Abstract
Although the biochemical targets of most drugs are known, the biological consequences of their actions are typically less well understood. In this study, we have used two whole-genome technologies in Saccharomyces cerevisiae to determine the cellular impact of the proteasome inhibitor PS-341. By combining population genomics, the screening of a comprehensive panel of bar-coded mutant strains, and transcript profiling, we have identified the genes and pathways most affected by proteasome inhibition. Many of these function in regulated protein degradation or a subset of mitotic activities. In addition, we identified Rpn4p as the transcription factor most responsible for the cell's ability to compensate for proteasome inhibition. Used together, these complementary technologies provide a general and powerful means to elucidate the cellular ramifications of drug treatment.
Cancer chemotherapy currently relies on drugs that have pleiotropic effects on both tumor and normal cells. Although the primary target site of many of these chemotherapies has been characterized, the key downstream events giving these agents their selective antitumor effect are poorly understood. This lack of understanding impedes progress both in studying new combinations of drugs and in developing new therapies.
Targeting the ubiquitin–proteasome pathway has recently emerged as a promising approach to the treatment of several diseases, including cancer and inflammatory disease (1, 2). Initial efforts have focused on the proteasome, a large multisubunit protease complex required for regulated protein turnover (3). In clinical trials, the reversible proteasome inhibitor PS-341 (Fig. 1a) has shown promising antitumor activity in multiple cancers. This compound is, to our knowledge, the first proteasome inhibitor to enter oncology clinical trials and represents a novel approach to cancer chemotherapy with broad therapeutic potential (1).
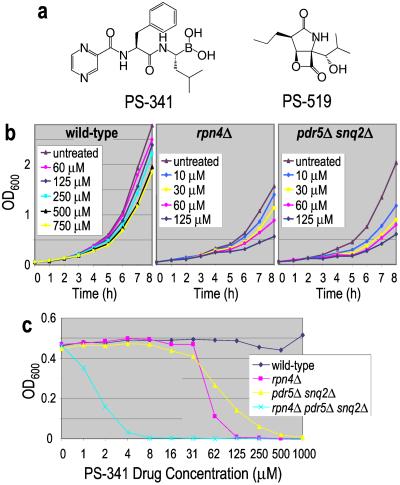
Figure 1.
Effect of the proteasome inhibitor PS-341 on yeast. (a) Structures of PS-341, a dipeptidyl boronic acid, and PS-519, a β-lactone. (b) Wild-type, rpn4Δ, and pdr5Δ snq2Δ strains were grown in various concentrations of PS-341, and cell growth was monitored by absorbance at 600 nm. All three strains display a concentration-dependent inhibition of growth. (c) These three strains and a rpn4Δ pdr5Δ snq2Δ triple mutant were grown in the presence of the indicated concentrations of PS-341 for 24 h at 30°C. Absorbance at 600 nm was used to determine cell density.
Despite intense interest in the ubiquitin–proteasome pathway, driven by its relevance to cell physiology and human disease, we have an incomplete understanding of how cells respond globally to perturbations of ubiquitin-dependent proteolysis. Whole-genome technologies in model organisms such as the yeast Saccharomyces cerevisiae offer a unique opportunity to study the global cellular response to inhibitors of this highly conserved pathway and to probe the specificity and possible secondary effects of these inhibitors. Herein, we combine two complementary approaches, whole-genome transcript profiling and a highly multiplexed competitive growth assay, to characterize the cellular response to proteasome inhibition.
Materials and Methods
Supporting Information.
Additional data and figures are published as supporting information on the PNAS web site, www.pnas.org, and at http://www.millennium.com/scitech/supplement/pnas_fleming02.html.
Yeast Strains.
All strains used in this study are diploid and congenic with the reference strain BY4743 (MATa/α ura3Δ0/ura3Δ0 leu2Δ0/leu2Δ0 his3Δ1/his3Δ1 met15Δ0/MET15 LYS2/lys2Δ0) (a gift of H. Bussey, McGill University, Montreal). All deletion strains were obtained from Research Genetics (Huntsville, AL), except pdr5Δ snq2Δ (MMB1489), rpn4ΔVT (MMB2301), and pdr5Δ snq2Δ rpn4ΔVT (MMB2301), which we constructed by deleting substantial portions of the relevant ORFs. All experiments were performed at 30°C.
Minimum Inhibitory Concentration (MIC) Determination.
We treated 6 × 103 yeast/well with 2-fold serial dilutions of drug in 96-well plates containing yeast extract/peptone/dextrose medium. After growth for 24 h, OD600 readings were taken on a SpectraMAX 250 (Molecular Dynamics). MIC50 is defined as the concentration of drug for which the OD600 is half of the OD600 of untreated cells.
Proteasome Activity Assay.
Yeast proteasome specific activity was determined as previously described for white blood cells (4, 5), except that yeast were disrupted in a MiniBead Beater 8 with 0.5-mm zirconia/silica beads (BioSpec Products, Bartlesville, OK). Typically, 1–3 μg of protein was analyzed in 110 μl of assay buffer.
DNA Microarray Production.
