Abstract
Plasma cell neoplasms in humans comprise plasma cell myeloma, otherwise known as multiple myeloma, Ig deposition and heavy chain diseases, and plasmacytoma (PCT). A subset of PCT, designated extramedullary PCT, is distinguished from multiple myeloma and solitary PCT of bone by its distribution among various tissue sites but not the bone marrow. Extramedullary (extraosseus) PCT are rare spontaneous neoplasms of mice but are readily induced in a susceptible strain, BALB/c, by treatment with pristane. The tumors develop in peritoneal granulomas and are characterized by Myc-activating T(12;15) chromosomal translocations and, most frequently, by secretion of IgA. A uniting feature of human and mouse plasma cell neoplasms is the critical role played by IL-6, a B cell growth, differentiation, and survival factor. To directly test the contribution of IL-6 to PCT development, we generated BALB/c mice carrying a widely expressed IL-6 transgene. All mice exhibited lymphoproliferation and plasmacytosis. By 18 months of age, over half developed readily transplantable PCT in lymph nodes, Peyer's patches, and sometimes spleen. These neoplasms also had T(12;15) translocations, but remarkably, none expressed IgA. Unexpectedly, ≈30% of the mice developed follicular and diffuse large cell B cell lymphomas that often coexisted with PCT. These findings provide a unique model of extramedullary PCT for studies on pathogenesis and treatment and suggest a previously unappreciated role for IL-6 in the genesis of germinal center-derived lymphomas.
Plasma cell disorders of humans range from premalignant monoclonal gammopathy of undetermined significance to highly malignant and often therapy-refractory multiple myeloma and plasmacytoma (PCT). Several studies have identified IL-6, a B cell growth, differentiation, and survival factor, as a crucial element in development of these human disorders. IL-6 has also been shown to play a central role in an experimental model of plasmacytomagenesis in which pristane-induced peritoneal granulomas provide a critical environment for PCT development in genetically susceptible BALB/c (C) mice (1). These understandings suggested that development of mice constitutively expressing IL-6 might bypass the requirement for pristane, thereby furthering our understanding of the pathogenesis of PCT in a model system.
Eight IL-6 transgenics have been described to date (see Table 2, which is published as supporting information on the PNAS web site, www.pnas.org; refs. 2–9). Strains resistant to pristane-induced PCT (10) and carrying IL-6 transgenes driven by promoters from either the metallothionine-1 (6) or the Ld gene of the major histocompatibility complex (H2-Ld) (7) are of particular interest. Histologic features of tissues from some of these mice were strongly suggestive of PCT, but direct demonstrations of malignancy through transplantation were not reported. The possibility that these models might be improved by expression of the IL-6 transgenes on the genetic background of PCT-sensitive mice was indicated by the observation that partially backcrossed C.B6-H2-Ld-IL-6 mice at generation N2 yielded transplantable PCT (9). The realization that multiple C genes contribute to the sensitivity of the strain to PCT induction (11, 12) suggested that further breeding to C should enhance the strength of the models. To investigate this possibility, we backcrossed the H2-Ld-IL-6 transgene (IL-6 Tg) onto the C background for 20 generations developing IL-6 Tg congenics (C.IL-6 mice) with a full complement of PCT susceptibility genes.
We found that 25 of 45 (56%) of C.IL-6 mice developed spontaneous PCTs in lymphoid tissues by 18 months of age. Furthermore, we demonstrated by in vivo transfer experiments that two-thirds of C.IL-6 mice contained transplantable PCT precursors in their hyperplastic lymphoid tissues. These findings established PCT development in strain C.IL-6 as a mouse model of plasma cell neoplasia that in contrast to peritoneal PCT in pristane-treated C mice does not depend on the artificial microenvironment of the inflammatory granuloma.
Materials and Methods
Mice, Tumor Diagnosis, and in Vivo Transfer of IL-6 Tg B Cells.
C.IL-6 congenics were derived from H2-Ld-IL-6 Tg C57BL/6 mice (9) by introgressive backcrossing of the transgene onto strain C to N20. Incipient PCT and B cell lymphomas were detected by monitoring C.IL-6 mice for splenomegaly and enlarged peripheral lymph nodes. Single-cell suspensions from lymphoid tissues of untreated C.IL-6 mice were transferred in vivo by giving 2.5 × 107 cells i.p. to pristane-treated C or C nude mice. All mice were bred and maintained in our conventional facility on the National Institutes of Health campus.
Histology and Immunohistochemistry.
