Abstract
The function of urokinase and its receptor is essential for cell migration in pathological conditions, as shown by the analysis of knockout mice phenotypes. How a protease of a fibrinolytic pathway can induce migration is not understood and no link between this protease and migration-promoting G protein-coupled receptors has been described. We now show that FPRL1/LXA4R, a G protein-coupled receptor for a number of polypeptides and for the endogenous lipoxin A4 (LXA4), is the link between urokinase-type plasminogen activator (uPA) and migration as it directly interacts with an activated, soluble, cleaved form of uPA receptor (uPAR) (D2D388–274) to induce chemotaxis. In this article we show that (i) both uPAR and FPRL1/LXA4R are necessary for the chemotactic activity of uPA whereas FPRL1/LXA4R is sufficient to mediate D2D388–274-induced cell migration. (ii) Inhibition or desensitization of FPRL1/LXA4R by antibodies or specific ligands specifically prevents chemotaxis induced by D2D388–274 in THP-1 cells and human peripheral blood monocytes. (iii) Desensitization of FPRL1/LXA4R prevents the activation of tyrosine kinase Hck induced by D2D388–274. (iv) D2D388–274 directly binds to FPRL1/LXA4R and is competed by two specific FPRL1/LXA4R agonists, the synthetic MMK-1 peptide and a stable analog of LXA4. Thus, a naturally produced cleaved form of uPAR is a unique endogenous chemotactic agonist for FPRL1/LXA4R receptor and its activity can be antagonized by specific ligands. These results provide the first direct link, to our knowledge, between the fibrinolytic machinery and the inflammatory response, demonstrating that uPA-derived peptide fragments can activate a specific chemotactic receptor.
Urokinase plasminogen activator (uPA) is a serine protease that activates plasminogen (Plg) to plasmin and binds to a specific high affinity cell surface receptor, uPAR (CD87) (1). The phenotype of uPA−/− and uPAR−/− mice is not caused only by the lack of Plg activation. Indeed, Plg−/− mice die early with multiple thrombosis and extensive fibrin deposits, whereas the uPA−/− and uPAR−/− mice live normally, showing rare thrombotic events and occasional fibrin deposits (2). Lack of uPA, however, causes impaired migration of lymphocytes and macrophages to tissue lesions, with impairment of the host defenses, bacterial spreading, and death (3), or resistance to the development of aneurysms in a mouse model (2). Deficient recruitment of peritoneal or lung neutrophils at inflammatory sites in Pseudomonas aeruginosa infection and deficient Mac-1 function in macrophages and neutrophils is also observed in uPAR-deficient mice (4, 5).
uPA binding to uPAR induces intracellular signaling affecting cell adhesion, migration, and proliferation. uPA binding to uPAR induces chemotaxis in a variety of cells, with activation of tyrosine kinases (Hck, Src), MEK, c-Raf, Tyk-3, PI-3-K, and Rac (1, 5–7).
uPAR is a high affinity cell surface receptor for uPA (1), formed by three extracellular domains (D1, D2, and D3), and anchored to the plasma membrane via a glycosylphosphatidylinositol anchor (8). Because uPAR lacks an intracellular domain, the existence of a trans-membrane adapter transmitting an intracellular signal initiated by the binding of uPA to uPAR has been hypothesized (8). Chemotaxis stimulated by uPA requires binding to uPAR (9, 10). However, in uPAR−/− cells, the addition of exogenous soluble uPAR, provided it is cleaved, reconstitutes migration, indicating that uPAR must be activated by uPA to induce chemotaxis. The induction of chemotaxis by “activated uPAR” in turn requires the existence of a trans-membrane adapter (9, 11). Activation of soluble recombinant uPAR is achieved in vitro by cleavage with chymotrypsin between domains D1 and D2, generating a carboxyl-terminal fragment starting at residue 88 (D2D388–274) (9). The amino acid residues 88–92 of uPAR is essential and sufficient for chemotaxis (11). This sequence is phenotypically relevant because cleaved (D2D3) forms of uPAR are naturally produced and found in tissues and in biological fluids (12, 13).
The trans-membrane adapter mediating uPA-dependent chemotaxis has not been identified. uPAR is known to directly interact with integrins (5–7, 14) and vitronectin (15, 16), affecting cell adhesion and signaling. However, because chemotaxis induced by uPA or D2D388–274 is inhibited by ADP-ribosylating pertussis toxin (9, 11), a different type of adaptor may exist, possibly a G protein-coupled receptor of the family of the chemotactic peptides or chemokine receptors.
