 1999;96:9491–9496. doi: 10.1073/pnas.96.17.9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunter W N, Kneale G, Brown T, Rabinovch D, Kennard O. J Mol Biol. 1986;190:605–618. doi: 10.1016/0022-2836(86)90246-9. [DOI] [PubMed] [Google Scholar]
- 24.Christopher J A. SPOCK: The Structural Properties Observation and Calculation Kit (Program Manual) College Station, TX: The Center for Macromolecular Design, Texas A&M University; 1998. [Google Scholar]
- 25.Bebenek K, Joyce C M, Fitzgerald M P, Kunkel T A. J Biol Chem. 1990;265:13878–13887. [PubMed] [Google Scholar]
- 26.Kunkel T A, Bebenek K. Biochim Biophys Acta. 1988;951:1–15. doi: 10.1016/0167-4781(88)90020-6. [DOI] [PubMed] [Google Scholar]
- 27.Osheroff W P, Beard W A, Wilson S H, Kunkel T A. J Biol Chem. 1999;274:20749–20752. doi: 10.1074/jbc.274.30.20749. [DOI] [PubMed] [Google Scholar]
- 28.Brown T, Kennard O, Kneale G, Rabinovich D. Nature (London) 1985;315:604–606. doi: 10.1038/315604a0. [DOI] [PubMed] [Google Scholar]
- 29.Hunter W N, Brown T, Anand N N, Kennard O. Nature (London) 1986;320:552–555. doi: 10.1038/320552a0. [DOI] [PubMed] [Google Scholar]
- 30.Patel D J, Kozlowski S A, Marky L A, Rice J A, Broka C, Dallas J, Itakura K, Breslauer K J. Biochemistry. 1982;21:437–444. doi: 10.1021/bi00532a003. [DOI] [PubMed] [Google Scholar]
- 31.Gao X, Patel D J. J Biol Chem. 1987;262:16973–16984. [PubMed] [Google Scholar]
- 32.Joyce C M, Sun X C, Grindley N D F. J Biol Chem. 1992;267:24485–24500. [PubMed] [Google Scholar]
- 33.Goodman M F, Creighton S, Bloom L B, Petruska J. Crit Rev Biochem Mol Biol. 1993;28:83–126. doi: 10.3109/10409239309086792. [DOI] [PubMed] [Google Scholar]
- 34.Morgan A R. Trends Biochem Sci. 1993;18:160–163. doi: 10.1016/0968-0004(93)90104-u. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Waksman G. Protein Sci. 2001;10:1225–1233. doi: 10.1110/ps.250101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guex N, Peitsch M C. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 37.Bell J B, Eckert K A, Joyce C M, Kunkel T A. J Biol Chem. 1997;272:7345–7351. doi: 10.1074/jbc.272.11.7345. [DOI] [PubMed] [Google Scholar]
- 38.Carroll S S, Cowart M, Benkovic S J. Biochemistry. 1991;30:804–813. doi: 10.1021/bi00217a034. [DOI] [PubMed] [Google Scholar]
- 39.Patel P H, Kawate H, Adman E, Ashbach M, Loeb L A. J Biol Chem. 2001;276:5044–5051. doi: 10.1074/jbc.M008701200. [DOI] [PubMed] [Google Scholar]
- 40.Shinkai A, Patel P H, Loeb L A. J Biol Chem. 2001;276:18836–18842. doi: 10.1074/jbc.M011472200. [DOI] [PubMed] [Google Scholar]
- 41.Zhou B-L, Pata J D, Steitz T A. Mol Cell. 2001;8:427–437. doi: 10.1016/s1097-2765(01)00310-0. [DOI] [PubMed] [Google Scholar]
- 42.Fersht A R, Shi J-P, Knill-Jones J, Lowe D M, Wilkinson A J, Blow D M, Brick P, Carter P, Waye M M Y, Winter G. Nature (London) 1985;314:235–238. doi: 10.1038/314235a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.