Sections (4 μm) of paraffin-embedded tissues were stained with hematoxylin and eosin, Giemsa (according to the protocol of Lennert), PAS, or methylgreen pyronine. Tissues were classified as exhibiting plasma cell abnormalities including: (i) plasmacytosis, accumulations of normal-appearing, and nondividing plasma cells; (ii) plasma cell hyperplasia (PCH), mixtures of normal and aberrant hyperchromatic, sometimes mitotically active plasma cells; (iii) microplasmacytoma (mPCT), isolated clusters of malignant plasma cells, presumptive precursors of overt PCT; (iv) PCT; and (v) anaplastic plasmacytoma (PCT-A), a mixture of immunoblasts and plasma cells with >90% of cells being immature forms of malignant plasma cells. Nonplasmacytic lymphoid neoplasms were classified as follicular B cell lymphoma (FBL) or diffuse large-cell B cell lymphoma (DLCL), using criteria described in a provisional system of nomenclature for mouse hematopoietic tumors (13, 14). Avidin-biotin immunoperoxidase techniques with antisera to IgL and IgH (Southern Biotechnology Associates), B220 (CD45R) (CalTag), and CD19 and CD138 (syndecan; PharMingen) were used for the determination of Ig production and surface marker expression as described (11).
Paraproteins.
Serum paraproteins were detected with the help of Paragon SPE electrophoresis kits (Beckman Coulter). Ig isotypes were determined by ELISA, using Immulon II plates (Dynex Technologies, Helsinki, Finland) and isotype-specific goat anti-mouse serum labeled with horseradish peroxidase (Southern Biotechnology Associates). Mouse serum samples were diluted from 10−3 to 1.28 × 10−5. Plates were read on a Molecular Dynamics microplate reader at 450 nm.
Detection of T(12;15).
Illegitimate genetic recombinations between IgH and Myc, the molecular indicators of T(12;15), were detected by long-template or high-fidelity PCR methods as described (15–17). For detection by Southern blot hybridization, genomic DNA was digested with KpnI, fractionated by electrophoresis on 0.7% agarose gels, transferred onto a nitrocellulose membrane, and hybridized to a 2.2-kb NheI/SpeI fragment of Myc that included exon 2, or to a 1.5-kb IgH HindIII/EcoRI probe (pJ11) that spanned JH2 and Eμ. Probes were labeled with 32P by random priming. For detection of T(12;15) by fluorescence in situ hybridization (FISH), BAC clones hybridizing to DH/JH/Cμ, Cα, or Myc were labeled by nick translation with Spectrum Orange, Rhodamine 110, or Cy5, respectively. Images were acquired with a DMRHC epifluorescence microscope (Leica, Deerfield, IL) equipped with a Sensys CCD camera (Roper Scientific, Trenton, NJ).
Results
Incidence of PCT and B Cell Lymphomas.
The incidence of PCT was determined in a group of 45 C.IL-6 mice followed to 18 months of age. PCT were first seen at 6 months and increased steadily, reaching 56% (25 of 45) by 18 months (Fig. 1A). Thirteen mice (29%) developed B cell lymphomas. However, these mice invariably also harbored incipient PCT that often took the form of multicentric mPCT in various lymphoid tissues. Seven mice (16%) remained tumor free, yet according to histological criteria they were at the transitional stage between plasma cell hyperplasia, mPCT, and frank PCT. In addition to lymphoid neoplasms, some transgenics died with renal disease and other pathologies of IL-6 disease, as described (9), such that almost all mice were dead with one disease or the other by 18 months (Fig. 1B).
Figure 1.
Incidence and transplantability of B cell lineage tumors in C.IL-6 mice (A) and survival of mice (B). Transplantability was determined in two studies in which 8 of 12 C.IL-6 mice and 11 of 17 C.IL-6 mice were successfully transplanted. Survival was determined in a group of untreated C.IL-6 mice (n = 63) and compared with control C mice (n = 80). Reduced survival in strain C.IL-6 was significant in χ2 analysis (P < 0.001).
Histological examination of 25 PCTs showed that the tumors occurred as three distinct subtypes. These included: (i) a typical mature plasmacytic form [medium sized sIg− cIg+ CD138+ plasma cells with pyroninophilic cytoplasm and a round eccentric nucleus with marginated chromatin (clock-face appearance) and one or several nucleoli (12 cases; Fig. 2 Top)]; (ii) a less mature plasmacytoid or plasmablastic form [medium to large-sized plasma cells with less cytoplasm and a more central nucleus and more prominent round nucleoli (seven cases)]; and (iii) a highly aggressive anaplastic form [less than 10% mature plasma cells on a mixed background of immunoblasts, plasmablasts, and intermediate cell forms that contained large nuclei with a thick nuclear membrane (five cases; Fig. 2 Middle)].
Figure 2.