In this study, we show that the seven-trans-membrane receptor FPR-like receptor-1/lipoxin A4 receptor (FPRL1/LXA4R), a functional receptor for a diverse array of exogenous and host-derived peptides (reviewed in ref. 17) as well as for the aspirin-triggered lipid mediator 15-epi-LXA4 (reviewed in ref. 18), is necessary and sufficient to mediate the chemotactic activity of D2D388–274. Our work identifies the D2D388–274 uPAR fragment as an endogenous ligand for FPRL1/LXA4R.
Materials and Methods
Cells and Reagents.
THP-1 cells (American Type Culture Collection, Rockville, MD) were grown in suspension in RPMI medium 1640 (GIBCO/BRL) with 10% FBS. Human peripheral blood monocytes were isolated from buffy coats enriched for mononuclear cells by two rounds of centrifugation (460 × g) over Ficoll and Percoll (Amersham Pharmacia) gradients (over 95% CD14-positive by cytofluorometry). Rat basophilic leukemia cells (RBL-2H3) and their transfectant with epitope-tagged FPR (ETFR cells), and the human embryonic kidney HEK293 cells and their transfectant with FPRL1 (HEK293/FPRL1 cells) have been described (19). Cells were grown as above; medium for transfected cells also contained 0.8 mg/ml of geneticin (GIBCO/BRL).
Production and purification of D2D388–274 was described (11). The rabbit polyclonal Ab that recognizes the LESIFRSLLFRVM sequence (residues 181–193) of FPRL-1/LXA4R has been described (20). The stable lipoxin analog 15-epi-16-parafluorophenoxy-LXA4-methyl ester (ZK223351) was a kind gift of J. Parkinson (Berlex Biosciences, Richmond, CA). The MMK-1 peptide has been described (21). The amino-terminal fragment of human uPA (ATF) was a kind gift of J. Henkin (Abbott). Nonspecific rabbit polyclonal immunoglobulins and the synthetic chemotactic peptide formyl-methyonyl-leucyl-proline (fMLP) were purchased from Sigma. Affinity-purified rabbit polyclonal anti-p56/p59hck was purchased from Santa Cruz Biotechnology. Type I collagen was purchased from Roche Molecular Biochemicals.
Chemotaxis Assay.
Migration of THP-1 cells, monocytes, human HEK293 cells, rat RBL-2H3 cells, or transfected cells (HEK293/FPRL1 and ETFR) was assessed by a 48-well microchemotaxis chamber (Neuroprobe, Cabin John, MD). Chemoattractants (26 μl), at different concentrations in serum-free RPMI medium 1640, were placed in the lower compartment of the chamber, and a 50-μl cell suspension (106 cells per ml) added to the upper compartment. The two compartments were separated by an uncoated filter of either a 5 μm pore size, in the case of monocytes and THP-1 cells, or by a collagen type I-coated (50 μg/ml for 2 h at 37°C) 8 μm pore size polycarbonate filter (Neuroprobe) for HEK293, RBL-2H3, ETFR, and HEK293/FPRL1 cells. Incubation at 37°C in humidified air with 5% CO2 was 90 min for monocytes and THP-1 cells, and 5 h for HEK293, HEK293FPRL1/LXA4R, RBL-2H3, and ETFR cells. The filter was removed, scraped, fixed, and stained with Diff-Quik (Dade Diagnostics, Aguada, PR), and migrated cells were counted by light microscopy in eight high-powered fields. Results, expressed as the mean (±SD) from triplicate samples, are representative of at least three experiments. Migration in the absence of chemoattractant was set to 100%. The chemotactic index is the no. of cells migrated in the presence vs. in the absence of chemoattractant.
Potential inhibitors of migration (i.e., Abs, desensitizers) were preincubated with cells for 30–60 min at 37°C, washed with medium, and the assay was performed as described above.
Tyrosine Kinase Assay.
The kinase assay was performed as described (11) with THP-1 cells (untreated or treated with fMLP, 10−4 M for 20 min at 37°C) metabolically labeled with Pro-mix L-[35S] (Amersham Biosciences). Cells were washed and incubated with 1 nM D2D388–274 for the indicated time at room temperature. Radiolabeled cell lysates were immunoprecipitated with a polyclonal anti-p56/p59hck Ab (9), and an aliquot was analyzed by SDS/PAGE. The remainder was used for an in vitro kinase assay [10 μCi of [γ-32P]ATP (Amersham Biosciences) for 15 min at room temperature] and resolved by SDS/PAGE and autoradiography.
Ligand Binding Assay.
125I-D2D388–274 (5 nM) (Iodogen, Pierce), specific activity 30 μCi/μg, was incubated with 1.5–2 × 106 cells in 100 μl of binding buffer (RPMI medium 1640, 0.5% BSA) for 30 min at room temperature in the presence or absence of increasing concentrations of unlabeled D2D388–274 or other competitors. The cells were washed once with 1 ml of RPMI medium 1640, 0.5% BSA, centrifuged through a 10% sucrose/PBS cushion in Eppendorf tubes, and the pellet-containing tips were cut off and counted in a gamma counter. Binding in the presence of 100-fold excess unlabeled D2D388–274 was subtracted. Binding competition is calculated as % Competition = 1 − (specific binding in the presence of competitor)/(specific binding in the absence of competitor) × 100.
