Abstract
Background
Poor preconception health has been associated with several pregnancy and childbirth-related complications, including perinatal mortality. Yet, the health and economic burden that inaction on preconception health places on societies remains under-researched, hindering efforts to address these issues effectively. This study aimed to quantify the economic burden of perinatal mortality attributable to five preconception risk factors in fifteen low and middle-income countries (LMICs).
Methods
We used a population-attributable fraction analysis to estimate the proportion of perinatal deaths in 2020 attributable to adolescent pregnancy, short birth intervals, intimate partner violence before pregnancy, pre-pregnancy overweight and obesity, and female genital mutilation. We then performed an economic impact analysis to quantify the foregone productivity and the societal costs due to these perinatal deaths, using both the human capital and the value of a statistical life-year approach.
Findings
More than 230,000 (20.7%) perinatal deaths were attributable to the five selected risk factors in the fifteen LMICs in 2020. The productivity losses were estimated at $INT21.3 billion, representing 0.7% of the combined GDP of those countries in 2020. The societal costs of perinatal mortality, the total economic burden was $INT51.0 billion.
Interpretation
Our findings indicate that inaction on preconception care potentially contributes to a substantial proportion of the burden of perinatal mortality, which, in turn, generates profound and long-term economic and societal losses in LMICs. These results highlight the need for effective preconception strategies and relevant policies, and further research is needed to explore the economic value of preconception care in these settings.
Introduction
Perinatal mortality, which encompasses deaths occurring during the period from twenty-two completed weeks of gestation to seven days after birth [1], remains a significant public health issue globally. Approximately 2.3 million newborn children died in 2022, making the neonatal period the most precarious for child survival [2]. The burden of neonatal mortality is disproportionately borne by low and middle-income countries (LMICs), notably in Sub-Saharan Africa and Central and Southern Asia. Despite the substantial progress that has been made since 2000, many LMICs will fall behind the Sustainable Development Goal (SDG) target of reducing neonatal mortality to at least 12 per 1,000 live births by 2030 [3]. Stillbirths represent a significant aspect of perinatal mortality, with nearly 2 million occurring around the world every year [4]. In 2014, the World Health Assembly endorsed the Every Newborn Action Plan (ENAP), which set a global target of 12 or fewer late stillbirths per 1,000 total births by 2030 [5]. However, current trends indicate that more than 50 countries, mainly LMICs, will not reach this target without accelerated action [4].
Effective strategies to reduce the burden of perinatal mortality include the provision of timely, regular, and good-quality antenatal care, accessible emergency obstetric care, birth attendance by trained health professionals, and immediate and high-quality care for newborns, including resuscitation and infection management. Additionally, educating mothers and families on maternal and newborn health plays a significant role in preventing perinatal deaths [5].
In the last decade, the promotion of preconception health has been highlighted as another way to reduce pregnancy-related complications and child morbidity and mortality [6–8]. This increased focus builds on the idea that various risk factors associated with adverse pregnancy, maternal, and child outcomes are rooted within the preconception period. By addressing these risk factors and ensuring better pregnancy readiness, women can embark on pregnancy in optimal health, ultimately leading to improved foetal development, reduced complications, and long-term well-being for both mothers and children [9]. The World Health Organization (WHO) identified many intervention areas, including but not limited to malnutrition, harmful substance use, early and rapid successive pregnancies, interpersonal violence, female genital mutilation, mental health disorders, or exposure to environmental risks [10].
In 2012, as part of a meeting to develop a global consensus on the topic, WHO recognised the need to integrate preconception care into the continuum of care that extends from pregnancy to the postnatal period [11]. However, global progress in developing comprehensive and integrated preconception strategies and policies has been limited since then. Enhanced advocacy is needed to foster better integration of preconception care into existing healthcare frameworks. Building the case for governments to increase their investment in preconception health can significantly support these efforts.
Assessing the cost and economic benefits of investing in preconception health is essential to garnering the needed political and societal support, informing decision-making, and guiding effective resource allocation. A preliminary step to such analysis is to better understand the health and economic burden that inaction on preconception health places on societies. Perinatal mortality is one of the main outcomes associated with poor preconception health. By decreasing future spending on goods and services, reducing tax revenues, and diminishing the potential labour force, perinatal deaths negatively affect future productivity and weaken the economy’s capacity to grow and develop [12,13].
This study estimates the economic burden of perinatal mortality due to inaction on five preconception risk factors in a selection of fifteen LMICs using 2020 data. It has two aims. The first is to measure the number of perinatal deaths attributable to adolescent pregnancy, short birth spacing, pre-pregnancy overweight and obesity, intimate partner violence, and female genital mutilation. The second is to estimate the productivity and societal costs of these perinatal deaths. By employing this two-step methodology, we assessed the costs that could theoretically be averted if these preconception risk factors were eliminated.