Morphology of B cell lineage lymphomas arising in C.IL-6 mice. (Top) Mature plasmacytic PCT (immunostain for κ light-chains); (Middle) plasmablastic PCT (hematoxylin/eosin); (Bottom) follicular B lymphoma (hematoxylin/eosin).
Studies of serum Ig levels revealed marked polyclonal increases in mice without lymphoid neoplasia and the presence of M-components in most mice with PCT. Sera of mice with anaplastic or plasmablastic PCT did not exhibit M-components. Immunohistochemical studies of nine PCT cases, using Ig class-specific Ab, demonstrated that seven produced IgG1 and two produced IgG2b. IgA-positive tumors were not detected.
The 13 cases of mature sIgM+ B220+ CD19+ B cell lymphomas included nine cases of FBL and four cases of DLCL. FBL were comprised of mixed populations of centrocytes and centroblasts (Fig. 2 Bottom), whereas DLCL were populated primarily by centroblasts with lesser numbers of immunoblasts and centrocytes.
Transplantation Studies.
To determine whether IL-6 transgenic PCT were transplantable, as an indication of transformation, cells from tumor nodules of 12 mice were transferred i.p. to C or C nude mice pretreated by i.p. inoculation with pristane or left untreated. Eight transfers were successful but only in pristane-primed recipients, either wild type or nude. This finding indicated that autocrine production of IL-6 was insufficient to sustain growth of transferred tumors, thereby suggesting a critical role for the pristane-conditioned environment for successful transfer. The production of IL-6 by granuloma cells (18) may be important in this regard because there is reason to believe that primary PCT depend on paracrine sources of IL-6, despite autocrine production of this cytokine (19).
To elucidate whether hyperplastic lymphoid tissues harbored latent PCT, a transplantation experiment with 17 C.IL-6 mice was carried out (Table 1). This transplantation was successful in 11 of 17 mice. Cell suspensions from 13 apparently tumor-free lymphoid tissues with plasma cell hyperplasia (PCH) by histology were transferred into pristane-primed C mice, resulting in outgrowth of PCT in all cases. Remarkably, seven of nine tissues that exhibited histologic evidence for B cell lymphoma also contained transplantable PCT. In three cases of FBL (8235, 8270, 8294), recipients developed FBL in lymphoid tissues but PCT in pristane granulomas. In three other cases of FBL (8198, 8293, 8299) and one case of DLCL (8191), all recipients developed PCT instead of the expected lymphoma. The results suggested that a significant proportion of tissues harboring lymphomas also contained inapparent PCT and that the environment of the pristane granuloma favored the outgrowth of PCTs over lymphomas.
Table 1.
Summary of transplantation study with 17 C.IL-6 donor mice
| Donor mice
|
Recipient mice
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Donor mouse* | Histological† diagnosis | Tissue‡ | IgH/Myc§
|
Tumor¶ | Recip.‖ | IgH/Myc§
|
Conserved IgH/Myc** | ||
| PCR | Southern | PCR | Southern | ||||||
| 8184 | PCT | PP | — | − | PCT | 4 | — | ++ | +(S) |
| PCH | MLN | — | − | PCT | 4 | — | ++ | +(S) | |
| PCH | PLN | — | − | PCT | 4 | — | ++ | — | |
| 8191 | DLCL | PP | (1) | + | PCT | 4 | (1) | − | +(P) |
| PCH | MLN | (1)+4 | − | PCT | 4 | (1) | − | +(P) | |
| PCH | PLN | (1)+1 | − | PCT | 4 | (1) | ++ | +(P) | |
| PCH | SPL | (1) | − | PCT | 4 | (1) | ++ | +(P) | |
| 8197 | FBL | MLN | 1 | − | FBL | 4 | — | − | — |
| 8198 | FBL | PP | (1) | + | PCT | 4 | (1) | + | +(S) |
| PCH | MLN | — | + | PCT | 4 | — | + | +(S) | |
| 8235 | FBL | PLN | (2) | − | FBL + PCT‡‡ | 2 | 1 | − | — |
| 8263 | PCT | MLN | (1) | − | PCT | 2 | 1 | + | — |
| PCH | PLN | — | − | PCT | 2 | 1 | + | — | |
| PCH | SPL | — | − | PCT | 2 | 1 | + | — | |
| 8270 | FBL | MLN | 3 | − | FBL + PCT‡‡ | 2 | 1 | + | +(P) |
| PCH | PLN | — | − | PCT | 2 | — | − | — | |
| PCH | SPL | — | − | PCT | 2 | — | − | — | |
| 8293 | FBL | PLN | 3 | − | PCT | 2 | 1 | + | +(P) |
| 8294 | FBL | MLN | 2 | − | FBL + PCT‡‡ | 2 | 1 | − | — |
| 8296 | FBL | PLN | 3 | − | FBL | 2 | — | + | — |
| PCH | PP | (1) | − | PCT | 2 | — | − | — | |
| PCH | SPL | 1 | − | PCT | 2 | 1 | + | +(P) | |
| 8299 | FBL | MLN | 1 | + | PCT | 2 | 1 | ++ | +(P) |
| PCH | PLN | 4 | − | PCT | 2 | 1 | ++ | +(P) | |
Mean age of donor mice was 275 ± 75 days (range 194 to 442 days).