DNAs containing the complete ORFs for 4,886 yeast genes (6) were obtained from Research Genetics. An additional 991 ORFs were represented by ≈500-bp fragments containing the 3′ ends of the ORFs (a gift from Cereon Genomics, Cambridge, MA). DNAs were PCR amplified and spotted onto nylon membranes (7).
Growth Conditions for Transcript Profiling.
Cells were grown in HR + HUL medium [HR powder medium (Oxoid, Basingstoke, U.K.) buffered with 0.15 M 4-morpholinepropanesulfonic acid, pH 6.5, and supplemented with 200 mg/liter of histidine, 200 mg/liter of uracil, and 300 mg/liter of leucine]. Cells in midlogarithmic phase (OD600 = 0.5) were pelleted, resuspended in fresh HR + HUL medium at OD600 = 0.3, grown with shaking in the presence of drug, and harvested by freezing on dry ice.
Wild-type cells treated with 0, 30, 80, and 250 μM PS-341, pdr5Δ snq2Δ cells treated with 0 and 30 μM PS-341, and rpn4Δ cells treated with 0, 3, 8, 30 (twice), and 80 μM PS-341 were harvested at 1, 2, and 4 h, with the exception of 3, 8, and 30 μM PS-341 rpn4Δ samples, in which the 1-h sample was not taken. Separately, pdr5Δ snq2Δ cells treated with 0 and 25 μM PS-341 and 0 and 12 μM PS-519 were harvested at 1 h.
Transcript Profiling.
Yeast total RNA was isolated by using Trizol (Invitrogen) according to the manufacturer's directions. Fifteen micrograms of total RNA and 1.5 μg of oligo(dT)12–18 were incubated with SuperScript II (Invitrogen) at 42°C for 1 h in the presence of 160 μM each dATP, dGTP, and dTTP, 1.6 μM dCTP, and 50 μCi α33P-dCTP (2,000–4,000 Ci/mmol). Labeled cDNA was purified (Micro Bio-Spin 6 column, Bio-Rad), treated with 50 mM NaOH at 68°C for 15 min, and split into separate tubes containing duplicate nylon arrays. Hybridization and washing conditions were performed essentially as previously described (8). Dried filters were exposed to PhosphoImager screens overnight. The hybridization signals were captured by a Fuji BAS 2500 PhosphoImager (Fuji Medical Systems, Stamford, CT) and quantified by grid guru software (Millennium Pharmaceuticals, Cambridge, MA). For each array, the distribution of intensities across all yeast genes was normalized to a median of 1. Intensity values for duplicate filters were then averaged. Data from drug-treated samples were ratioed on a per-gene basis to the appropriate control sample.
Parallel Analysis of Deletion Strains.
Homozygous diploid deletion strains (4,650) were obtained from Research Genetics, grown individually in deep-well 96-well plates, pooled, and frozen in aliquots in the presence of 15% glycerol. Aliquots were added to yeast extract/peptone/dextrose medium, grown until logarithmic phase, diluted to OD600 = 0.05–0.1, and split into tubes with or without drug. Cultures were maintained in logarithmic phase growth by periodic dilution. At intervals, aliquots of cells were harvested for genomic DNA preparation. UPTAG and DOWNTAG sequences were separately amplified from genomic DNA by PCR using biotin-labeled primers as described previously (9), except that all primers were labeled. The amplification products were combined and hybridized to Tags3 arrays (Affymetrix, Santa Clara, CA) according to the genflex chip protocol provided by Affymetrix (no Streptavidin was in the hybridization mixture). After overnight hybridization, chips were washed in an Affymetrix Fluidics Station 400 using the genflex_s protocol and scanned in an Agilent GeneArray Scanner (Palo Alto, CA). The images were quantified by using the Affymetrix microarray suite software.
Treatment with PS-341 (260 μM) was done twice in independent experiments. In experiment 1, samples were quantified after 3, 7, and 12 generations of growth. In experiment 2, which also included a PS-519 treated culture, cells were harvested after 15 generations.
UPTAG and DOWNTAG values were separately normalized, ratioed (treated sample signal/control), and filtered for intensities above background. PM probe values for the UPTAGs and CPM probe values for the DOWNTAGs were used for this analysis, as these provided the highest value information (unpublished observations).
Results
Growth and Proteasome Inhibition Assays.
We initially examined whether PS-341 affected the growth or proteasome activity of yeast. Growth curve analysis of the BY4743 strain (henceforth the wild-type strain) showed that PS-341 partially impaired cell growth at concentrations >100 μM (Fig. 1b), although after extended incubation the cultures reached saturation (Fig. 1c). Analysis of the proteasome activity in these cells revealed that despite the limited effect on growth, treatment with 30–300 μM PS-341 resulted in a sustained 75% inhibition of proteasome activity (data not shown).
Next we asked whether strains carrying certain gene deletions are hypersensitive to proteasome inhibition (Fig. 1 b and c). Indeed, loss of the major drug efflux pumps, Pdr5p and Snq2p, significantly increased sensitivity to PS-341, as did loss of Rpn4p, a transcription factor that functions in part to maintain basal levels of proteasome gene expression (10, 11). Consistent with the latter result, we find that the rpn4Δ strain has lowered proteasome activity compared with the wild-type or pdr5Δ snq2Δ strains (4, 7, and 7 nmol 7-amino-4-methyl coumarin min−1⋅mg−1, respectively). Finally, loss of both Rpn4p and the drug efflux pumps results in much greater sensitivity to PS-341 relative to either mutant strain alone, indicating that these resistance mechanisms act independently (Fig. 1c).
