Abstract
We test whether the dysfunction with age of carnitine acetyltransferase (CAT), a key mitochondrial enzyme for fuel utilization, is due to decreased binding affinity for substrate and whether this substrate, fed to old rats, restores CAT activity. The kinetics of CAT were analyzed by using the brains of young and old rats and of old rats supplemented for 7 weeks with the CAT substrate acetyl-l-carnitine (ALCAR) and/or the mitochondrial antioxidant precursor R-α-lipoic acid (LA). Old rats, compared with young rats, showed a decrease in CAT activity and in CAT-binding affinity for both substrates, ALCAR and CoA. Feeding ALCAR or ALCAR plus LA to old rats significantly restored CAT-binding affinity for ALCAR and CoA, and CAT activity. To explore the underlying mechanism, lipid peroxidation and total iron and copper levels were assayed; all increased in old rats. Feeding old rats LA or LA plus ALCAR inhibited lipid peroxidation but did not decrease iron and copper levels. Ex vivo oxidation of young-rat brain with Fe(II) caused loss of CAT activity and binding affinity. In vitro oxidation of purified CAT with Fe(II) inactivated the enzyme but did not alter binding affinity. However, in vitro treatment of CAT with the lipid peroxidation products malondialdehyde or 4-hydroxy-nonenal caused a decrease in CAT-binding affinity and activity, thus mimicking age-related change. Preincubation of CAT with ALCAR or CoA prevented malondialdehyde-induced dysfunction. Thus, feeding old rats high levels of key mitochondrial metabolites can ameliorate oxidative damage, enzyme activity, substrate-binding affinity, and mitochondrial dysfunction.
Aging appears to be due, in part, to damage caused by the oxidants produced by mitochondria as by-products of normal metabolism (1–10). Aging is associated with a decrease in cellular enzyme or receptor activities. Some enzyme or receptor inactivation is due to an increase in Km for their substrates or cofactors (B.N.A., J.L., and I. Elson-Schwab, unpublished work). For example, Feuers (11) found that in female mice, the Vmax of mitochondrial complexes III and IV significantly decreased with age, in parallel with a decrease of ubiquinol or cytochrome c substrate-binding affinity. Dietary restriction, which reduces the generation of oxidants and oxidative damage, effectively reversed these decreases in complex activity and substrate affinity. On the other hand, the activity of many enzymes decreases with age but shows no change in Km (12–14) (B.N.A., J.L., and I. Elson-Schwab, unpublished work).
As many as one-third of mutations in a gene result in the corresponding enzyme having a poorer binding affinity (an increased Km) for its coenzyme, which in turn lowers the rate of the reaction (15–17). When the concentration of the coenzyme is increased by feeding the corresponding vitamin at high levels, the enzyme activity is partially restored, and the disease phenotype is cured or ameliorated (18). Thus, we hypothesize (18–20) that during aging, mitochondrial oxidants deform proteins because of direct oxidation, changes in membranes, and adduction of aldehyde by-products from lipid peroxidation. This deformation in turn decreases the binding affinity of many enzymes for their substrates or coenzymes. Feeding high doses of enzyme substrates or coenzymes can overcome the deficiencies of those enzymes with decreased binding affinity and restore enzyme function. Oxidative decay is particularly acute in mitochondria (1, 4, 8). Thus, feeding high levels of several mitochondrial substrates and vitamin precursors of coenzymes might reverse some of the mitochondrial decay of aging (5–7, 9, 18, 21, 22).
l-Carnitine is a betaine required in the mitochondria for transporting in long chain fatty acids for β oxidation and ATP production, as well as for transporting out excess short and medium chain fatty acids (23). Feeding old rats an acetyl-l-carnitine (ALCAR)-supplemented diet restores tissue levels of free and acyl carnitines to that found in plasma and brain tissues of younger animals (20, 24). This diet-induced increase in carnitine levels in older animals results in a reversion of liver and heart mitochondria to a more youthful state, both structurally and functionally (6, 9, 14, 25–28).
R-α-lipoic acid (LA) is a coenzyme for pyruvate dehydrogenase and α-ketoglutarate dehydrogenase in mitochondria. Dihydrolipoic acid, the reduced form of LA, is a potent antioxidant that can recycle other antioxidants, such as vitamins C and E, and raise the levels of intracellular glutathione, which is critical for neuronal function (29, 30). LA supplementation restores long-term potentiation, a synaptic analogue of learning and memory, in aged rodents (31) and partially restores ambulatory activity and memory lost during aging (5, 32, 33).