Histological analysis of donor tissues from which cell suspensions for in vivo transfer were prepared.
Mesenteric lymph node (MLN), peripheral lymph node (PLN), Peyer's patch (PP), and spleen (SPL).
Detection of Myc/IgH junctions by PCR and/or Southern analysis. Numbers indicate how many cell clones with distinct Myc/IgH junctions were found in the same tissue. Numbers without parenthesis: reciprocal Myc/IgH junctions indicative of der 12 and der 15 were detected. Numbers in parenthesis: only Myc/IgH junctions indicative of der 15 were detected. Southern blotting resulted not in detection of Myc rearrangements (−), detection of unique Myc rearrangements present in one tissue (+) or detection of the same Myc rearrangement in various tissues (++).
Type of B-cell tumor that arose in recipient mouse. Mean latency between cell transfer and tumor take was 128 ± 66 days (range 34 to 326 days). Genotyping for IL-6 transgene demonstrated that all tumors were of donor origin.
All recipient mice (no IL-6 transgene) were primed with pristane.
Precursor–product relationship between donor cells and recipient tumor was established by PCR analysis of clonotypic IgH/Myc rearrangements (P) or by Southern blotting (S).
Outgrowth of FBL in lymphoid tissues and PCT in pristane granulomas.
T(12;15).
Pristane-induced PCT are characterized by T(12;15) in 90% of cases with 3′-Cα enhancers on chromosome (Chr) 12 leading to deregulation of Myc on the apposed sequences from Chr 15. To determine whether IL-6 transgenic PCT and lymphomas also featured 12;15 translocations of this sort, we first examined cells for the translocation by fluorescence in situ hybridization (FISH) followed by studies of extracted DNA by using Southern analysis and PCR. Of the 59 PCT and 9 FBL observed in transplanted mice, 24 PCT and 5 FBL were chosen for molecular and cytogenetic analyses. All PCT, except the tumors derived from peripheral lymph node and spleen of mouse 8270 and Peyer's patch of mouse 8296, harbored T(12;15) (Figs. 2–4, and data not shown). Two cases of FBL (including 8296 in Fig. 2) were also found to contain T(12;15), indicating that translocation can occur before B cells differentiate into plasma cells. PCR analysis of FACS-sorted cell fractions from these cases supported this interpretation by demonstrating that B220+ B cells were T(12;15)+, whereas CD138+ plasma cells were T(12;15)− (data not shown).
Figure 4.
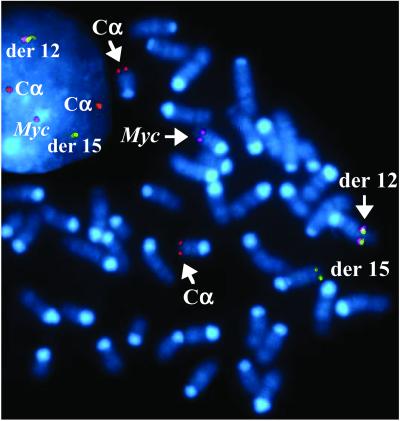
Detection of T(12;15) by fluorescence in situ hybridization (FISH) in PCT 8184. The Myc activating product of translocation, der 12, is visualized by colocalization of probes for Myc, DH/JH/Cμ, and Cα. The reciprocal product of translocation, der 15, is visualized by the DH/JH/Cμ signal. Normal chromosome (Chr) 15 (one copy) and Chr 12 (two copies) are visualized by Myc and Cα signals, respectively.