Transcript Profiling.
With the knowledge that PS-341 was having a substantive effect on yeast growth and proteasome activity, we turned to transcript profiling to provide a genome-wide view of the cellular response to this proteasome inhibitor. In a pilot experiment, we profiled cells treated with PS-341 or the β- lactone proteasome inhibitor PS-519 (Fig. 1a). As these molecules have distinct chemical structures and properties (3), expression level changes produced by both should reflect the impact of proteasome inhibition and not the particular pharmacological characteristics of either compound. Indeed, the transcriptional response to these two inhibitors was very similar (Fig. 5, which is published as supporting information on the PNAS web site, www.pnas.org), indicating that the vast majority of the observed effects are caused by proteasome inhibition.
To gain a better understanding of the kinetics and breadth of the transcriptional response to PS-341 treatment, we performed a time and dose study with the wild-type, pdr5Δ snq2Δ, and rpn4Δ strains (see Fig. 2 and www.millennium.com/scitech/supplement/pnas_fleming02.html). These mutant strains allow us to characterize the drug's impact in the absence of two distinct compensatory mechanisms. General patterns of the response were analyzed by use of hierarchical clustering (12), and the resulting heat map is shown in Fig. 3. Wild-type cells treated with concentrations of PS-341 that have little effect on growth nonetheless displayed a pronounced transcriptional response (Figs. 2 and 3). Higher doses of the drug increased the number of genes affected and the strength of their response. Interestingly, for all doses, the scope of the transcriptional changes decreased over time, suggesting a return to homeostasis (Figs. 2 and 3). The transcriptional response of the pdr5Δ snq2Δ strain was similar to the response of wild-type cells but occurred at lower concentrations of drug (Figs. 2 and 3). This result confirms that these drug efflux pumps primarily affect the concentration of PS-341 required to effectively inhibit the proteasome and not other aspects of the cellular response to the drug. In contrast, rpn4Δ cells treated with the same concentrations of PS-341 have a markedly subdued transcriptional response (Figs. 2 and 3).
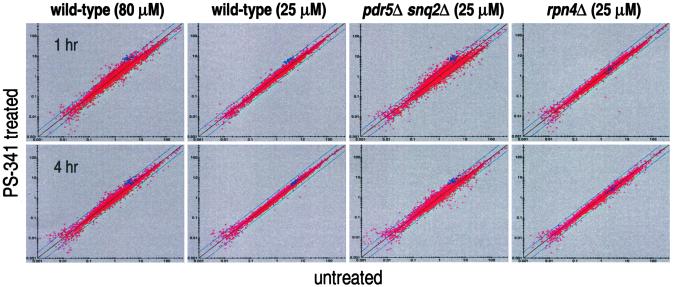
Figure 2.
Scatterplots of PS-341-treated samples and their appropriate controls. Normalized intensity values from untreated samples are plotted on the x axis and PS-341 treated samples on the y axis. Blue squares indicate those genes that are subunits of the 26S proteasome. Proteasome subunit genes in a given cell type have similar expression levels and are concomitantly induced in the wild-type and pdr5Δ snq2Δ strains when proteasome function is impaired. In the rpn4Δ strain, these genes have a lower level of expression in untreated cells, and this level does not change during drug treatment. Blue diagonal lines denote 2-fold changes in expression level.
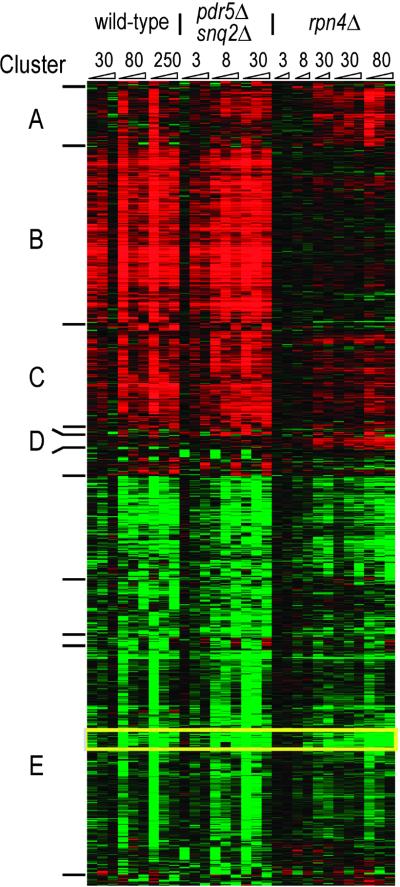
Figure 3.