Carnitine acetyltransferase (CAT) (EC 2.3.1.7) catalyses the reversible conversion of acetyl-CoA and carnitine to acetylcarnitine and CoA. CAT's essential functions are to regenerate CoA, which allows peroxisomal β-oxidation to proceed, and to facilitate transport of acetyl moieties to mitochondria for oxidation (34). More than 70% of CAT is located in the mitochondrial matrix, and it appears to be present in all mammalian tissues (34–36). An age-associated decrease of CAT activity has been reported in rat soleus, diaphragm, and heart (37, 38) and in brain and muscles in vitamin E-deficient rats (39), although Moret et al. (40) did not find altered CAT activity in the brain of Long–Evans rats with age in healthy animals over a moderate age range. CAT activity was found to decrease in Alzheimer's patient brain microvessels and cerebellum (41, 42), although there are contrary findings (43). CAT activity was also found to decrease in fatal ataxic encephalopathy (44), mitochondrial encephalomyopathy (45), and severe peripheral vascular disease (46). Sulfhydryl reactive agents cause a decrease in CAT-binding affinity for substrates (increase in Km) and in CAT activity, and the addition of CAT substrates or antioxidants such as mercaptoethanol prevents or partially reverses CAT inhibition (47). No study has attempted to examine the age-associated changes in CAT substrate-binding affinity and the effects of substrates or antioxidant treatments on the Km of CAT.
The present study was designed to test the Km hypothesis by assaying the kinetics of CAT in the brains of young and old rats, and old rats fed ALCAR and/or LA. The mechanism for the change in kinetics was also explored.
Materials and Methods
Materials.
ALCAR (hydrochloride salt) was a gift of Sigma Tau (Pomezia, Italy) and LA (the natural R-isomer), of ASTA Medica (Frankfurt/Main, Germany). All other reagents were reagent grade or the highest quality available from Sigma, unless otherwise indicated.
Animals.
Fischer 344 male rats were obtained from the National Institute on Aging. Control animals were fed an AIN93M diet from Dyets (Bethlehem, PA) and MilliQ (Millipore) water (pH 5.2). The rats in the experimental groups were fed either 0.5% ALCAR in their drinking water, 0.2% LA in AIN93M diet, or both, for 7 weeks. The young rats were 4.5 months, and the old ones were 24.5 months at the start of experiment; they were more than 7 weeks older when they were killed with ether anesthesia. The brains were removed, immediately put into liquid nitrogen, and stored in a −80°C freezer until analysis.
Kinetic Analysis.
Because there are no brain regional differences in CAT activity as well as Km in rats (40) or in rabbits (36), we used the whole brain. Brain tissue was homogenized with 50 mM Tris-buffered saline (pH 7.5) containing 2 mM EDTA, 5 mM MgCl2, 0.8 mM DTT, 1 μM protease inhibitor mixture, and 0.25 mM phenylmethylsulfonyl fluoride (freshly made in acetone and added to the homogenizing tube before homogenization). Homogenates were then sonicated on ice for 3 × 10 s and centrifuged at 3,500 × g for 5 min to obtain the mitochondrial and microsomal portion containing more than 90% of the enzyme (36). The CAT activity was assayed immediately after the centrifugation as described (48, 49). The assay medium contained about 0.5 mg of protein/ml brain homogenate supernatant, 50 mM Tris, 2 mM EDTA, 25 mM malate, 0.25 mM NAD, 12.5 μg/ml of rotenone, 12.5 μg/ml of malate dehydrogenase, 50 μg/ml of citrate synthase, and 0.04% Triton-100. The kinetics were determined over a range of ALCAR concentrations from 0.015 to 5 mM with a constant concentration of 1.25 mg/ml of CoA (Km for ALCAR) or over a range of CoA concentrations from 6.25 to 400 μM with a constant concentration of 2 mM acetyl-l-carnitine (Km for CoA). The results were plotted with the double-reciprocal plot of reciprocal rate 1/v against reciprocal substrate concentration 1/S. Results were also calculated by the direct linear plot with the equation of Vmax = v + (v/S)Km (50).
Ex Vivo Oxidation of Rat-Brain Homogenate.