Results
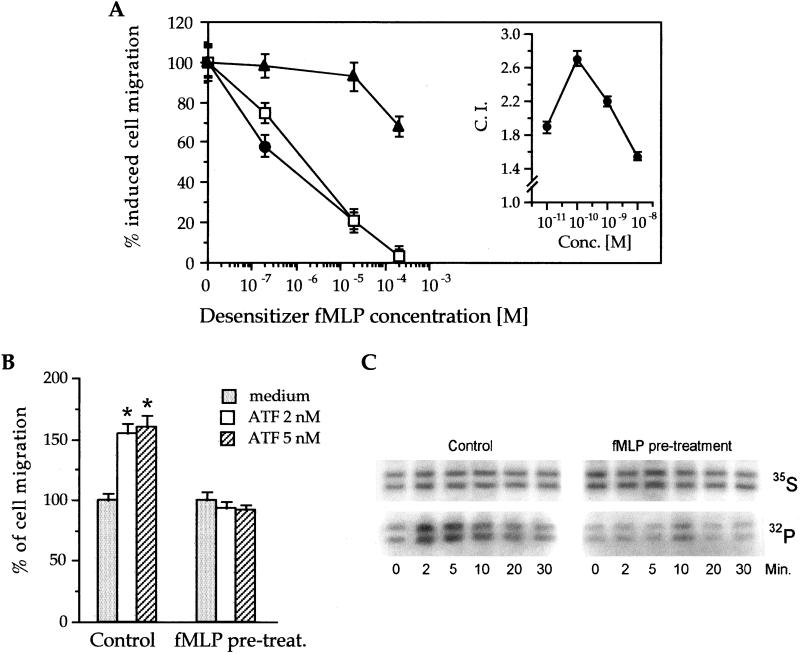
An activated uPAR fragment, D2D388–274, has chemotactic activity between 0.01–1.0 nM (ref. 11; Fig. 1A Inset). To understand the molecular basis of the chemotactic activity of D2D388–274, we performed desensitization experiments. Fig. 1A shows that when THP-1 cells were preincubated with different concentrations of fMLP, their response to both fMLP and D2D388–274 was inhibited, with complete desensitization at 0.2 mM fMLP. Pretreatment with fMLP, however, had a much lower effect on monocyte chemoattractant protein-1 (MCP-1)-induced chemotaxis. Fig. 1B shows that a pretreatment with 0.2 mM fMLP also desensitized the response of THP-1 cells to ATF.
Figure 1.
(A) FMLP specifically desensitizes D2D388–274 chemotaxis in THP-1 cells. Inset shows the chemotactic index (CI) of D2D388–274-induced THP-1 cells at different attractant concentration. For desensitization, cells were preincubated (30 min at 37°) with different concentrations of fMLP (abscissae), and then their chemotactic responses were tested toward 10 nM fMLP (open squares), 1 nM D2D388–274 (filled circles), or 2 nM MCP-1 (filled triangles) (see Materials and Methods). A value of 100% cell migration (ordinates) corresponds to the CI obtained in the absence of desensitizer, i.e., about 2.5 for MCP-1 and 2.0 for fMLP or D2D388–274. The value of 0% cell migration corresponds to a CI of 1, i.e., no stimulation of chemotaxis. (B) Desensitization of ATF-induced chemotaxis by FMLP. THP-1 cells were pretreated with or without 0.2 mM fMLP (see above) and tested for their migration induced by the indicated concentrations of ATF for 90 min at 37°C. *, P ≤ 0.0005 (Student's t test). (C) FMLP pretreatment prevents HcK activation by D2D388–274. 35S-labeled cells were pretreated with or without 0.2 mM fMLP for 20 min at 37°C, washed, and challenged with 0.1 nM D2D388–274 for the indicated times at room temperature (see Materials and Methods). Cells were then pelleted and lysed, and the lysate was immunoprecipitated with anti-HcK Ab. The immunoprecipitate was supplemented with [γ-32P]ATP and then subjected to SDS/PAGE and autoradiography (32P line). In parallel, equivalent amounts of immunoprecipitate were run in SDS/PAGE as a loading control and autoradiographed (35S).