Materials and methods
Approach
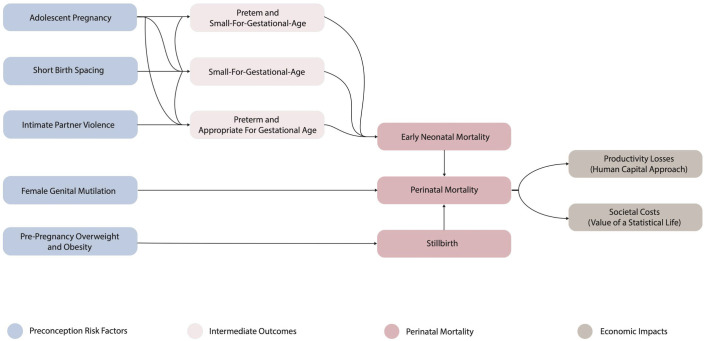
We combined a population-attributable fraction analysis and an economic impact analysis to estimate the productivity losses and the societal costs of perinatal mortality attributable to adolescent pregnancy, short birth spacing, pre-pregnancy overweight and obesity, intimate partner violence before pregnancy, and female genital mutilation in a selection of fifteen countries in 2020 (Fig 1). While these preconception risk factors have been associated with various adverse pregnancy-related outcomes (i.e., low birth weight, postpartum haemorrhage, etc.), we focused on perinatal mortality because evidence linking these five risk factors to this outcome is both consistent and robust. We estimated the foregone productivity using a human capital approach, whilst a value of a statistical life-year approach was utilised to model the societal costs of perinatal mortality. To allow comparisons between countries, all the costs are provided in 2020 INT$, using purchasing power parity (PPP) conversion factors. Future costs were discounted at 3%, a standard rate in global health economic analyses [14]. This process reflects the time value of money, capturing the principle that one dollar today is worth more than a dollar in the future.
Fig 1. Conceptual framework.
Guidelines
We followed the Guidelines for Accurate and Transparent Health Estimates Reporting [15] to guide the reporting of our methodology and ensure transparency and reproducibility of our analysis (S1 Appendix).
Selected countries
The productivity and societal costs of perinatal deaths attributable to the selected preconception risk factors were estimated for Afghanistan, Burundi, Benin, Chad, Côte d’Ivoire, Guinea, Gambia, Kenya, Liberia, Lesotho, Mali, Mauritania, Nigeria, Pakistan, and Sierra Leone. We used two inclusion criteria to select these countries. First, the neonatal mortality rate exceeded 30 neonatal deaths per 1,000 live births. Second, a Demographic Health Survey (DHS) had to be published in the country after 2014 [16]. DHS country data were used as the primary source of information to determine the prevalence of short birth spacing, overweight and obesity, intimate partner violence and female genital mutilation, and this was to ensure that relevant data were available and were sufficiently recent.
Population attributable fraction analysis
We employed a five-step methodology to estimate the number of perinatal deaths attributable to the selected preconception risk factors. First, we determined the prevalence of the preconception risk factors based on available evidence. The prevalence of adolescent pregnancy in primiparous women was determined by combining data from the UN World Population Prospects [17] and the DHS [16]. The prevalence of short birth spacing, overweight and obesity, intimate partner violence, and female genital mutilation was obtained from the DHS. Intimate partner violence was defined as sexual and physical violence committed by the husband or partner in the 12 months preceding the survey. We only considered the female genital mutilation of type II and III, whose association with perinatal mortality is supported by robust evidence. According to WHO’s classification, female genital mutilation type II is defined by the excision of the clitoris with partial or total removal of the labia minora. In contrast, female genital mutilation type III refers to the excision of part or all of the external genitalia and stitching or narrowing of the vaginal opening [18]. The prevalence of female genital mutilation was not available in Afghanistan, Burundi, Pakistan, and Lesotho, either because it was too low, it was not collected, or it was not reported. Therefore, the perinatal deaths attributable to female genital mutilations of type II and III were calculated for the eleven other countries only. We used prevalence rates disaggregated by five-year age intervals to capture a maximum nuance in the results (S1 Appendix).
Second, the relative risks (RRs) measuring the association between the risk factors and perinatal mortality were derived from five meta-analyses and one prospective study conducted in Ghana, Burkina Faso, Nigeria, Kenya, Senegal, and Sudan [18–23] (S1 Appendix). We considered RRs of perinatal mortality, stillbirths or early neonatal deaths. For adolescent pregnancy, short birth spacing, and intimate partner violence, we used RRs for preterm birth (PTB-AGA), preterm birth with small-for-gestational-age (PTB-SGA), and small-for-gestational-age (SGA) as intermediate outcomes. In two meta-analyses, the risk relationships were expressed in odd ratios (ORs). We converted these ORs into RRs using the methodology described by Zhang and Yu [24] (S1 Appendix). We used RR estimates adjusted for potential confounding factors, except for the relationship between intimate partner violence before pregnancy and preterm birth. In this case, the authors reported unadjusted data due to a lack of available evidence [22].
Third, we estimated the population-attributable fraction (PAF) of perinatal deaths due to the preconception risk factors. The PAF represents the proportion of perinatal deaths that could theoretically be prevented if all five preconception risk factors were eliminated. We calculated it using Levin’s formula [25]:
Where Pe is the prevalence of the risk factor, and RR represents the relative risk of a health outcome in the population exposed to the risk factor compared to those not exposed. As mentioned above, we calculated the PAF of perinatal deaths due to adolescent pregnancy, short birth spacing and intimate partner violence in two stages. Firstly, we estimated the PAF of PTB-AGA, PTB-SGA and SGA due to these risk factors. Then, we calculated the PAF of perinatal deaths due to cases of PTB-AGA, PTB-SGA and SGA attributable to the three preconception risk factors. Following the methodology described by Bryce et al. [26], we used additional formulas to ensure that estimates of the PAF accounted for the interactions and combined effects of multiple risk factors (e.g., adolescent pregnancy and intimate pregnancy). The detailed methodology is presented in the supplementary information (S1 Appendix).
Fourth, we calculated the total cases of perinatal deaths by adding the cases of stillbirths and early neonatal deaths. The cases of stillbirths were estimated by combining fertility indicators from the UN World Population Prospects and stillbirth rates from the WHO Global Health Observatory [27]. To estimate the number of early neonatal rates, we used the neonatal mortality rate from the WHO Global Health Observatory and the proportion of neonatal deaths occurring in the first seven days after childbirth derived from the Institute of Health Metrics Global Burden of Disease 2019 [28].