The young-rat-brain homogenate was incubated with FeSO4 for 15 min at 37°C. The kinetics were assayed as described above, and the oxidation was assayed by measuring malondialdehyde (MDA) with a gas chromatography–mass spectrometric method (51, 52).
In Vitro Oxidation of Purified CAT with Iron.
CAT (purified from pigeon breast muscle) was obtained from Sigma. The enzyme was diluted with PBS and used immediately. The enzyme (0.36 μg/ml) was incubated with PBS and various concentrations of FeSO4 with or without metal chelators/antioxidants at room temperature for 15 min. CAT activity was assayed immediately by using the method described above (CAT at 0.036 μg/ml of protein).
MDA- and 4-Hydroxynonenal (HNE)-Induced Inactivation and Km Change of Purified CAT in Vitro.
MDA was prepared by derivatization of 1,1,3,3-tetramethoxypropane with 0.01 M HCl and stored 3–6 weeks at 4°C. The concentration of MDA was determined at 245 nm by using an extinction coefficient of 13,700/M (52). HNE was obtained from Calbiochem, and its concentration was determined at 224 nm by using an extinction coefficient of 13,750/M (53). Similar to the above incubation, CAT (0.36 μg/ml) was incubated in PBS or Tris-buffered saline (TBS) with MDA or HNE at room temperature. The CAT kinetics were assayed immediately as above (CAT at 0.036 μg/ml of protein).
Total Metal Assay with Inductively Coupled Plasma Spectrometry (ICP).
Six metals (iron, copper, calcium, magnesium, manganese, and zinc) were analyzed by using ICP with modification of a reported method (54, 55).
Lipid Peroxidation Assay.
Lipid peroxidation was assayed by using a gas chromatography–mass spectrometric method to measure the level of MDA (51, 52).
Results
CAT Kinetics.
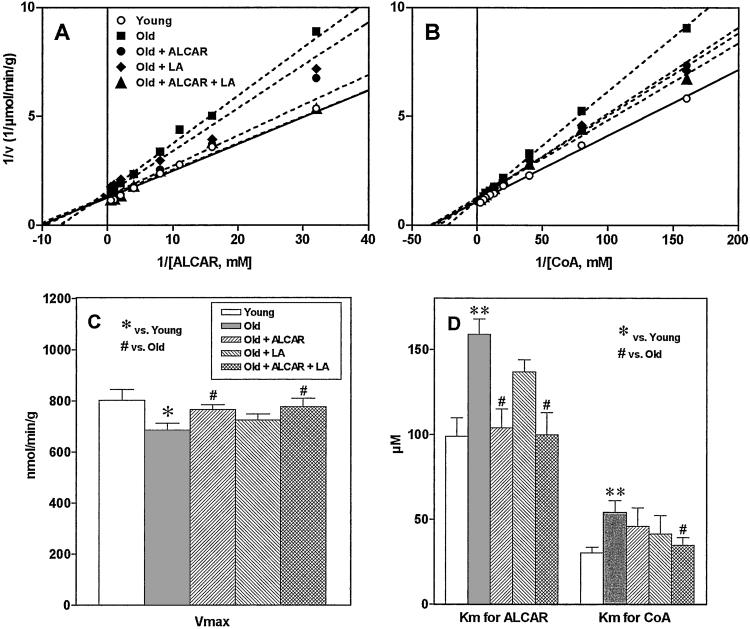
The double-reciprocal plots of CAT reaction velocity versus ALCAR or CoA concentration in rat brain are shown in Fig. 1 A and B. The values of Vmax and apparent Km are shown in Fig. 1 C and D. Compared with young rats, old rats showed a moderate decrease in enzyme activity (Vmax) (14%, Fig. 1C) and an increase in Km [160% of Km for ALCAR and 180% of Km for CoA (Fig. 1D)], suggesting a decrease in substrate-binding affinity. Supplementation with ALCAR in old rats significantly increased the binding affinity for ALCAR. Supplementation with LA showed a small increase in binding affinity that was not statistically significant. The combination of ALCAR and LA significantly elevated enzyme activity and binding affinity for both substrates (Km for ALCAR, P = 0.019; Km for CoA, P = 0.018). The combination also significantly increased CAT activity (P = 0.04).
Figure 1.