In THP-1 cells and neutrophils, neither uPA nor D2D388–274 (not shown) induce calcium flux unless after the artificial clustering of uPAR (22). Because uPAR clustering would increase the complexity of our experimental set up, we tested the effect of fMLP pretreatment on another signaling event induced by uPA in THP-1 cells, i.e., the D2D388–274-induced phosphorylation of p56–59hck tyrosine kinase, which is required for cell migration (9). As shown in Fig. 1C, D2D388–274 rapidly activated p56–59hck; however, pretreatment of the cells with 0.2 mM fMLP blocked the effect. Overall, these data suggest that D2D388–274 may use a receptor for induction of chemotaxis that recognizes fMLP with low affinity.
fMLP binds two receptors, FPR and FPRL1/LXA4R (23), with high and low affinity, respectively. The high concentrations of fMLP required for complete desensitization of cell response to D2D388–274 (Fig. 1A) suggest that D2D388–274 might use FPRL1/LXA4R rather than FPR. To test this hypothesis, we exposed THP-1 cells to the synthetic MMK-1 peptide, a high affinity agonist of FPRL1/LXA4R (21). As shown in Table 1, MMK-1 completely suppressed the cell response to D2D388–274, indicating that FPRL1/LXA4R is involved in D2D388–274 signaling.
Table 1.
MMK-1 peptide desensitizes THP-1 cells to D2D388–274
| Stimulants in lower well | Pretreatment of cells
|
||
|---|---|---|---|
| No addition | 250 nM MMK-1 | 500 nM MMK-1 | |
| No addition | 100 ± 3.6 | 102 ± 3.6 | 96 ± 3.3 |
| D2D3, 1 nM | 161 ± 11.1* | 96 ± 4.4 | 99 ± 4.1 |
| D2D3, 0.1 nM | 182 ± 10.2* | 108 ± 5.4 | 98 ± 5.9 |
| D2D3, 0.01 nM | 205 ± 12.2* | 118 ± 4.9 | 108 ± 4.2 |
Chemotaxis assays were carried out in triplicate as described in Materials and Methods. The value 100% refers to the migration of cells in the absence of chemoattractants. Data points are the mean ± SEM of three experiments. Desensitization was carried out by preincubating cells with the indicated concentration of MMK-1 peptide for 20 min at 37°C; MMK-1 was also present in the upper well during the assay.
, P ≤ 0,0001, Student's t test.
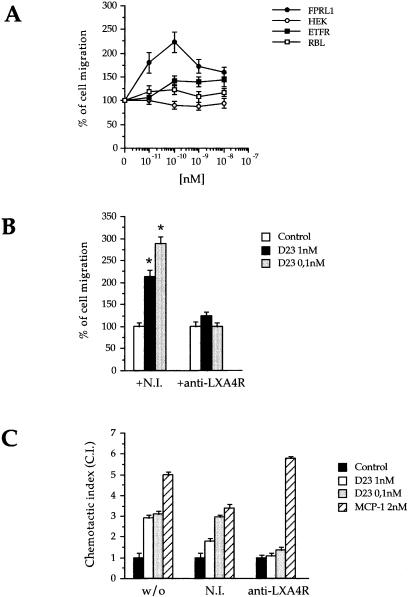
If this was the case, D2D388–274 should not induce migration of cells lacking FPRL1/LXA4R. Indeed HEK293 cells, which express neither uPAR nor FPRL1/LXA4R (data not shown), failed to respond to uPA, fMLP (not shown), and D2D388–274 (Fig. 2A). In contrast, D2D388–274 induced a potent chemotactic response of HEK293 cells transfected with FPRL1/LXA4R (Fig. 2A), with a maximum of activity at 0.1 nM. Thus, the presence of FPRL1/LXA4R on the cell surface is sufficient for D2D388–274-induced chemotaxis. We have excluded that the high affinity fMLP receptor, FPR, plays a role in this event, because neither rat basophilic leukemia cells, which lack FPR (RBL-2H3), nor the same cells transfected with FPR cDNA (ETFR) were affected by D2D388–274 (Fig. 2A). These cells did, however, differentially respond to 10 nM fMLP in chemotaxis assays (data not shown).
Figure 2.
FPRL1-LXA4R is necessary and sufficient to mediate D2D388–274 chemotactic activity. (A) D2D388–274 induces chemotaxis in 293FPRL1-LXA4R, but not in HEK293 (which express no FPRL1-LXA4R), nor in RBL-2H3 (which do not express FPR), nor in EFTR (RBL-2H3 expressing FPR). The 100% value is the migration in the absence of chemoattractants. The data are the average of three experiments (RBL-2H3 and EFTR cells) or of six experiments (293FPRL1- LXA4R and HEK293 cells), each in triplicate. (B) An Ab against FPRL1-LXA4R specifically blocks D2D388–274 chemotactic activity in THP-1 cells. NI, nonimmune serum. The data represent the average of three experiments in triplicate. 100% represents the extent of migration observed in the absence of D2D388–274. *, P ≤ 0.0001 (Student's t test). (C) An Ab against FPRL1-LXA4R blocks D2D388–274 but not MCP-1 chemotaxis in human fresh peripheral blood monocytes. The data refer to one single experiment carried out in triplicate.