Fifth, we calculated the number of perinatal deaths attributable to adolescent pregnancy, short birth spacing, pre-pregnancy overweight and obesity, intimate partner violence before pregnancy and female genital mutilation by multiplying the total number of perinatal deaths by the corresponding PAFs.
Economic impact analysis
We employed two distinct methodologies to assess the economic impact of perinatal mortality attributable to the selected preconception risk factors: (1) the human capital approach and (2) the value of a statistic life-year (VSLY) approach. The human capital approach quantifies the foregone productivity due to the premature deaths of individuals who would have participated in the workforce had they survived. This method estimates tangible economic losses, emphasising the direct impact of perinatal mortality on national productivity. The VSLY approach builds on society’s willingness to pay for reducing mortality risks to estimate the value of every additional year of life. Unlike methods that focus solely on lost productivity, the VSLY captures the broader societal cost of mortality by valuing life itself. This approach provides a more comprehensive assessment of the economic burden, accounting for both monetary and non-monetary impacts of perinatal mortality.
We used the following formula to estimate the future productivity losses due to perinatal mortality [29]:
where D is the perinatal deaths attributable to the preconception risk factors, L is the labour force participation rate, E is the employment rate, S is the age when individuals would have started working, R is the retirement age, GDPW is the gross domestic product (GDP) per working-age population, and r is the discount rate. We obtained the labour force participation and the employment rate from the International Labour Organisation database (ILOSTAT) [30]. To calculate the GDP per working-age population, we sourced the GDP from the World Bank online database [31] and divided it by the working-age population. We assumed the minimum working age was 15 and the retirement age was 64 in all the selected countries (S1 Appendix).
We calculated the societal costs of perinatal mortality using the formula as follows [32]:
where D is the perinatal deaths attributable to the preconception risk factors, LE is the life expectancy at birth, VSLY is the income-adjusted value of a statistical life-year, and r is the discount rate. The life expectancy at birth was obtained from the WHO Global Health Observatory [27]. Following the methodology described by Viscusi and Masterman [33], we estimated the value of a statistical life (VSL) in each country by transferring it from an estimate of the VSL in the United States in 2020 [34]. The transfer was realised using the GNI per capita from the World Bank database [31] and an income elasticity of 1.103. We then annualised the VSL by dividing it by the life expectancy of an individual of median age in each country, which we obtained from the UN World Population Prospects [17] and the WHO Global Health Observatory [27] (S1 Appendix).
Sensitivity analysis
Because PAF estimates are highly sensitive to RRs, we conducted the analysis using the lower and upper bounds of the confidence intervals of the RRs. We also explored variations in the results when using a discount rate of 5% and 7% [35].
Results
In 2020, there were 21,503,945 births reported in the fifteen countries, with 65.5% of these occurring in Pakistan and Nigeria. The total number of perinatal deaths was estimated at 1,125,296, which represents an average rate of 53.7 perinatal deaths per 1,000 live births. The average GDP per working-age population is $INT5,699 (SD = $INT2,779), with substantial variations from $INT1,476 in Burundi to $INT10,151 in Mauritania. The average income-adjusted VSL is $INT437,051 (SD = $INT237,882). It ranges from $INT93,605 in Burundi to $INT809,889 in Côte d’Ivoire and Mauritania (S1 Appendix).
We estimated that 20.7% of all perinatal deaths in the fifteen selected countries were attributable to adolescent pregnancies, short birth spacing, pre-pregnancy overweight and obesity, intimate partner violence before pregnancy, and female genital mutilation in 2020 (Table 1). Sierra Leone had the largest PAF (34.8%), followed by Guinea (32.2%) and Mauritania (31.7%). The smallest PAFs were found in Burundi (7.5%) and Lesotho (10.6%), two countries in which female genital mutilation is officially not practised. When taking into consideration the countries for which the impact of female genital mutilation was modelled, the smallest PAF was observed in Benin (12.5%). Overall, we estimated that 233,347 perinatal deaths were attributable to the selected preconception risk factors in 2020, including 61,029 in Nigeria and 98,080 in Pakistan.
Table 1. Proportion attributable fraction (PAF) of perinatal deaths for the selected preconception risk factors in 2020.
| Country | Total perinatal deaths | Perinatal deaths attributable to the preconception risk factors | PAF (%) |
|---|---|---|---|
| Afghanistan* | 71,621 | 15,972 | 22.3% |
| Burundi* | 14,747 | 1,100 | 7.5% |
| Benin | 20,875 | 2,617 | 12.5% |
| Côte d’Ivoire | 44,313 | 10,765 | 24.3% |
| Guinea | 21,278 | 6,841 | 32.2% |
| Gambia | 3,774 | 1,212 | 32.1% |
| Kenya | 49,127 | 8,101 | 16.5% |
| Liberia | 7,490 | 1,767 | 23.6% |
| Lesotho* | 2,785 | 294 | 10.6% |
| Mali | 44,791 | 12,626 | 28.2% |
| Mauritania | 5,176 | 1,642 | 31.7% |
| Nigeria | 391,372 | 61,029 | 15.6% |
| Pakistan* | 399,530 | 98,080 | 24.5% |
| Sierra Leone | 12,300 | 4,279 | 34.8% |
| Chad | 36,118 | 7,021 | 19.4% |
| Total** | 1,125,296 | 233,347 | 20.7% |
*Indicates countries where female genital mutilation is not practiced and/or officially reported.