Double-reciprocal plots of reaction velocity versus substrate ALCAR (A) or CoA (B) concentrations in rat brain. (C) Vmax; (D) apparent Km for ALCAR and CoA. All values are mean ± SE of 10 animals for young and old groups, 5 for the LA group, and 6 for the ALCAR and ALCAR plus LA groups. Significant difference was calculated by using Student's t test between young and old groups (*, P < 0.05, **, P < 0.01) and by using one-way ANOVA with Dunnett's multiple comparison test between old and other treated groups (#, P < 0.05).
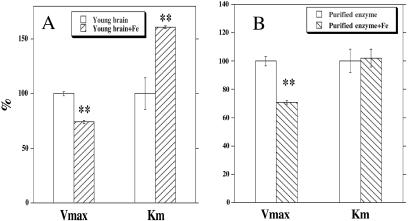
Ex Vivo Oxidation of Brain Homogenate from Young Rats.
Incubation of Fe(II) (1–10 mM) with brain homogenate from young rats for 15 min induced a concentration-dependent inactivation of CAT. The inactivation was accompanied by a significant decrease in substrate-binding affinity (Fig. 2A), similar to that associated with aging (Fig. 1). Incubation of rat-brain homogenate with Fe(II) induced a marked increase in membrane lipid peroxidation, as shown by the level of MDA (control, 15.2 ± 0.2; addition of 0.2 mM FeSO4, 134 ± 1.6 pmol/mg of protein). As expected, metal chelators such as EDTA at 1 mM (MDA 48.6 ± 0.6 pmol/mg) and deferoxamine at 1 mM (MDA 8.5 ± 0.2 pmol/mg) protected lipid membranes from peroxidation.
Figure 2.
The kinetic parameters (Vmax and Km for ALCAR) of CAT for young-rat brain with and without 5 mM Fe(II) (A) and for purified CAT (from pigeon breast muscle) with and without 0.1 mM Fe(II) (B). Significant difference was calculated with Student's t test (**, P < 0.01).
In Vitro Oxidation of Purified CAT.
Incubation of purified enzyme with Fe(II) (0.1–1 mM) induced a concentration-dependent inactivation of CAT with a 50% inactivation at a concentration of 95 μM at 37°C for 15 min (0.5 units/ml). Unlike ex vivo oxidation of rat-brain homogenate, this oxidation induced a decrease in enzyme activity but not in the substrate-binding affinity (Fig. 2B). FeSO4 at 200 μM concentration caused 80% inactivation of CAT. As expected, metal chelators EDTA and deferoxamine, at 1 mM concentration, protected the enzyme from inactivation by 70 and 94% respectively, as did the sulfhydryl antioxidants (all at 1 mM), reduced glutathione (23%), DTT (32%), and dihydrolipoic acid (35%), although they were less effective. Catalase (1 mg/ml) also showed protection (87%), suggesting that enzyme inactivation is mediated by Fenton chemistry. The substrates of the enzyme protected against Fe(II)-induced inactivation: ALCAR at 1 mM, 92%; l-carnitine at 1 mM, 36%; CoA at 0.6 mM, 91%; and acetyl-CoA at 0.6 mM, 50%.
In Vivo Lipid Peroxidation Levels in Rat Brain.
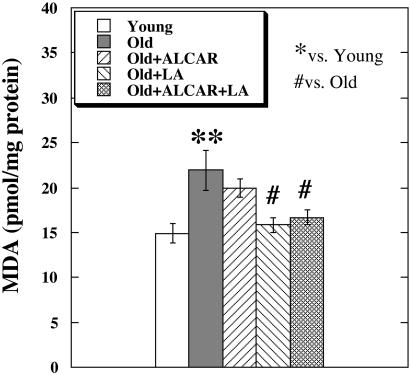
Compared with young rats, old rats showed a significant increase in brain MDA, a major product of lipid peroxidation. Old rats fed LA or LA plus ALCAR had significantly lowered levels of brain MDA (Fig. 3).
Figure 3.
MDA levels in the rat brain measured with a gas chromatography–mass spectrometric assay. The values are mean of seven animals for the young and old groups, three for the LA group, and five for the ALCAR and ALCAR plus LA groups. Significant difference was calculated by using Student's t test between young and old groups (**, P < 0.01), and by using one-way ANOVA with Dunnett's multiple comparison test between old and other treated groups (#, P < 0.05).