Time, dose, and strain response to PS-341 treatment. The hierarchical clustering of the 941 genes whose level of transcription changed greater than 1.7-fold in at least 3 of the 30 experiments is presented in heat-map form. Intensity of color correlates with degree of up-regulation (red) or down-regulation (green). Numbers at top indicate concentration of PS-341 used (micromolar) and wedges represent increasing time. Essentially all proteasome subunits are in Cluster B. A tightly clustered subset of genes (outlined in yellow), down-regulated in all strains, contains genes involved in fatty acid metabolism (FAS1, FAS2, FAA1, and OLE1).
We next focused on the genes whose expression level is altered in response to PS-341 treatment. Clustering analysis identified four main groupings of up-regulated genes. Cluster B (Fig. 3) consists of 215 genes whose induction depends on Rpn4p, as they are induced only in the wild-type and pdr5Δ snq2Δ strains. This cluster contains essentially all of the proteasome subunit genes as well as many genes involved in ubiquitination, establishing that Rpn4p functions to mediate the induction of ubiquitin–proteasome genes in the face of proteasome inhibition.
Rpn4p is known to bind to the 8-bp PACE box promoter element found upstream of many proteasome genes (10) and, as expected, almost all are in Cluster B. However, only 18% of the genes in Cluster B contain a PACE box. For example, this cluster contains seven of the eight members of the Cct chaperonin complex, but only one of these genes contains a PACE box, suggesting either that Rpn4p has alternative binding sites or that some of the genes in Cluster B are activated by additional transcription factors under the control of Rpn4p. In support of the latter, the transcription factor gene YAP1, which contains a PACE box, is present in Cluster B, as are several genes under its control (13), all of which lack this promoter element. Thus, there appears to be a cascade of transcriptional responses that are initiated by the activity of Rpn4p.
Cluster C contains 115 genes whose regulation appears to depend partially on Rpn4p. Classes of genes significantly up-regulated in this group include those involved in protein folding, cell stress, and several mitochondrial functions. In addition, genes involved in DNA repair, particularly nucleotide excision repair, were found in this group as well as in Cluster B, consistent with the findings of Jelinsky et al. (14).
Cluster A consists of 70 genes whose regulation is independent of Rpn4p. This group contains carbohydrate [including trehalose (15)] metabolism genes and a set of mitochondrial genes that function mainly in translation. Cluster D contains 15 genes that are induced primarily in the rpn4Δ strain. The majority of these are also involved in mitochondrial function.
It is notable that Clusters A, C, and D have significant numbers of nuclear-encoded genes involved in mitochondrial function. Although the role of the proteasome in mitochondrial processes is not fully understood, various mutations in ubiquitin-mediated proteolysis, such as pre4, ump1, rpn11, and rsp5, impair mitochondrial-dependent growth on glycerol and perturb mitochondrial morphology and inheritance (16–18). Our transcriptional results strengthen the connection between mitochondria and proteasome activity.
Genes down-regulated by PS-341 treatment clustered according to the time course of the drug treatment rather than the strain genotype. Many of these are involved in amino acid metabolism, protein synthesis, and small-molecule transport. Genes in these classes are typically down-regulated in cells treated with antifungal compounds (unpublished observations). However, one notable exception is a highly correlated set of genes that is involved in fatty acid biosynthesis (Fig. 3, outlined in yellow). The implications of this observation are described below.
Lastly, an unexpected finding of the transcript profiling is that, even after 4 h of drug treatment, the transcriptional response of cells is almost completely confined to that initially mediated by Rpn4p. Given the large number of cellular processes impacted by the proteasome and that the proteasome activity of these cells has been significantly impaired during the entire interval, it is striking that there is a dearth of secondary transcriptional effects. In rpn4 mutants, the majority of the transcriptional changes observed in wild-type cells are strongly attenuated. Thus our results identify Rpn4p as a key factor in the ability of cells to sense proteasome inhibition and adjust their transcriptional programs accordingly.
Population Genomics Studies.
To identify pathways that are highly sensitive to proteasome impairment, we sought to identify additional mutants with altered growth properties in the presence of PS-341. The rapid identification of such mutants on a genome-wide scale is made possible by a previously described approach (9, 19–21), which we term “population genomics” (Fig. 6, which is published as supporting information on the PNAS web site). Homozygous diploid strains (4,650) (see Table 2, http://www.millennium.com/scitech/supplement/pnas_fleming02.html), each deleted for a single nonessential gene, are simultaneously grown in liquid culture and treated en masse. Each strain typically contains two unique 20-bp sequences (called UPTAG and DOWNTAG) that serve as strain identifiers. These tags can be PCR amplified from the genomic DNA isolated from the culture and then quantified using Affymetrix chip technology. The fitness of any given strain in the population can be determined by comparing its tag level in the treated culture with that in an untreated culture grown in parallel (Fig. 7, which is published as supporting information on the PNAS web site). The population genomics data exhibited excellent reproducibility (see Fig. 8, which is published as supporting information).
Hypersensitive Strains.
For our experiments, we treated cultures with a dose of PS-341 that is permissive for the growth of wild-type cells. Although the vast majority of the strains were unaffected by the presence of the drug, ≈2% of the mutants displayed altered fitness (Fig. 4). As with transcript profiling, we used PS-519 in a parallel experiment to identify strains impacted specifically by proteasome inhibition and, as before, there was extensive overlap in the results obtained with the two drugs (Fig. 4). Using MIC analysis to confirm the chip results, we identified 52 mutants that were reproducibly and selectively hypersensitive to both proteasome inhibitors (Table 1).