**Due to rounding, the totals presented in this table may differ slightly from the sum of individual country values.
We estimated that perinatal deaths attributable to the selected preconception risk factors generated productivity losses estimated at INT$21.3 billion in 2020 (Table 2). This foregone productivity results from the future loss of more than 7.9 million years of productive life. These productivity losses represent 0.7% of the fifteen countries’ combined 2020 GDP. Pakistan bears the highest economic burden (INT$11.1 billion, 0.9% of GDP), followed by Nigeria (INT$5.6 billion, 0.5% of GDP). These two countries account for about 78.6% of the total economic burden. Relative to its GDP, the productivity losses were the highest in Mali (1.5%) and Chad (1.0%). On average, the productivity losses represent a cost of $INT668 (SD = $INT432) per live birth.
Table 2. Present value in 2020 of foregone productivity due to perinatal mortality attributable to selected preconception risk factors (measured in $INT and as a percentage of 2020 GDP).
| Country | Years of productive life lost (YPLLs) | Foregone productivity ($INT) | Foregone productivity (% of 2020 GDP) |
|---|---|---|---|
| Afghanistan* | 467,200 | 602,016,365 | 0.7% |
| Burundi* | 41,890 | 20,653,296 | 0.2% |
| Benin | 86,184 | 178,383,446 | 0.4% |
| Côte d’Ivoire | 373,618 | 1,197,157,129 | 0.8% |
| Guinea | 200,829 | 337,105,709 | 0.9% |
| Gambia | 36,774 | 49,324,612 | 0.9% |
| Kenya | 284,161 | 772,850,997 | 0.3% |
| Liberia | 65,478 | 56,649,791 | 0.8% |
| Lesotho* | 8,754 | 11,488,527 | 0.2% |
| Mali | 483,007 | 723,725,830 | 1.5% |
| Mauritania | 41,829 | 141,833,900 | 0.6% |
| Nigeria | 1,763,001 | 5,644,202,314 | 0.5% |
| Pakistan* | 3,657,186 | 11,101,222,082 | 0.9% |
| Sierra Leone | 111,560 | 109,159,881 | 0.8% |
| Chad | 239,837 | 355,650,386 | 1.0% |
| Total** | 7,861,309 | 21,301,424,263 | 0.7% |
*Indicates countries where female genital mutilation is not practiced and/or officially reported.
**Due to rounding, the totals presented in this table may differ slightly from the sum of individual country values.
Table 3 shows the societal costs of perinatal mortality attributable to the preconception risk factors. Overall, we estimated these costs to be INT$51.0 billion in 2020. The estimate reflects the societal value of 10.8 million years of life lost (YLLs). As per the productivity losses, Pakistan and Nigeria generate most of the burden, with a contribution of 53.6% and 24.8%, respectively. On average, the societal losses represent a cost of $INT1,616 (SD = $INT1,101.9) per live birth.
Table 3. Present value in 2020 of societal costs due to perinatal mortality attributable to selected preconception risk factors (measured in $INT and as a percentage of 2020 GDP).
| Country | Years of life lost (YLLs) | Societal costs ($INT) |
|---|---|---|
| Afghanistan* | 622,242 | 1,725,471,607 |
| Burundi* | 56,049 | 42,283,869 |
| Benin | 116,877 | 394,677,229 |
| Côte d’Ivoire | 501,804 | 3,225,217,782 |
| Guinea | 262,692 | 863,203,531 |
| Gambia | 50,219 | 123,030,916 |
| Kenya | 390,386 | 1,973,094,734 |
| Liberia | 89,935 | 128,104,596 |
| Lesotho* | 9,537 | 51,238,952 |
| Mali | 649,012 | 1,443,629,656 |
| Mauritania | 60,621 | 341,567,181 |
| Nigeria | 2,395,999 | 12,660,517,988 |
| Pakistan* | 5,107,449 | 27,364,841,228 |
| Sierra Leone | 148,173 | 226,673,596 |
| Chad | 308,816 | 464,341,633 |
| Total** | 10,769,812 | 51,027,894,497 |
*Indicates countries where female genital mutilation is not practiced and/or officially reported.
**Due to rounding, the totals presented in this table may differ slightly from the sum of individual country values.
On average, adolescent pregnancy made up 29.0% of the economic burden (Table 4). It was the largest contributor, followed by short birth interval (26.1%) and pre-pregnancy overweight and obesity (24.6%). It is important to note that the average contribution of short birth intervals was strongly driven by the distribution in Pakistan, where it accounts for 41.7% of the economic burden. 6.0% of the foregone productivity or societal losses were generated by intimate partner violence before pregnancy. The average contribution of female genital mutilation was 14.3%, with substantial variations between countries.
Table 4. Distribution of the economic burden by preconception risk factor.
| Country | Adolescent pregnancy | Short birth interval | Intimate partner violence | Pre-pregnancy overweight and obesity | Female genital mutilation type II and III |
|---|---|---|---|---|---|
| Afghanistan* | 31.6% | 25.9% | 9.5% | 32.9% | 0.0% |
| Burundi* | 37.9% | 20.4% | 22.2% | 19.5% | 0.0% |
| Benin | 38.8% | 11.1% | 8.4% | 27.1% | 14.6% |
| Côte d’Ivoire | 32.6% | 7.5% | 4.9% | 18.0% | 37.1% |
| Guinea | 20.7% | 4.6% | 5.4% | 11.6% | 57.7% |
| Gambia | 15.5% | 4.2% | 3.0% | 16.6% | 60.7% |
| Kenya | 25.8% | 9.4% | 6.6% | 36.9% | 21.2% |
| Liberia | 36.2% | 6.9% | 9.6% | 20.3% | 27.0% |
| Lesotho* | 9.3% | 8.9% | 11.7% | 70.1% | 0.0% |
| Mali | 24.9% | 9.7% | 6.1% | 12.0% | 47.3% |
| Mauritania | 19.1% | 13.1% | 2.1% | 26.3% | 39.5% |
| Nigeria | 35.7% | 16.9% | 7.7% | 22.6% | 17.0% |
| Pakistan* | 25.7% | 41.7% | 4.1% | 28.6% | 0.0% |
| Sierra Leone | 16.8% | 3.6% | 6.1% | 10.4% | 63.1% |
| Chad | 30.9% | 18.8% | 7.9% | 7.4% | 35.1% |
| Total | 29.0% | 26.1% | 6.0% | 24.6% | 14.3% |
*Indicates countries where female genital mutilation is not practiced and/or officially reported.