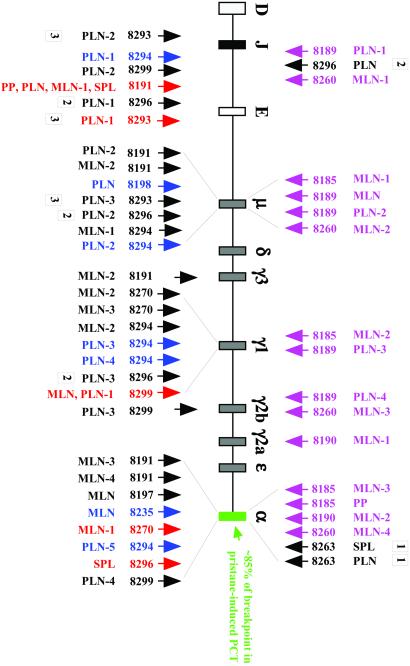
The most commonly occurring IgH breakpoints in pristane-induced PCT localize to the switch region, Sα, of the Cα locus (Fig. 5, textbox). To evaluate whether hyperplastic lymphoid tissues of IL-6 transgenic mice contained T(12;15)+ plasma cell clones with the same molecular feature, PCR analysis of Myc/IgH junctions was carried out. Tissue samples from nine successfully transplanted mice included in Table 1 contained 30 clones (Fig. 5, arrows pointing up). Four mice that were not successfully transplanted (not included in Table 1) harbored 15 clones (Fig. 5, magenta arrows pointing down). The sample of 45 unique translocation breakpoints clustered in four regions of IgH on the Myc-activating product of translocation, der 12: Cα (12 cases), Cγ1 (9 cases), Cμ (11 cases), and JH/Eμ (8 cases). The occurrence of many Myc rearrangements 5′ of Cμ (19/45;11 Cμ junctions plus 8 JH/Eμ junctions) and the categorization of all breakpoints in Myc as class I (ref. 20; results not shown) agreed with previous findings on the fine structure of translocations occurring in younger tumor-free C.IL-6 mice (17). The considerable portion of Cγ1/Myc rearrangements (9/45) that are rarely seen in PCT of pristane-treated C mice was presumably related to the propensity of IL-6 transgenic B cells to use Cγ1 for isotype switching (8, 9, 21, 22), because Ig isotype switch recombination is thought to be involved in the origin of T(12;15) (23–25). The relatively infrequent utilization of breakpoints in Sα/Cα (12/15, 27%) contrasted with the predominant utilization of this locus in pristane-induced PCT (≈85%).
Figure 5.
Map of T(12;15) breakpoints in IgH. Break sites with Myc are indicated by arrows. Each arrow represents a distinct T(12;15)+ cell clone. Mouse numbers and tissue designations are the same as in Table 1. Upwards pointing arrows designate Myc rearrangements in hyperplastic donor tissues: blue, rearrangements occurred in tissues that were not transplantable; black, rearrangements were not redetected in recipient tumors, yet they occurred in tissues that gave rise to tumors with different Myc junctions; red, rearrangements were detected in corresponding donor and recipient tissues. Three Myc rearrangements found in recipient tumors (down-pointing black arrows labeled 1 or 2) are included to illustrate two points made in the last paragraph of Results. The Myc rearrangements of 19 additional recipient tumors (Cμ, 2 cases; Cγ1, 3 cases; Cα, 3 cases; JH/Eμ, 11 cases) were analyzed by PCR, but were not included in the figure for clarity of presentation. Samples with hyphenated numbers contained multiple T(12;15)+ clones in the same tissue (e.g., three clones in the PLN sample of mouse 8293; arrows labeled 3).
To directly demonstrate that specific T(12;15)+ cells can give rise to transplanted PCT with the same clonotypic translocation breakpoint, paired samples of donor tissues and transplanted tumors were analyzed by PCR for the presence of matching Myc/IgH junctions. Three different relationships of donor and recipient translocation were observed. First, a recipient tumor contained a translocation, whereas none were found in the donor tissues (Fig. 5, arrows labeled 1). Second, there were unrelated clonal translocations in donor and recipient mice. For example, the PLN of mouse 8296 contained three translocations, but they were different from the translocation found in the recipient tumor (Fig. 5, arrows labeled 2). Third, the same translocations were observed in donor and recipient tissues (Fig. 5, red arrows), thereby establishing precursor–product relationships between T(12;15)+ donor clones and transplanted PCT. In mice 8191 and 8299, the donor clones must have been disseminated because transfer of cell suspensions from four separate tissues in 8191 and two in 8299 gave rise to tumors with the same T(12;15) in multiple recipients (eight mice in case 8191 and four mice in case 8299).