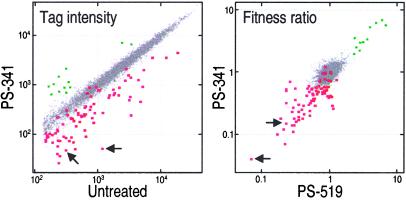
Figure 4.
Population genomics identifies deletion strains impacted by proteasome inhibition. (Left) Normalized tag intensities from untreated cells compared with those from PS-341-treated (260 μM) samples at 15 generations. (Right) Fitness ratios for tags comparing the results from PS-519 (8 μM) and PS-341 (260 μM) treatments at 15 generations. In both Right and Left, UPTAG and DOWNTAG data are shown, and arrows identify tags from the rpn4Δ strain. Data points for which the tag intensity of the untreated sample was below the detection threshold have been omitted. Red and green boxes identify points for strains classified in Table 1 as hypersensitive or resistant, respectively. These were individually validated by MIC testing. The selection of a given strain for subsequent validation studies was based on data from multiple experiments. The presence of colored data points interspersed among the bulk of unaffected strain tags reflects experimental and biological variability (21).
Table 1.
Deletion strains showing hypersensitivity or increased fitness in the presence of proteasome inhibitors
| Strain | MIC50, μM
|
Strain | MIC50, μM
|
||
|---|---|---|---|---|---|
| PS-341 | PS-519 | PS-341 | PS-519 | ||
| Wild type | >1,300 | 1,200 | |||
| Strains showing decreased fitness | |||||
| Group 1: Regulated protein degradation | Group 3: DNA repair | ||||
| Proteasome | apn1 | 250 | 170 | ||
| rpn4 | 50 | 10 | rad50 | 250 | 250 |
| rpn10 | 200 | 40 | rad51 | 400 | 170 |
| pre9 | 650 | 150 | Group 4: Nuclear import/morphology | ||
| Vacuole | nup2 | 250 | 200 | ||
| apg17 | 250 | 120 | nem1 | 250 | 350 |
| vam3 | 800 | 400 | spo7 | 250 | 500 |
| doa4 | 1,000 | 200 | ymr048W | 1,000 | 200 |
| Group 2: Mitosis | Group 5: General transcription factors | ||||
| Kinetochore and microtubule function | snf2 | 130 | 40 | ||
| vik1 | 130 | 120 | sin3 | 130 | 40 |
| ctf19 | 250 | 120 | swi3 | 150 | 40 |
| bik1 | 250 | 120 | esc4 | 600 | 400 |
| mcm21 | 400 | 200 | ymr263W | 1,000 | 200 |
| pfd1 | 600 | 120 | hta1 | 1,000 | 600 |
| iml3 | 600 | 400 | Group 6: Intracellular drug concentration/membrane composition | ||
| chl4 | 1,000 | 250 | pdr5 | 200 | 200 |
| Mitotic progression/exit | erg3 | 250 | 250 | ||
| clb2 | 250 | 100 | erg2 | 650 | 200 |
| chl1 | 250 | 100 | erg4 | 1,000 | 80 |
| ccr4 | 250 | 170 | Other | ||
| lte1 | 400 | 120 | pro1 | 200 | 60 |
| ctf8 | 400 | 170 | yml013W | 200 | 250 |
| sic1 | 500 | 200 | yor258W | 200 | 400 |
| cdc26 | 1,200 | 400 | ylr386W | 250 | 50 |
| clb5 | 1,200 | 500 | ycl016C | 250 | 200 |
| Other cell cycle role | ypl017C | 400 | 170 | ||
| scp160 | 450 | 350 | idp1 | 500 | 200 |
| she1 | 500 | 200 | swm1 | 500 | 250 |
| whi3 | 500 | 400 | yer083c | 650 | 600 |
| src1 | 1,200 | 800 | tof1 | 1,200 | 350 |
| Strains showing increased fitness | |||||
| ydr136C, ypt6, ric1, bem4, yme1, tub3, ylr068W | |||||
Strikingly, nearly all of these genes fall into only six functional classes on the basis of their cellular activities (Table 1).
Group 1: Protein Degradation.
We found that the rpn4Δ strain was the most sensitive mutant to both PS-341 and PS-519 (Table 1). This result strongly reinforces the critical importance of the Rpn4p-mediated transcriptional response to proteasome inhibition that we observed in Fig. 3. Deletion of nonessential proteasome subunits, because of compromised proteasome activity, may also result in hypersensitivity, which was confirmed by the identification of the rpn10 and pre9 mutants. However, another nonessential subunit mutant, rpn13, had unaltered fitness (data not shown), indicating that the various subunits have different contributions to proteasome activity. The sensitivity of the other mutants in this group (doa4, apg17, and vam3) may relate to the role of ubiquitin in vacuolar targeting (22).
Group 2: Mitosis.