Table 5 shows the results of our sensitivity analysis. We re-conducted the analysis using the lower and upper bounds of the relative risks. The total foregone productivity varied from INT$11.2 (−47.4%) billion to INT$37.1 billion (+74.2%), and the societal costs from INT$27.0 billion (−47.1%) to INT$88.3 billion (+73.1%). Using 5% and 7% discount rates reduced the productivity losses by 46.5% and 69.5%, respectively. The societal losses were reduced by 33.1% with a 5% discount rate, and 50.8% with a 7% discount rate. These results indicate that the magnitude of the economic burden is dependent on the relative risks and the discount rates utilised.
Table 5. Sensitivity analysis.
| Country | Present value in 2020 of foregone productivity ($INT, Billion) | Present value in 2020 of societal costs ($INT, Billion) |
|---|---|---|
| Base Scenario (BS) | 21.3 | 51.0 |
| Mean (BS) | ||
| Lower Bound | 11.2 | 27.0 |
| Upper Bound | 37.1 | 88.3 |
| Discount Rate: 3% (BS) | ||
| Discount Rate: 5% | 11.4 | 34.1 |
| Discount Rate: 7% | 6.5 | 25.1 |
Discussion
The study results indicated that more than 233,000 cases of perinatal deaths were attributable to the five preconception risk factors in 2020, equivalent to 20.7% of all cases reported in the fifteen countries. This mortality burden, which translates into 10.8 million years of life lost and 7.9 million years of productive life lost, had profound economic and societal implications. In 2020, the foregone productivity was estimated at $INT21.3 billion, representing 0.7% of the fifteen countries’ combined GDP. The use of a value of a statistical life-year approach enabled us to capture the economic burden from a societal perspective, revealing a cost of $INT51.0 billion.
To our knowledge, this study is the first to assess the economic burden of various preconception risk factors through their impact on perinatal mortality in LMICs. Therefore, we do not have prior studies to compare our findings directly. To estimate the PAF of early neonatal deaths for adolescent pregnancy and short birth interval, we used preterm births, preterm births with small-for-gestational-age, and small-for-gestational-age births as intermediate outcomes. These outcomes are among the main causes of early neonatal mortality with congenital anomalies, birth asphyxia, and infections, such as pneumonia or neonatal sepsis [36]. We found that 9.9% of preterm births were attributable to adolescent pregnancy, and 3.9% to short birth spacing in the thirteen Sub-Saharan African countries included in our model. Additionally, 5.9% of small-for-gestational-age births were attributable to adolescent pregnancy and 2.4% to short birth spacing. These estimates are higher than the PAFs reported in two recently published studies [26,37]. These differences might be partly because, unlike our study, these two studies did not consider preterm births with small-for-gestational-age as an independent category. Additionally, those studies adjusted for a larger number of potential confounders, which could further explain the lower PAFs they reported. Our estimates of the PAF of stillbirths attributable to pre-pregnancy overweight and obesity are coherent with those of a previous study conducted in Australia [38]. Nevertheless, this study used maternal body mass index and not pre-pregnancy body mass index, which, once again, limits the comparison. With regard to the economic burden analysis, we did not find studies with which a comparison would have been relevant. However, it is worth mentioning the study by Kirigia et al. [12], in which the authors estimated the non-health GDP loss due to under-five mortality in 47 African countries to be INT$150.3 billion in 2013. Similar to our study, a large share of the economic burden was borne by Nigeria.
We found that PAFs varied substantially between countries, with the lowest being found in Burundi (7.5%) and the highest in Sierra Leone (34.8%). As mentioned above, the variations are amplified by the fact that the impact of female genital mutilation was null in four out of the fifteen selected countries. Female genital mutilation is reportedly not practised in Burundi and Lesotho [39]. In Pakistan, the practice is not officially reported, though it is believed that it exists or has existed marginally, likely confined to a few isolated communities [40]. The situation in Afghanistan remains unclear. In 2015, a study conducted in Iran found that women of Afghan origin had higher risks of experiencing female genital mutilation [41], but no study has comprehensively assessed the prevalence of this practice in Afghanistan, which is not officially reported. The observed variations in PAFs also reflect substantial differences in the magnitude of exposure to the selected preconception risk factors. These disparities are influenced by a complex interplay of socio-economic, cultural, and healthcare-related factors that vary across different regions and populations. Since PAFs are highly sensitive to risk exposure levels, even minor differences in prevalence rates can lead to significant differences between countries, subsequently influencing the economic burden.