Discussion
This study describes the development of transplantable T(12;15)+ IgG PCT in lymphoid tissues of untreated C.IL-6 mice. These findings extend previous observations defining the importance of IL-6 in the classical model of pristane-induced peritoneal PCT in conventional C mice (26–28). They also complement earlier studies showing that C mice homozygous for an IL-6-null allele are resistant to peritoneal PCT induced by pristane (29) and accelerated peritoneal PCT induced by a Myc/Raf retrovirus plus pristane (30). In addition, they are in full agreement with studies on PCT driven by an Abl/Myc virus (31) demonstrating that the combination of these oncogenes can override the block in PCT development in C.IL-6−/− mice by inducing constitutive activation of Stat3 (32), a key component in the IL-6 signaling cascade (33). Collectively, the results of this and previous studies demonstrate that IL-6 is critical for all forms of PCT in C mice. An unexpected finding was that IL-6 also plays a role in the pathogenesis of follicular and diffuse large cell B cell lymphomas in C mice.
It is of interest that the preferred isotype of IL-6 transgenic PCT was IgG, which contrasts with the marked bias toward IgA in conventional pristane-induced PCT. Similarly, the 5′ Cμ region was the preferred CH locus for recombinations with Myc in IL-6 transgenic PCT, which contrasts with the predominant utilization of the 5′ Cα region in pristane-induced PCT. These differences could be taken to support the hypothesis (34) that peritoneal PCT are derived from IgA-committed, evolutionary primitive B1 B cells that develop in Peyer's patches and recirculate through the peritoneal cavity (35), whereas IL-6 transgenic PCT may rather be derived from conventional follicular B2 B cells that under conditions of constitutive IL-6 signaling are channeled primarily into the IgG1 compartment (8, 9, 21, 22).
In light of reports that IL-6 signaling via Stat3 is fully sufficient to activate Myc in B cells (36), it was not necessarily expected that virtually all IL-6 transgenic PCT examined in this study harbored the Myc-activating T(12;15) translocation. The Stat3-dependent pathway of Myc activation is apparently insufficient for PCT development because it is invariably replaced by the Stat3-independent activation of Myc via chromosomal translocation (17). Recent findings in multiple myeloma (MM) suggest that MYC activation by chromosomal aberrations may be similarly important for plasma cell tumors in humans (37). Diverse karyotypic abnormalities of the MYC locus that frequently juxtaposed MYC to IGH or IGL have been demonstrated in 19 of 20 MM cell lines and 7 of 14 advanced primary MM. Eight of eight informative MM cell lines exhibited monoallelic expression of MYC, an indication of deregulated gene expression (37). The presence of Ig/MYC translocations in both MM as a component of tumor progression (37) and in PCT as an initiating mutation (23, 24) defines an interesting parallel in the natural history of plasma cell tumors in both species. Our observation that IgH/Myc translocations were also found in two cases of FBL in C.IL-6 mice established another parallel to the corresponding disease in humans, as MYC translocations have been associated with the progression of FBL to higher grade in humans (38, 39).
In conclusion, C.IL-6 mice offer a model of PCT development that in contrast to pristane-induced PCT in conventional C mice does not depend on treatment with an exogenous proinflammatory stimulus (1). Strain C.IL-6 may be useful as a preclinical model for testing antibody or drug based intervention strategies that attempt to inhibit growth and survival of malignant plasma cells by interrupting IL-6 signaling, a promising approach in the treatment of multiple myeloma (40). Strain C.IL-6 may also permit fundamentally new insights into the role of negative IL-6 signaling during plasmacytomagenesis and, thereby, define new targets for therapy. One envisioned experiment along this line predicts that PCT development will be suppressed in C.IL-6 mice that overexpress the suppressor of cytokine signaling 1 (SOCS-1) gene, whereas it will be accelerated in C.IL-6 mice that harbor two null alleles of SOCS-1 (41–43).
Supplementary Material
Figure 3.
Detection of Myc and VDJ rearrangements by Southern analysis. Corresponding pairs of donor tissues (Left) and transplanted tumors (Right) are presented. Myc rearrangements that cohybridized upon stripping and reprobing to the JH2/Eμ probe are indicated by arrows in Upper. Corresponding VDJ fragments of matching size are also indicated by arrows in Lower. The fragment pairs represent rearrangements between Myc and the 5′ Cμ region on der 12. Asterisks denote Myc rearrangements that did not cohybridize to the JH2/Eμ probe. These rearrangements occurred presumably 3′ of Cμ. The rectangles indicate a case in which the Myc- and VDJ-rearranged clone in the tumor-free donor tissue with PCH was sufficiently expanded to permit detection by Southern analysis. Note the presence of several expanded B cell clones (multiple VDJ rearrangements) in many tissues. GL, germ-line fragment.
Acknowledgments
We thank Elizabeth B. Mushinski for immunostainings of tissue sections, Dr. Konrad Huppi for providing probe pJ11, and Wendy duBois and Lisa Craig for animal husbandry. This work was supported in part by a National Cancer Institute Intramural Research Award (to S.J.).