Pretreatment with D2D388–274 or with peptide 3, a synthetic peptide (AVTYSRSRYLEC) reproducing the 84–95 residues of uPAR and showing full chemotactic activity (11), each resulted in desensitization of HEK293FPRL1/LXA4R cells to fMLP (Table 2). Instead, a scrambled version of peptide 3 had no effect.
Table 2.
Effect of pretreatment of HEK293 FPRL1/LXA4R cells with activated uPAR on the chemotactic response to fMLP
| Desensitizers | % of migration | n |
|---|---|---|
| None | 100 ± 7 | 4 |
| D2D3, 0.01 μM | 57 ± 3 | 4 |
| D2D3, 0.1 μM | 49 ± 3 | 2 |
| Peptide 3*, 10 μM | 71 ± 4 | 3 |
| Peptide 3, 100 μM | 2 ± 0.6 | 3 |
| Scrambled peptide 3, 10 μM | 96 ± 5 | 3 |
| Scrambled peptide 3, 100 μM | 65 ± 4 | 3 |
Chemotaxis assays were carried out in triplicate as described in Materials and Methods. Cells were preincubated (60 min at 37°C) with the desensitizers indicated and then were assayed for chemotaxis. The value 100% refers to a chemotactic index of 2.0 observed in the presence of 0.2 mM fMLP in the lower well in the absence of desensitizers. The 0 value refers to a chemotactic index of 1, i.e., no stimulation. Data are the mean ± SEM of three experiments.
Peptide 3 is the synthetic chemotactic peptide located between domains 1 and 2 (sequence: AVTYSRSRYLEC).
If FPRL1/LXA4R is necessary for D2D388–274-induced chemotaxis, specific inhibiting Abs should block its activity. Therefore, we tested the effect of an FPRL1/LXA4R antiserum (20) on THP-1 cells or human monocytes, both expressing (not shown) uPAR, FPR, and FPRL1, and migrating in response to uPA, fMLP (not shown), and D2D388–274 (Fig. 2B). These cells also express the chemokine receptor CCR2 and respond to its ligand MCP-1 (Fig. 2B). The anti-FPRL1/LXA4R antiserum (20) specifically blocked the effect of D2D388–274 on THP-1 cells (Fig. 2B) as well as on peripheral blood monocytes, although it did not alter MCP-1-induced chemotaxis (Fig. 2C). A preimmune serum had no effect on either D2D388–274 or MCP-1. Thus, in cells that express multiple chemotactic receptors, the FPRL1/LXA4R Ab selectively blocked the response to D2D388–274. Taken together, these results show that FPRL1/LXA4R is both necessary and sufficient for the chemotactic activity of D2D388–274.
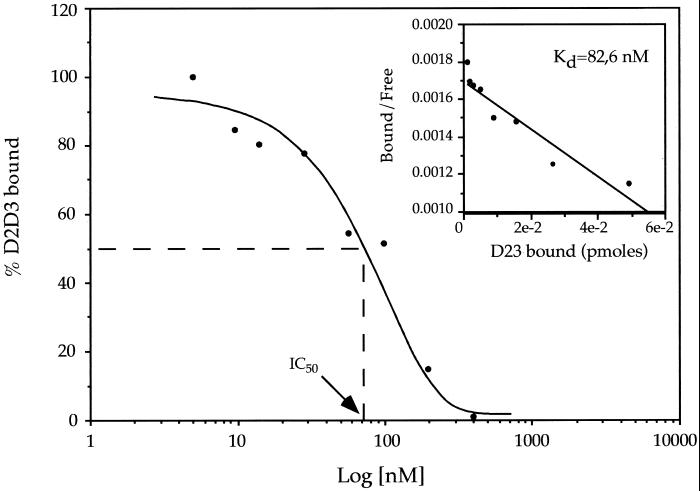
To investigate further the interaction of D2D388–274 with the receptor, we performed binding assays of iodinated D2D388–274 with HEK293FPRL1/LXA4R cells. Binding was specific, time-dependent, and saturable (not shown). Moreover, equilibrium-binding studies showed that D2D388–274 bound to HEK293FPRL1/LXA4R cells with an apparent Kd of 83 nM (Fig. 3A). Similar results were obtained with THP-1 cells (not shown). Binding was specific (Table 3), as it was displaced by the unlabeled D2D388–274, but not by ΔD2D3, a chemotactically inactive uPAR fragment starting at residue 92 and thus lacking the amino-terminal 5-residues-long minimal chemotactic epitope (11). Two established FPRL1/LXA4R ligands, the peptide MMK-1 (21, 24) and the stable LXA4 analog 15-epi-16-parafluorophenoxy-LXA4 methyl ester (25), inhibited the binding of D2D388–274 to HEK293FPRL1/LXA4R cells by 60% at the concentration of 100 nM. As expected, domain D1 of uPAR, lacking the chemotactic epitope, was unable to compete for binding of D2D388–274 to THP-1 cells (not shown). Collectively, these results clearly indicate that D2D388–274 binds to FPRL1/LXA4R and that the chemotactic region at its amino terminus (residues 88–92) is required for the interaction. fMLP at 0.2 mM had no effect.