In this study, we used preterm birth with or without small-for-gestational-age as intermediate outcomes to capture the influence of early and repeated pregnancies and intimate partner violence before pregnancy on perinatal mortality. Research has shown that babies born prematurely and/or small for gestational age are more likely to die within the first seven days of life due to higher susceptibility to complications [42]. Several explanations have been advanced to explain why children born from adolescent mothers are at higher risks of prematurity and small for gestational age, including increased competition for nutrients between the mother and the foetus, inadequate maternal nutritional intake, physiological immaturity, higher prevalence of anaemia, and increased likelihood of pregnancy-induced hypertension and other obstetric complications in adolescents [43]. Short birth intervals have also been associated with increased risks of preterm births and small-for-gestational-age births [44]. Pregnancies that are closely spaced together impose physiological stress on mothers that hinders adequate recovery and replenishment of nutritional reserves, impairing foetal growth and maturation. In women with previous caesarean deliveries, short birth intervals may lead to incomplete healing of the uterine scar, resulting in complications such as preterm labour. Additionally, shorter interpregnancy intervals may elevate the probability of maternal infections being transmitted to the foetus due to the maternal immune system having insufficient time to fully recover between pregnancies. The mechanisms linking abuse before pregnancy and preterm labour remain unclear. One possible explanation is that women with such a history are more likely to engage in behaviours associated with increased risks of prematurity, including smoking, drug abuse, or alcohol use, and to receive inadequate prenatal care [22,45]. We also hypothesise that exposure to abuse is more likely to continue during pregnancy if initiated beforehand, further compounding the risk of prematurity and adverse pregnancy outcomes.
Female genital mutilation persists in many countries and is driven by a complex interplay of cultural traditions, sociocultural norms, and economic pressures. The mechanisms by which female genital mutilation might cause adverse obstetric outcomes, including stillbirth, are not fully understood. It is possible that the presence of scar tissue from female genital mutilation increases the risks of obstruction and tears during labour, which can prolong the second stage of labour. This, in turn, increases the risks of perineal injury and postpartum haemorrhage, ultimately leading to higher risks of stillbirth [18]. Pre-pregnancy overweight and obesity have also been linked to higher risks of stillbirths. The biological pathways include increased risks of diabetes, hypertension, and hyperlipidaemia, as well as more frequent apnoeic hypoxemia events that reduce blood flow to the foetus. Overweight and obese women may also have a reduced perception of foetal movement, which delays timely medical intervention and increases the risk of adverse outcomes [21].
Our findings suggest that investing in interventions and policies that address the selected risk factors may be an avenue to reduce perinatal mortality. However, it is important to recall that the PAFs reported in our study represent the proportion of perinatal deaths that could be prevented if the associated risk factors were entirely eliminated, which is an idealised scenario. In reality, completely eradicating these risk factors is highly unlikely due to a variety of challenges, including socioeconomic constraints, health system limitations, cultural practices, and individual behaviours. Therefore, while our findings underscore the potential impact of targeting these five preconception risk factors, they should be interpreted cautiously. Achieving significant reductions in perinatal mortality through the preconception pathway will require multifaceted approaches that combine public health policies, education, healthcare improvements, and social support systems.
This study has several limitations that must be acknowledged. First, we focused on five preconception risk factors, although many others, such as malnutrition, infections, poor mental health, or substance abuse, are also linked to perinatal mortality. Similarly, we did not account for other important outcomes, including maternal mortality, pregnancy and childbirth-related complications or long-term impacts like childhood stunting, which also contribute to economic and societal losses. We chose to limit this analysis to perinatal mortality to maintain a clear narrative but also because it is likely to be the main contributor to the economic burden of poor preconception health. Second, the analysis was limited to fifteen countries with available and relatively recent DHS, restricting the generalizability of our findings to other LMICs with different socio-economic contexts. Third, we did not adjust the PAFs for confounding risk factors. However, we minimised this limitation by using adjusted relative risks wherever possible. Fourth, our model assumed that all women have an equal chance of becoming pregnant, regardless of their exposure to overweight and obesity, female genital mutilation, and intimate partner violence. This assumption overlooks the nuanced reality that some risk factors may influence fertility rates, either enhancing or diminishing a woman’s likelihood of conception. Unfortunately, it was not possible to differentiate fertility rates in exposed and not-exposed women due to data limitations. Fifth, we did not factor in potential changes in the GDP per working-age population or the VSLY over time. Introducing such projections could have increased uncertainty around our estimates, so we adopted a more conservative approach by not adjusting these parameters over time.
Despite these limitations, and given the scarce published evidence in this area, our study provides new insights into the economics of preconception health and care in LMICs. It might be beneficial to investigate this further by extending the scope of this study, whether in terms of risk factors, outcomes or selected countries. In addition, further research should focus on building an investment case to assess the economic value of investing in preconception health and care, which could guide policymaking and resource allocation in LMICs.
In LMICs, a substantial proportion of perinatal deaths is attributable to risk factors that can be addressed before pregnancy starts, such as early and rapidly repeated pregnancies, overweight and obesity or exposure to violence. In turn, these perinatal deaths cause significant economic losses at the country level. Results from this study provide insights into the profound and long-term economic implications that arise from inaction on preconception health and care. They also highlight the potential of preconception care interventions and policies for curbing the burden of stillbirths and neonatal mortality while contributing to the achievement of the SDGs. To stimulate interest from policymakers and the public, it is essential to pursue research efforts on the cost and benefits of preconception care in LMICs.