Abbreviations
- FBL
follicular B-cell lymphoma
- DLCL
diffuse large-cell B-cell lymphoma
- PCT
plasmacytoma
- C
BALB/c
References
- 1.Potter M, Wiener F. Carcinogenesis. 1992;13:1681–1697. doi: 10.1093/carcin/13.10.1681. [DOI] [PubMed] [Google Scholar]
- 2.Turksen K, Kupper T, Degenstein L, Williams I, Fuchs E. Proc Natl Acad Sci USA. 1992;89:5068–5072. doi: 10.1073/pnas.89.11.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell I L, Abraham C R, Masliah E, Kemper P, Inglis J D, Oldstone M B A, Mucke L. Proc Natl Acad Sci USA. 1993;90:10061–10065. [Google Scholar]
- 4.DiCosmo B F, Geba G P, Picarella D, Elias J A, Rankin J A, Stripp B R, Whitsett J A, Flavell R A. J Clin Invest. 1994;94:2028–2035. doi: 10.1172/JCI117556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fattori E, Lazzaro D, Musiani P, Modesti A, Alonzi T, Ciliberto G. Eur J Neurosci. 1995;7:2441–2449. doi: 10.1111/j.1460-9568.1995.tb01042.x. [DOI] [PubMed] [Google Scholar]
- 6.Fattori E, Della Rocca C, Costa P, Giorgio M, Dente B, Pozzi L, Ciliberto G. Blood. 1994;83:2570–2579. [PubMed] [Google Scholar]
- 7.Woodroofe C, Muller W, Ruther U. DNA Cell Biol. 1992;11:587–592. doi: 10.1089/dna.1992.11.587. [DOI] [PubMed] [Google Scholar]
- 8.Suematsu S, Matsuda T, Aozasa K, Akira S, Nakano N, Ohno S, Miyazaki J, Yamamura K, Hirano T, Kishimoto T. Proc Natl Acad Sci USA. 1989;86:7547–7551. doi: 10.1073/pnas.86.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suematsu S, Matsusaka T, Matsuda T, Ohno S, Miyazaki J, Yamamura K, Hirano T, Kishimoto T. Proc Natl Acad Sci USA. 1992;89:232–235. doi: 10.1073/pnas.89.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potter M, Pumphrey J G, Bailey D W. J Natl Cancer Inst. 1975;54:1413–1417. doi: 10.1093/jnci/54.6.1413. [DOI] [PubMed] [Google Scholar]
- 11.Potter M, Mushinski E B, Wax J S, Hartley J, Mock B A. Cancer Res. 1994;54:969–975. [PubMed] [Google Scholar]
- 12.Zhang S L, DuBois W, Ramsay E S, Bliskovski V, Morse H C, III, Taddesse-Heath L, Vass W C, DePinho R A, Mock B A. Mol Cell Biol. 2001;21:310–318. doi: 10.1128/MCB.21.1.310-318.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morse H C, III, Qi C F, Chattopadhyay S K, Hori M, Taddesse-Heath L, Ozato K, Hartley J W, Taylor B A, Ward J M, Jenkins N A, Copeland N G, Fredrickson T N. Leuk Res. 2001;25:719–733. doi: 10.1016/s0145-2126(01)00022-4. [DOI] [PubMed] [Google Scholar]
- 14.Hartley J W, Chattopadhyay S K, Lander M R, Taddesse-Heath L, Naghashfar Z, Morse H C, III, Fredrickson T N. Lab Invest. 2001;80:159–169. doi: 10.1038/labinvest.3780020. [DOI] [PubMed] [Google Scholar]
- 15.Kovalchuk A L, Müller J R, Janz S. Oncogene. 1997;15:2369–2377. doi: 10.1038/sj.onc.1201409. [DOI] [PubMed] [Google Scholar]
- 16.Kovalchuk A L, Mushinski E B, Janz S. Leukemia. 2000;14:909–921. doi: 10.1038/sj.leu.2401676. [DOI] [PubMed] [Google Scholar]
- 17.Kovalchuk A L, Kishimoto T, Janz S. Leukemia. 2000;14:1127–1135. doi: 10.1038/sj.leu.2401767. [DOI] [PubMed] [Google Scholar]
- 18.Hinson R M, Williams J A, Shacter E. Proc Natl Acad Sci USA. 1996;93:4885–4890. doi: 10.1073/pnas.93.10.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rawat R, Rainey G J, Thompson C D, Frazier-Jessen M R, Brown R T, Nordan R P. Blood. 2000;96:3514–3521. [PubMed] [Google Scholar]
- 20.Cory S. Adv Cancer Res. 1986;47:189–234. doi: 10.1016/s0065-230x(08)60200-6. [DOI] [PubMed] [Google Scholar]
- 21.Oka Y, Rolink A G, Suematsu S, Kishimoto T, Melchers F. Eur J Immunol. 1995;25:1332–1337. doi: 10.1002/eji.1830250530. [DOI] [PubMed] [Google Scholar]