More than a third of the hypersensitive strains are mutated in genes with mitotic function. These genes encode spindle-associated proteins, a tubulin chaperone, components of the kinetochore, and genes that promote mitotic progression and mitotic exit. Mutants in kinetochore function can partially activate the spindle assembly checkpoint, resulting in mitotic delay (23). Partial inhibition of the proteasome degradation of Pds1p, a spindle assembly checkpoint protein that inhibits sister chromatid separation, could then reinforce this checkpoint, resulting in a more profound mitotic arrest. Similarly, mutations in proteins that normally promote exit from mitosis [Lte1p, Sic1p, or Ccr4p (24)] could result in partial activation of the spindle position checkpoint. This activation would then be reinforced by the lack of proteasome-mediated degradation of the B-type cyclins, thus inhibiting mitotic exit. Taken together with previous studies demonstrating the critical role of ubiquitin-dependent proteolysis in mitotic progression (25), our findings indicate that these mitotic activities are particularly susceptible to modest proteasome disruption and that they represent the most sensitive essential processes dependent on proteasome activity. Consistent with this interpretation, we found by FACS analysis that cells arrest in G2/M when treated with PS-341 (data not shown).
Group 3: DNA Repair.
It is intriguing that transcript profiling has shown that the nucleotide excision repair pathway is under Rpn4p control (ref. 14 and above), whereas the mutants identified by population genomics function in recombination repair. Furthermore, there are links between protein ubiquitination and the postreplicative DNA repair pathway (26). Perhaps in the face of proteasome inhibition, the burden of DNA repair is shifted to the recombination repair pathway that is not known to be ubiquitin-dependent. Alternatively, proteasome inhibition may induce genomic instability by perturbing mitotic events (see above), and the mutants in this group may be hypersensitive to this effect.
Group 4: Nuclear Import/Morphology.
In fission yeast, proper localization of the proteasome to the nuclear envelope appears to be important for chromosome segregation, spindle dynamics, and cytokinesis (27). Thus, an explanation for the sensitivity of these mutants to PS-341 is that they cause an altered association of the proteasome with the nuclear membrane. Alternatively, these mutants may impair the nuclear import of Rpn4p and thereby diminish the cell's ability to compensate for PS-341 activity.
Group 5: General Transcription Factors.
Deletion of these genes presumably affects the cell's ability to transcriptionally respond to proteasome inhibition. A plausible explanation is that Rpn4p requires these factors for its activity in PS-341-treated cells.
Group 6: Intracellular Drug Concentration/Membrane Composition.
The only efflux pump strain identified as hypersensitive was pdr5Δ, and thus it is the sensitizing mutation in the pdr5Δ snq2Δ strain (the snq2Δ strain showed unaltered fitness). Mutants in the ergosterol biosynthesis pathway (erg2, 3, and 4) also have increased sensitivity to both proteasome inhibitors. In addition, the erg6 strain, which was undetectable in our population studies because of low tag signals, proved to be hypersensitive in subsequent testing. Interestingly, Kaur and Bachhawat (28) found that the altered sterol composition caused by these erg mutants impairs Pdr5p function, suggesting that the observed hypersensitivity of the erg genes may be because of their effect on this efflux pump rather than membrane permeability per se.
Strains with Increased Fitness.
In contrast to the above, seven strains had increased fitness in the presence of PS-341 and PS-519 (Table 1, Fig. 4). Subsequent genetic testing (Fig. 9, which is published as supporting information on the PNAS web site) indicated that sub-MIC levels of these drugs suppress the slow-growth phenotype of the mutants. In corroboration, it has been observed (29) that yme1 mutations are suppressed by defects in RPT3, a subunit of the 26S proteasome. In other genetic experiments, it was observed that impaired growth caused by protein synthesis inhibition could be suppressed by proteasome mutations (30). Among our more fit mutants, ric1 and ypt6 function in the same pathway and are known to reduce ribosomal protein and RNA expression (31–33). Thus, the indirect inhibition of protein synthesis in these mutants may be the mechanism underlying their increased fitness in the presence of PS-341.
Discussion
This manuscript presents an integration of transcript profiling and population genomics for compound characterization, and we have used these two genome-wide technologies to investigate the cellular response to proteasome inhibition.
Both PS-341 and PS-519 target the proteolytic activity of the proteasome, and thus it was not unexpected that they should elicit overlapping responses in our experiments. Still it is notable that these two chemically distinct drugs should produce nearly identical results with both technologies. Equally striking is the limited set of pathways identified as perturbed or sensitized by the treatments. At the concentrations studied here, there is no indication of nonproteasomal targets for the drugs.
Although the power of transcript profiling is well documented, this paper represents the first application for drug characterization of the bar-coded homozygous diploid strains coupled with Affymetrix chip technology. This coupling, as refined by the Davis lab (9, 20, 21), provides a facile and powerful means to identify sensitizing mutations. Its efficacy was confirmed by the fact that half of the identified mutants are in pathways expected to be affected by proteasome inhibitors, namely regulated protein degradation and mitosis. Further validating the approach, we recovered mutants in proteins known to interact directly: Swi3p⋅Snf2p (34), Nem1p⋅Spo7p (35), Sin3p⋅YMR263Wp (36), Ctf19p⋅Mcm21p (37), Chl4p⋅Iml3p (38), and Ypt6p⋅Ric1p (39). The independent identification of interacting protein partners adds strong support to the inclusion of their respective pathways in the affected list.