Supporting information
(DOCX)
Data Availability
No original data were collected for this study. Data used in this analysis are presented in the supporting information and/or are available in the public domain.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.World Health Organization. Mortality database. Perinatal conditions. World Health Orgnaization; 2024. [cited 2024 Aug 30]. https://platform.who.int/mortality/themes/theme-details/topics/topic-details/MDB/perinatal-conditions [Google Scholar]
- 2.World Health Organization. Newborn mortality. World Health Organization; 2024. [cited 2024 Jul 19]. https://www.who.int/news-room/fact-sheets/detail/newborn-mortality [Google Scholar]
- 3.Hug L, Alexander M, You D, Alkema L, UN Inter-agency Group for Child Mortality Estimation. National, regional, and global levels and trends in neonatal mortality between 1990 and 2017, with scenario-based projections to 2030: a systematic analysis. Lancet Glob Health. 2019;7(6):e710–20. doi: 10.1016/S2214-109X(19)30163-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Stillbirth. World Health Organization; 2024. [cited 2024 Jul 19]. https://www.who.int/health-topics/stillbirth#tab=tab_1 [Google Scholar]
- 5.World Health Organization. Every Newborn: an action plan to end preventable deaths. Geneva: World Health Organization; 2014. [cited 2024 Jul 19]. https://iris.who.int/bitstream/handle/10665/127938/9789241507448_eng.pdf?sequence=1 [Google Scholar]
- 6.Berglund A, Lindmark G. Preconception health and care (PHC)-a strategy for improved maternal and child health. Ups J Med Sci. 2016;121(4):216–21. doi: 10.1080/03009734.2016.1191564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khekade H, Potdukhe A, Taksande AB, Wanjari MB, Yelne S. Preconception Care: A Strategic Intervention for the Prevention of Neonatal and Birth Disorders. Cureus. 2023;15(6):e41141. doi: 10.7759/cureus.41141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephenson J, Schoenaker DA, Hinton W, Poston L, Barker M, Alwan NA, et al. A wake-up call for preconception health: a clinical review. Br J Gen Pract. 2021;71(706):233–6. doi: 10.3399/bjgp21X715733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poix S, Elmusharaf K. Investigating the pathways from preconception care to preventing maternal, perinatal and child mortality: A scoping review and causal loop diagram. Prev Med Rep. 2023;34:102274. doi: 10.1016/j.pmedr.2023.102274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Preconception care: Maximizing the gains for maternal and child health. Geneva: World Health Organization; 2013. [cited 2024 Aug 21]. https://iris.who.int/bitstream/handle/10665/340533/WHO-FWC-MCA-13.02-eng.pdf?sequence=1 [Google Scholar]
- 11.World Health Organization. Meeting to Develop a Global Consensus on Preconception Care to Reduce Maternal and Childhood Mortality and Morbidity. Meeting Report: 6–7 February 2012. Geneva: World Health Organization; 2012. [cited 2024 Aug 18]. https://iris.who.int/bitstream/handle/10665/78067/9789241505000_eng.pdf [Google Scholar]
- 12.Kirigia JM, Muthuri RDK, Nabyonga-Orem J, Kirigia DG. Counting the cost of child mortality in the World Health Organization African region. BMC Public Health. 2015;15:1103. doi: 10.1186/s12889-015-2465-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stenberg K, Axelson H, Sheehan P, Anderson I, Gülmezoglu AM, Temmerman M, et al. Advancing social and economic development by investing in women’s and children’s health: a new Global Investment Framework. Lancet. 2014;383(9925):1333–54. doi: 10.1016/S0140-6736(13)62231-X [DOI] [PubMed] [Google Scholar]
- 14.Neumann PJ, Ganiats TG, Russell LB, Sanders GD, Siegel JE. Cost-Effectiveness in Health and Medicine. 2nd ed. New York: Oxford University Press; 2016. [Google Scholar]
- 15.Stevens GA, Alkema L, Black RE, Boerma JT, Collins GS, Ezzati M, et al. Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement. Lancet. 2016;388(10062):e19–23. doi: 10.1016/S0140-6736(16)30388-9 [DOI] [PubMed] [Google Scholar]
- 16.ICF International. STATcompiler. Rockville, Maryland: ICF International; 2024. [cited 2024 Apr 01]. http://www.statcompiler.com [Google Scholar]
- 17.United Nations. World Population Prospects 2022, Online Edition. New York: United Nations; 2024. [cited 2024 Apr 04]. https://population.un.org/wpp/ [Google Scholar]
- 18.WHO study group on female genital mutilation and obstetric outcome, Banks E, Meirik O, Farley T, Akande O, Bathija H, et al. Female genital mutilation and obstetric outcome: WHO collaborative prospective study in six African countries. Lancet. 2006;367(9525):1835–41. doi: 10.1016/S0140-6736(06)68805-3 [DOI] [PubMed] [Google Scholar]
- 19.Kozuki N, Lee ACC, Silveira MF, Sania A, Vogel JP, Adair L, et al. The associations of parity and maternal age with small-for-gestational-age, preterm, and neonatal and infant mortality: a meta-analysis. BMC Public Health. 2013;13 Suppl 3(Suppl 3):S2. doi: 10.1186/1471-2458-13-S3-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozuki N, Lee ACC, Silveira MF, Victora CG, Adair L, Humphrey J, et al. The associations of birth intervals with small-for-gestational-age, preterm, and neonatal and infant mortality: a meta-analysis. BMC Public Health. 2013;13 Suppl 3(Suppl 3):S3. doi: 10.1186/1471-2458-13-S3-S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vats H, Saxena R, Sachdeva MP, Walia GK, Gupta V. Impact of maternal pre-pregnancy body mass index on maternal, fetal and neonatal adverse outcomes in the worldwide populations: A systematic review and meta-analysis. Obes Res Clin Pract. 2021;15(6):536–45. doi: 10.1016/j.orcp.2021.10.005 [DOI] [PubMed] [Google Scholar]