Figure 3.
D2D388–274 binds specifically to 293FPRL1-LXA4R cells. Iodinated D2D388–274 (5 nM) was incubated with 2 million cells in the presence or absence of different concentrations of unlabeled D2D388–274 for 30 min at room temperature. The abscissae show the concentration of unlabeled D2D388–274, and the ordinates show the percentage of bound radioactivity. The data shown are from one experiment representative of four carried out with the same results (less than 10% variation). Inset shows the same data presented as a Scatchard plot.
Table 3.
Binding specificity of iodinated D2D388–274 on HEK293/FPRL1–LXA4R cells
| Unlabeled competitor | % competition | n |
|---|---|---|
| D2D3, 1 μM | 100 ± 6.5 | 8 |
| MMK-1, 100 nM | 55.5 ± 5 | 5 |
| 15-epi-16-parafluorophenoxy-LXA4 methyl ester, 100 nM | 57.5 ± 5.5 | 3 |
| Δ-D2D3, 1 μM | 23.7 ± 1.5 | 2 |
| fMLP, 0.2 mM | 23.5 ± 1 | 1 |
Ligand binding assays were performed in triplicate. Iodinated D2D388–274 was used at 5 nM. Cells were preincubated for 20 min at room temperature with the unlabeled competitors, then labeled D2D388–274 was added to the samples and incubated for 30 min at room temperature. Specific binding was measured as described in Materials and Methods.
Discussion
We show that the peptides fMLP and MMK-1, low and high-affinity agonists of FPRL1/LXA4R, respectively, interfere with the chemotactic activity of D2D388–274 and uPA-ATF and that fMLP also prevents D2D388–274-induced Hck activation. In addition, we also present evidence that the expression and the activity of FPRL1/LXA4R are required for D2D388–274 and ATF chemotaxis. D2D388–274 specifically binds to FPRL1/LXA4R-expressing cells, and the binding requires the specific chemotactic epitope of uPAR and is displaced by the stable LXA4 analog and by the synthetic MMK-1 peptide, both high affinity agonists for FPRL1/LXA4R. Our findings show that the naturally occurring uPAR fragment is an endogenous ligand and agonist of FPRL1/LXA4R.
FMLP is low affinity (20) whereas the synthetic MMK-1 peptide and LXA4 are high affinity ligands for FPRL1/LXA4R (24). The binding determinant for the latter two ligands resides in the seventh transmembrane domain and adjacent sequences, distinguishable from that of fMLP located in the first extracellular loop (24). Because the stable LXA4 analog and MMK-1 displace the binding of D2D388–274 to FPRL1/LXA4R, it is likely that the seventh transmembrane domain of FPRL1/LXA4R is also a recognition site for D2D388–274.
The interaction of activated uPAR with FPRL1/LXA4R, which explains the uPAR-dependent chemotactic activity of uPA, provides a link between the fibrinolytic cascade and inflammation. Fibrinolysis, in particular uPA and uPAR, has long been tied to cancer in view of its overexpression and function in this disease (26).
Elevated levels of serum uPAR represent a strong negative prognostic marker in tumors, independently of the type of cells (stromal or cancer) in which uPAR is expressed (26, 27). The interaction of uPAR fragments with FPRL1/LXA4R, therefore, provides a novel molecular and functional link between cancer and stromal cells in invasion. Indeed, fragments such as D2D388–274 are overproduced in cancer (12, 28). Also, in transgenic mice, the increased uPAR cleavage caused by uPA overexpression leads to pathological consequences (29).
Elevated levels of soluble uPAR in serum have a negative prognostic significance also in diseases with strong inflammatory components, like AIDS (30). Increased serum uPAR may reflect release from overactivated, overproduced, and rapidly turned over cells, at stages where the disease is not yet full-blown. In this respect, it is interesting to notice that although the lack of uPA in mice prevents an efficient defense against microbial infections (3), it causes an exacerbated reaction in antigen-induced arthritis (31).