- 22.Raynal M C, Liu Z Y, Hirano T, Mayer L, Kishimoto T, Chen-Kiang S. Proc Natl Acad Sci USA. 1989;86:8024–8028. doi: 10.1073/pnas.86.20.8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janz S, Müller J, Shaughnessy J, Potter M. Proc Natl Acad Sci USA. 1993;90:7361–7365. doi: 10.1073/pnas.90.15.7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Müller J R, Potter M, Janz S. Proc Natl Acad Sci USA. 1994;91:12066–12070. doi: 10.1073/pnas.91.25.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müller J R, Janz S, Potter M. Cancer Res. 1995;55:5012–5018. [PubMed] [Google Scholar]
- 26.Nordan R P, Potter M. Science. 1986;233:566–569. doi: 10.1126/science.3726549. [DOI] [PubMed] [Google Scholar]
- 27.Degrassi A, Hilbert D M, Rudikoff S, Anderson A O, Potter M, Coon H G. Proc Natl Acad Sci USA. 1993;90:2060–2064. doi: 10.1073/pnas.90.5.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vink A, Coulie P, Warnier G, Renauld J C, Stevens M, Donckers D, Van Snick J. J Exp Med. 1990;172:997–1000. doi: 10.1084/jem.172.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lattanzio G, Libert C, Aquilina M, Cappelletti M, Ciliberto G, Musiani P, Poli V. Am J Pathol. 1997;151:689–696. [PMC free article] [PubMed] [Google Scholar]
- 30.Hilbert D M, Kopf M, Mock B A, Kohler G, Rudikoff S. J Exp Med. 1995;182:243–248. doi: 10.1084/jem.182.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Largaespada D A, Kaehler D A, Mishak H, Weissinger E, Potter M, Mushinski J F, Risser R. Oncogene. 1992;7:811–819. [PubMed] [Google Scholar]
- 32.Hilbert D M, Migone T S, Kopf M, Leonard W J, Rudikoff S. Immunity. 1996;5:81–89. doi: 10.1016/s1074-7613(00)80312-x. [DOI] [PubMed] [Google Scholar]
- 33.Hirano T, Ishihara K, Hibi M. Oncogene. 2001;19:2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 34.Potter M, Wax J S, Hansen C T, Kenny J J. Int Immunol. 1999;11:1059–1064. doi: 10.1093/intimm/11.7.1059. [DOI] [PubMed] [Google Scholar]
- 35.Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T. Nature (London) 2001;413:639–643. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
- 36.Kiuchi N, Nakajima K, Ichiba M, Fukada T, Narimatsu M, Mizuno K, Hibi M, Hirano T. J Exp Med. 1999;189:63–73. doi: 10.1084/jem.189.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shou Y, Martelli M L, Gabrea A, Qi Y, Brents L A, Roschke A, Dewald G, Kirsch I R, Bergsagel P L, Kuehl W M. Proc Natl Acad Sci USA. 2000;97:228–233. doi: 10.1073/pnas.97.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yano T, Jaffe E S, Longo D L, Raffeld M. Blood. 1992;80:758–767. [PubMed] [Google Scholar]
- 39.de Jong D, Voetdijk B M, Beverstock G C, van Ommen G J, Willemze R, Kluin P M. N Engl J Med. 1988;318:1373–1378. doi: 10.1056/NEJM198805263182106. [DOI] [PubMed] [Google Scholar]
- 40.Barille S, Bataille R, Amiot M. Eur Cytokine Network. 2001;11:546–551. [PubMed] [Google Scholar]
- 41.Narazaki M, Fujimoto M, Matsumoto T, Morita Y, Saito H, Kajita T, Yoshizaki K, Naka T, Kishimoto T. Proc Natl Acad Sci USA. 1998;95:13130–13134. doi: 10.1073/pnas.95.22.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naka T, Fujimoto M, Kishimoto T. Trends Biochem Sci. 1999;24:394–398. doi: 10.1016/s0968-0004(99)01454-1. [DOI] [PubMed] [Google Scholar]
- 43.Naka T, Matsumoto T, Narazaki M, Fujimoto M, Morita Y, Ohsawa Y, Saito H, Nagasawa T, Uchiyama Y, Kishimoto T. Proc Natl Acad Sci USA. 1998;95:15577–15582. doi: 10.1073/pnas.95.26.15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.