In the experiments presented, we were able to evaluate three-quarters of the yeast genes for altered fitness. Strains not examined in this study include those corresponding to the 1,100 essential genes, those with poor growth rates, and strains whose tags have poor molecular biology properties (9). Even so, it is likely that representative genes from most if not all pathways were included in our analysis, and that we have obtained a substantive account of the cellular impact of these drugs.
Transcript profiling and population genomics are complementary technologies, and their combination provides deeper insights than either alone. For example, although population genomics identified the rpn4Δ mutant as the strain most sensitive to the proteasome inhibitors, transcript profiling elucidated the basis for this impact on survival. The profiles of the mutant compared with wild-type showed that Rpn4p is responsible for the majority of the transcriptional response to drug treatment observed in the wild-type cells. Thus, without this transcription factor, the cells are significantly impaired in their ability to compensate for the effects of the drug. We anticipate that the identification of key transcription factors by population genomics will be a hallmark of this technology, and the use of this information in guiding transcript profiling experiments will prove extremely powerful.
As a second example of the synergy of the two technologies, our profiling data revealed that PS-341 treatment leads to the down-regulation of fatty acid biosynthesis genes including FAS1, FAS2, OLE1, and FAA4. Recent studies have shown that OLE1 is up-regulated by Spt23p and Mga2p, two transcription factors activated by proteasome-mediated cleavage (40). Our results support this finding and suggest that other genes clustering with OLE1 are also regulated by these transcription factors. Further, because PS-341 causes down-regulation of fatty acid biosynthesis genes, proteasome inhibition may cause phenotypes similar to mutations in these genes, such as perturbations in membrane fluidity (41), nuclear morphology (41, 42), or mitochondrial inheritance (43). This hypothesis then could provide alternative explanations for the lowered fitness of the Group 4 and Group 6 genes (Table 1) in the presence of PS-341.
In conclusion, by using S. cerevisiae, we have identified several key elements that determine cellular sensitivity to clinically relevant proteasome inhibitors. These results improve our understanding of the impact of PS-341 and suggest further experiments to study the effects on tumor cells. For example, one could investigate whether a response similar to that mediated by Rpn4p exists in humans or whether defects in the specific mitotic pathways identified here will determine the predilection of PS-341 for inducing mitotic arrest and apoptosis in tumor cells. We anticipate that the combination of these genome-wide technologies will prove equally powerful for a broad spectrum of drugs.
Supplementary Material
Acknowledgments
We are grateful for contributions and/or advice from the following individuals and organizations: the TRACE group at Millennium, Julian Adams, Dan Finley, Chris Groves, Michael Kauffman, Mark Macera, Andrew Murray, Margaret Read, Chris Wilkes, Elizabeth Winzeler, Affymetrix, Research Genetics, and ApotheCom.
Abbreviation
- MIC
minimum inhibitory concentration
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Adams J, Palombella V J, Sausville E A, Johnson J, Destree A, Lazarus D D, Maas J, Pien C S, Prakash S, Elliott P J. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- 2.Lee D, Goldberg A. J Biol Chem. 1996;271:27280–27284. doi: 10.1074/jbc.271.44.27280. [DOI] [PubMed] [Google Scholar]
- 3.Myung J, Kim K, Crews C. Med Res Rev. 2001;21:245–273. doi: 10.1002/med.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lightcap E S, McCormack T A, Pien C S, Chau V, Adams J, Elliott P J. Clin Chem. 2000;46:673–683. [PubMed] [Google Scholar]
- 5.Elliott P J, McCormack T A, Pien C S, Adams J, Lightcap E S. Methods in Molecular Medicine. Totowa, NJ: Humana Press; 2002. , in press. [Google Scholar]
- 6.Hudson J, Jr, Dawson E, Rushing K, Jackson C, Lockshon D, Conover D, Lanciault C, Harris J, Simmons S, Rothstein R, Fields S. Genome Res. 1997;7:1169–1173. doi: 10.1101/gr.7.12.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang L, Grenier J, Ettwiller L, Jenkins L, Ficenec D, Martin J, Jin F, DiStefano P, Wood A. Proc Natl Acad Sci USA. 2001;98:2814–2819. doi: 10.1073/pnas.051630598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Church G M, Gilbert W. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winzeler E A, Shoemaker D D, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke J D, Bussey H, et al. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- 10.Mannhaupt G, Schnall R, Karpov V, Vetter I, Feldmann H. FEBS Lett. 1999;450:27–34. doi: 10.1016/s0014-5793(99)00467-6. [DOI] [PubMed] [Google Scholar]