FPRL1/LXA4R is expressed in monocytes, lymphocytes, and neutrophils, where it is also up-regulated by several cytokines and differentiation factors like retinoic acid and mediates the chemotactic activity of HIV-derived peptides (reviewed in ref. 17). The presence of FPRL1/LXA4R has been reported in many other cells, including astrocytes, fibroblasts, epithelial cells, mesangial cells, hepatocytes, smooth muscle, and tumor cells. The widespread expression of FPRL1/LXA4R is in agreement with the broad range of cells that respond to uPA/uPAR chemotactic signals.
The present results raise several intriguing questions regarding the relationship of inflammation to the activation of uPAR (1). In this regard, LXA4 inhibits neutrophils while activating chemotaxis in monocytes via FPRL1/LXA4R (5, 7, 27), that in vivo culminates in an antiinflammatory response when presented either topically and/or via i.v. injection (25). uPA clearly stimulates chemotaxis with both monocytes and neutrophils (1). Because both monocytes and neutrophils express uPA and uPAR, it is important to examine whether uPAR activation interferes with the signaling pathways elicited by LXA4 and aspirin-triggered 15-epi-LXA4 that use the FPRL1/LXA4R system. In this regard, it is possible that these two sets of ligands, lipid and peptide, can stimulate heterologous desensitization (32). However, the in vivo correlate of desensitization, as established in in vitro studies using intracellular receptor crosstalk, remains to be established. On the basis of the present results, LXA4 and aspirin-triggered LXA4 might interfere with uPA and uPAR in invasive cancer phenotype with FPRL1/LXA4R.
Acknowledgments
We thank Dr. Anna Mondino, Nicolai Sidenius, Roberta Mazzieri, Maria Pia Protti, Ruggero Pardi, and Jacopo Meldolesi for advice and critical discussions. We are grateful to Dr. John Parkinson for the generous gift of the stable LXA4 analog. This work was supported by grants (to F.B.) from the Italian Association for Cancer Research (AIRC), the Italian Ministry of Education (PRIN 99), and by the European Union 5th Framework Program (Contract No. QLRT-1999-31131). C.N.S. acknowledges support by National Institutes of Health Grant GM 38765.
Abbreviations
- uPA
urokinase-type plasminogen activator
- uPAR
uPA receptor
- ATF
amino-terminal fragment of uPA
- D2D388–274
carboxyl-terminal fragment of soluble uPAR starting at residue 88 and obtained by cleavage with chymotrypsin
- fMLP
formyl-methyonyl-leucyl-proline
- FPRL1/LXA4R
FPR-like receptor-1/lipoxin A4 receptor
- FPR
fMLP receptor
- LXA4
lipoxin A4
- MCP-1
monocyte chemoattractant protein-1
- ETFR
epitope-tagged FPR
References
- 1.Blasi F. Immunol Today. 1997;18:415–417. doi: 10.1016/s0167-5699(97)01121-3. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Moons L, Dewerchin M, Mackman N, Luther T, Breier G, Ploplis V, Muller M, Nagy A, Plow E, et al. Ann NY Acad Sci. 1997;811:191–206. doi: 10.1111/j.1749-6632.1997.tb52002.x. [DOI] [PubMed] [Google Scholar]
- 3.Gyetko M R, Chen G H, McDonald R A, Goodman R, Huffnagle G B, Wilkinson C C, Fuller J A, Toews G B. J Clin Invest. 1996;97:1818–1826. doi: 10.1172/JCI118611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gyetko M R, Sud S, Kendall T, Fuller J A, Newstead M W, Standiford T J. J Immunol. 2000;165:1513–1519. doi: 10.4049/jimmunol.165.3.1513. [DOI] [PubMed] [Google Scholar]
- 5.Preissner K, Kanse S M, May A E. Curr Opin Cell Biol. 2000;12:621–628. doi: 10.1016/s0955-0674(00)00141-1. [DOI] [PubMed] [Google Scholar]
- 6.Chapman H A. Curr Opin Cell Biol. 1997;9:714–724. doi: 10.1016/s0955-0674(97)80126-3. [DOI] [PubMed] [Google Scholar]
- 7.Ossowski L, Aguirre-Ghiso J A. Curr Opin Cell Biol. 2000;12:613–620. doi: 10.1016/s0955-0674(00)00140-x. [DOI] [PubMed] [Google Scholar]