- 22.Nesari M, Olson JK, Vandermeer B, Slater L, Olson DM. Does a maternal history of abuse before pregnancy affect pregnancy outcomes? A systematic review with meta-analysis. BMC Pregnancy Childbirth. 2018;18(1):404. doi: 10.1186/s12884-018-2030-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katz J, Lee AC, Kozuki N, Lawn JE, Cousens S, Blencowe H, et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet. 2013;382(9890):417–25. doi: 10.1016/S0140-6736(13)60993-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1. doi: 10.1001/jama.280.19.1690 [DOI] [PubMed] [Google Scholar]
- 25.Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum. 1953;9(3):531–41. [PubMed] [Google Scholar]
- 26.Bryce E, Gurung S, Tong H, Katz J, Lee AC, Black RE, et al. Population attributable fractions for risk factors for spontaneous preterm births in 81 low- and middle-income countries: A systematic analysis. J Glob Health. 2022;12:04013. doi: 10.7189/jogh.12.04013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. The Global Health Observatory. World Health Organization; 2024. [cited 2024 Jun 06]. https://www.who.int/data/gho [Google Scholar]
- 28.Global Burden of Disease Collaborative Network. Global Burden of Disease 2019 (GBD 2019). Seattle: Institute for Health Metrics and Evaluation (IHME); 2024. [cited 2024 June 06]. https://www.healthdata.org/research-analysis/about-gbd [Google Scholar]
- 29.Elmusharaf K, Grafton D, Jung JS, Roberts E, Al-Farsi Y, Al Nooh AA, et al. The case for investing in the prevention and control of non-communicable diseases in the six countries of the Gulf Cooperation Council: an economic evaluation. BMJ Glob Health. 2022;7(6):e008670. doi: 10.1136/bmjgh-2022-008670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.International Labour Organization. ILOSTAT. International Labour Organization; 2024; [cited 2024 Jun 06]. https://ilostat.ilo.org/data/ [Google Scholar]
- 31.The World Bank. World Bank Open Data. The World Bank; 2024. [cited 2024 Jun 06]. https://data.worldbank.org [Google Scholar]
- 32.Robinson LA, Hammitt JK, Cecchini M, Chalkidou K, Claxton K, Cropper M, et al. Reference Case Guidelines for Benefit-Cost Analysis in Global Health and Development. 2019. https://content.sph.harvard.edu/wwwhsph/sites/2447/2019/05/BCA-Guidelines-May-2019.pdf [Google Scholar]
- 33.Viscusi WK, Masterman CJ. Income Elasticities and Global Values of a Statistical Life. J Benefit Cost Anal. 2017;8(2):226–50. doi: 10.1017/bca.2017.12 [DOI] [Google Scholar]
- 34.U.S. Department of Transportation. Departmental guidance on valuation of a statistical life in economic analysis. Washington, DC: US Department of Transport; 2024. https://www.transportation.gov/office-policy/transportation-policy/revised-departmental-guidance-on-valuation-of-a-statistical-life-in-economic-analysis [Google Scholar]
- 35.Haacker M, Hallett TB, Atun R. On discount rates for economic evaluations in global health. Health Policy Plan. 2019;35(1):107–14. doi: 10.1093/heapol/czz127 [DOI] [PubMed] [Google Scholar]
- 36.Lehtonen L, Gimeno A, Parra-Llorca A, Vento M. Early neonatal death: A challenge worldwide. Semin Fetal Neonatal Med. 2017;22(3):153–60. doi: 10.1016/j.siny.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 37.Gurung S, Tong HH, Bryce E, Katz J, Lee AC, Black RE, et al. A systematic review on estimating population attributable fraction for risk factors for small-for-gestational-age births in 81 low- and middle-income countries. J Glob Health. 2022;12:04024. doi: 10.7189/jogh.12.04024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheney K, Farber R, Barratt AL, McGeechan K, de Vries B, Ogle R, et al. Population attributable fractions of perinatal outcomes for nulliparous women associated with overweight and obesity, 1990-2014. Med J Aust. 2018;208(3):119–25. doi: 10.5694/mja17.00344 [DOI] [PubMed] [Google Scholar]
- 39.UNFPA, UNICEF. Global annual report - reimagining resilience: eliminating female genital mutilation in the context of the polycrisis. UNFPA, UNICEF; 2023. [Google Scholar]
- 40.Syyed H. Lack of data and dialogue on female genital mutilation in Pakistan. Journal of International Women’s Studies. 2022;24(1). [Google Scholar]
- 41.Dehghankhalili M, Fallahi S, Mahmudi F, Ghaffarpasand F, Shahrzad ME, Taghavi M, et al. Epidemiology, Regional Characteristics, Knowledge, and Attitude Toward Female Genital Mutilation/Cutting in Southern Iran. J Sex Med. 2015;12(7):1577–83. doi: 10.1111/jsm.12938 [DOI] [PubMed] [Google Scholar]
- 42.Ashorn P, Ashorn U, Muthiani Y, Aboubaker S, Askari S, Bahl R, et al. Small vulnerable newborns-big potential for impact. Lancet. 2023;401(10389):1692–706. doi: 10.1016/S0140-6736(23)00354-9 [DOI] [PubMed] [Google Scholar]
- 43.Gibbs CM, Wendt A, Peters S, Hogue CJ. The impact of early age at first childbirth on maternal and infant health. Paediatr Perinat Epidemiol. 2012;26 Suppl 1(0 1):259–84. doi: 10.1111/j.1365-3016.2012.01290.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conde-Agudelo A, Rosas-Bermudez A, Castaño F, Norton MH. Effects of birth spacing on maternal, perinatal, infant, and child health: a systematic review of causal mechanisms. Stud Fam Plann. 2012;43(2):93–114. doi: 10.1111/j.1728-4465.2012.00308.x [DOI] [PubMed] [Google Scholar]
- 45.Alhusen JL, Ray E, Sharps P, Bullock L. Intimate partner violence during pregnancy: maternal and neonatal outcomes. J Womens Health (Larchmt). 2015;24(1):100–6. doi: 10.1089/jwh.2014.4872 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
No original data were collected for this study. Data used in this analysis are presented in the supporting information and/or are available in the public domain.