- 11.Xie Y, Varshavsky A. Nature (London) 2001;98:3056–3061. doi: 10.1073/pnas.071022298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisen M, Spellman P, Brown P, Botstein D. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J, Godon C, Lagniel G, Spector D, Garin J, Labarre J, Toledano M B. J Biol Chem. 1999;274:16040–16046. doi: 10.1074/jbc.274.23.16040. [DOI] [PubMed] [Google Scholar]
- 14.Jelinsky S A, Estep P, Church G M, Samson L D. Mol Cell Biol. 2000;20:8157–8167. doi: 10.1128/mcb.20.21.8157-8167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee D H, Goldberg A L. Mol Cell Biol. 1998;18:30–38. doi: 10.1128/mcb.18.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rinaldi T, Ricci C, Porro D, Bolotin-Fukuhara M, Frontali L. Mol Biol Cell. 1998;9:2917–2931. doi: 10.1091/mbc.9.10.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisk H A, Yaffe M P. J Cell Biol. 1999;145:1199–1208. doi: 10.1083/jcb.145.6.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutz M, Ellis S, Martin N. Genetics. 2000;154:1013–1023. doi: 10.1093/genetics/154.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hensel M, Shea J, Gleeson C, Jones M, Dalton E, Holden D. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- 20.Shoemaker D, Lashkari D, Morris D, Mittmann M, Davis R. Nat Genet. 1996;14:450–456. doi: 10.1038/ng1296-450. [DOI] [PubMed] [Google Scholar]
- 21.Giaever G, Shoemaker D, Jones T, Liang H, Winzeler E, Astromoff A, Davis R. Nat Genet. 1999;21:278–283. doi: 10.1038/6791. [DOI] [PubMed] [Google Scholar]
- 22.Katzmann D, Babst M, Emr S. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 23.Skibbens R V, Hieter P. Annu Rev Gen. 1998;32:307–337. doi: 10.1146/annurev.genet.32.1.307. [DOI] [PubMed] [Google Scholar]
- 24.Hoyt M A. Cell. 2000;102:267–270. doi: 10.1016/s0092-8674(00)00031-3. [DOI] [PubMed] [Google Scholar]
- 25.Morgan D O. Nat Cell Biol. 1999;1:E47–E53. doi: 10.1038/10039. [DOI] [PubMed] [Google Scholar]
- 26.Ulrich H D, Jentsch S. EMBO J. 2000;19:3388–3397. doi: 10.1093/emboj/19.13.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatebe H, Yanagida M. Curr Biol. 2000;10:1329–1338. doi: 10.1016/s0960-9822(00)00773-9. [DOI] [PubMed] [Google Scholar]
- 28.Kaur R, Bachhawat A. Microbiology. 1999;145:809–818. doi: 10.1099/13500872-145-4-809. [DOI] [PubMed] [Google Scholar]
- 29.Campbell C, Tanaka N, White K, Thorsness P. Mol Biol Cell. 1994;5:899–905. doi: 10.1091/mbc.5.8.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCusker J, Haber J. Genetics. 1998;119:303–315. doi: 10.1093/genetics/119.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li B, Nierras C, Warner J. Mol Cell Biol. 1999;19:5393–5404. doi: 10.1128/mcb.19.8.5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizutka K, Park J, Sugiyama M, Nishiyama M, Warner J. Gene. 1997;187:171–178. doi: 10.1016/s0378-1119(96)00740-8. [DOI] [PubMed] [Google Scholar]
- 33.Li B, Warner J. J Biol Chem. 1996;271:16813–16819. doi: 10.1074/jbc.271.28.16813. [DOI] [PubMed] [Google Scholar]
- 34.Peterson C, Tamkun J. Trends Biochem Sci. 1995;20:143–146. doi: 10.1016/s0968-0004(00)88990-2. [DOI] [PubMed] [Google Scholar]
- 35.Siniossoglou S, Santos-Rosa H, Rappsilber J, Mann M, Hurt E. EMBO J. 1998;17:6449–6464. doi: 10.1093/emboj/17.22.6449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Sun Z W, Iratni R, Erdjument-Bromage H, Tempst P, Hampsey M, Reinberg D. Mol Cell. 1998;1:1021–1031. doi: 10.1016/s1097-2765(00)80102-1. [DOI] [PubMed] [Google Scholar]
- 37.Ortiz J, Stemmann O, Rank S, Lechner J. Genes Dev. 1999;13:1140–1155. doi: 10.1101/gad.13.9.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghosh S K, Poddar A, Hajra S, Sanyal K, Sinha P. Mol Genet Gen. 2001;265:249–257. doi: 10.1007/s004380000408. [DOI] [PubMed] [Google Scholar]
- 39.Siniossoglou S, Peak-Chew S, Pelham H. EMBO J. 2000;19:4885–4894. doi: 10.1093/emboj/19.18.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoppe T, Matuschewski K, Rape M, Schlenker S, Ulrich H, Jentsch S. Cell. 2000;102:577–586. doi: 10.1016/s0092-8674(00)00080-5. [DOI] [PubMed] [Google Scholar]
- 41.Zhang S, Skalsky Y, Garfinkel D. Genetics. 1999;151:473–483. doi: 10.1093/genetics/151.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneiter R, Kohlwein S. Cell. 1997;88:431–434. doi: 10.1016/s0092-8674(00)81882-6. [DOI] [PubMed] [Google Scholar]
- 43.Stewart L, Yaffe M. J Cell Biol. 1991;115:1249–1257. doi: 10.1083/jcb.115.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.