- 8.Ploug M, Ellis V, Dano K. Biochemistry. 1994;33:8991–8997. doi: 10.1021/bi00196a017. [DOI] [PubMed] [Google Scholar]
- 9.Resnati M, Guttinger M, Valcamonica S, Sidenius N, Blasi F, Fazioli F. EMBO J. 1996;15:1572–1582. [PMC free article] [PubMed] [Google Scholar]
- 10.Webb D J, Nguyen D H, Gonias S L. J Cell Sci. 2000;113:123–134. doi: 10.1242/jcs.113.1.123. [DOI] [PubMed] [Google Scholar]
- 11.Fazioli F, Resnati M, Sidenius N, Higashimoto Y, Appella E, Blasi F. EMBO J. 1997;16:7279–7286. doi: 10.1093/emboj/16.24.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sidenius N, Sier C F, Blasi F. FEBS Lett. 2000;475:52–56. doi: 10.1016/s0014-5793(00)01624-0. [DOI] [PubMed] [Google Scholar]
- 13.Solberg H, Rømer J, Brünner N, Holm A, Sidenius N, Danø K, Høyer-Hansen G. Int J Cancer. 1994;58:877–881. doi: 10.1002/ijc.2910580622. [DOI] [PubMed] [Google Scholar]
- 14.Wei Y, Eble J A, Wang Z, Kreidberg J A, Chapman H A. Mol Biol Cell. 2001;12:2975–2986. doi: 10.1091/mbc.12.10.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei Y, Lukashev M, Simon D I, Bodary S C, Rosenberg S, Doyle M V, Chapman H A. Science. 1996;273:1551–1555. doi: 10.1126/science.273.5281.1551. [DOI] [PubMed] [Google Scholar]
- 16.Deng G, Curriden S A, Wang S, Rosenberg S, Loskutoff D J. J Cell Biol. 1996;134:1563–1571. doi: 10.1083/jcb.134.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Y, Li B, Gong W, Shen W, Hu J, Dunlop N M, Oppenheim J J, Wang J M. Immunol Rev. 2000;177:185–194. doi: 10.1034/j.1600-065x.2000.17704.x. [DOI] [PubMed] [Google Scholar]
- 18.Serhan C N, Prescott S M. J Exp Med. 2000;192:F5–F8. doi: 10.1084/jem.192.3.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Y, Gong W, Tiffany H L, Tumanov A, Nedospasov S, Shen W, Dunlop N M, Gao J L, Murphy P M, Oppenheim J J, Wang J M. J Neurosci. 2001;21:RC123. doi: 10.1523/JNEUROSCI.21-02-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiore S, Serhan C N. Biochemistry. 1995;34:16678–16686. doi: 10.1021/bi00051a016. [DOI] [PubMed] [Google Scholar]
- 21.Klein C, Paul J I, Sauve K, Schmidt M M, Arcangeli L, Ransom J, Trueheart J, Manfredi J P, Broach J R, Murphy A J. Nat Biotechnol. 1998;16:1334–1337. doi: 10.1038/4310. [DOI] [PubMed] [Google Scholar]
- 22.Sitrin R G, Pan P M, Harper H A, Todd R F, 3rd, Harsh D M, Blackwood R A. J Immunol. 2000;165:3341–3349. doi: 10.4049/jimmunol.165.6.3341. [DOI] [PubMed] [Google Scholar]
- 23.Murphy P M. Cytokine Growth Factor Rev. 1996;7:46–64. doi: 10.1016/1359-6101(96)00009-3. [DOI] [PubMed] [Google Scholar]
- 24.Chiang N, Fierro I M, Gronert K, Serhan C N. J Exp Med. 2000;191:1197–1207. doi: 10.1084/jem.191.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takano T, Clish C B, Gronert K, Petasis N, Serhan C N. J Clin Invest. 1998;101:819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnsen M, Lund L R, Roemer J, Almholt K, Danoe K. Curr Opin Cell Biol. 1998;10:667–671. doi: 10.1016/s0955-0674(98)80044-6. [DOI] [PubMed] [Google Scholar]
- 27.Stephens R W, Nielsen H J, Christensen I J, Thorlacius-Ussing O, Sorensen S, Dano K, Brunner N. J Natl Cancer Inst. 1999;91:869–874. doi: 10.1093/jnci/91.10.869. [DOI] [PubMed] [Google Scholar]
- 28.Mustjoki S, Sidenius N, Sier C F, Blasi F, Elonen E, Alitalo R, Vaheri A. Cancer Res. 2000;60:7126–7132. [PubMed] [Google Scholar]
- 29.Zhou H-M, Nichols A, Meda P, Vassalli J-D. EMBO J. 2000;19:4817–4826. doi: 10.1093/emboj/19.17.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sidenius N, Sier C F, Ullum H, Pedersen B K, Lepri A C, Blasi F, Eugen-Olsen J. Blood. 2000;96:4091–4095. [PubMed] [Google Scholar]
- 31.Busso N, Péclat V, van Ness K, Kolodziesczyk E, Degen J, Bugge T, So A. J Clin Invest. 1998;102:41–50. doi: 10.1172/JCI2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali H, Richardson R M, Haribabu B, Snyderman R. J Biol Chem. 1999;274:6027–6030. doi: 10.1074/jbc.274.10.6027. [DOI] [PubMed] [Google Scholar]