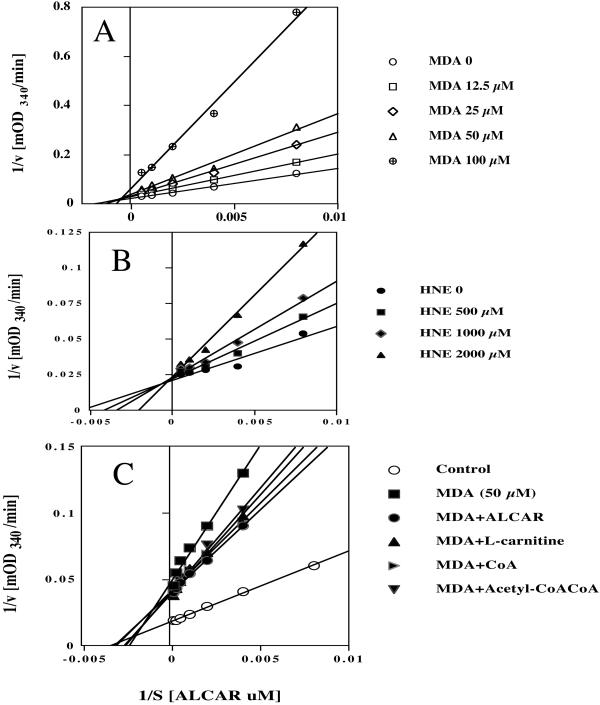
Effect of MDA and HNE on the Km of Purified CAT.
Fig. 4 A and B show the effects of MDA and HNE in PBS on reciprocal plots of CAT for the substrate ALCAR. Both MDA and HNE caused a concentration-dependent inactivation of CAT accompanied by an increase in Km when incubated in PBS (Fig. 4) or in TBS (data not shown). MDA was a more powerful inhibitor than HNE. In PBS incubation, MDA at 25, 50, and 100 μM inhibited CAT activity to 69, 54, and 30% and increased the Km for ALCAR to 135, 152, and 259%, whereas HNE at 0.5, 0.75, 1.0, and 2.0 mM inhibited CAT activity to 96, 88, 79, and 60% and increased the Km for ALCAR to 135, 164, and 269%). The concentration required for 50% inhibition of CAT activity by MDA is 45 μM in PBS solution and 400 μM in TBS solution. Preincubation with substrates, ALCAR, l-carnitine, CoA, and acetyl-CoA protected MDA-induced CAT inactivation and increase in Km (Fig. 4C).
Figure 4.
Concentration-dependent effects of MDA (A) and HNE (B) in PBS on reciprocal plots of CAT for substrate ALCAR. Different concentrations of MDA or HNE were incubated with 0.36 μg/ml of CAT enzyme for 1 h at room temperature in PBS. The kinetics were assayed with an assay mixture containing 0.036 μg/ml of CAT. (C). The protection of substrate ALCAR, l-carnitine, CoA, and acetyl-CoA on reciprocal plots of CAT for substrate ALCAR (0.5 mM), l-carnitine (0.25 mM), CoA (25 μM), and acetyl-CoA (50 μM). The MDA used was 50 μM with 1-h incubation. The substrates were added before MDA.
Total Metal Content in Rat Brain.
Compared with young rats, old rats had a significant increase in total iron (young, 66.4 ± 1.8, and old, 81.6 ± 2.2 ng/mg of dry tissue; P < 0.001) and copper (young, 10.3 ± 0.2, and old, 17.4 ± 0.6 ng/mg of dry tissue; P < 0.001) in the brain; no changes in Ca, Mg, Zn, and Mn were found (data not shown). Supplementing with ALCAR and/or LA did not cause a significant decrease in the levels of total iron (old + ALCAR, 85.1 ± 4.2; old + LA, 80 ± 1.7; and old + ALCAR + LA, 76.3 ± 4.5 ng/mg of dry tissue) or total copper (data not shown).
Discussion
Old-rat brain is shown to have a moderate age-associated decrease in CAT activity and a marked decrease in binding affinity for the substrates ALCAR (young 100 μM, old 150 μM; Fig. 1D) and CoA. Feeding old rats ALCAR for 7 weeks, which elevates the level of free and acyl carnitines in blood and brain to a level of about 100 μM (20), significantly restored this age-associated decrease in binding affinity for ALCAR; the combination of ALCAR and LA significantly restored both CAT activity and its binding affinity for the substrates ALCAR and CoA to the levels observed in young rats. CAT has two separate binding sites: one for CoA/acetyl-CoA involving the sulfhydryl group of a cysteine residue and a second for l-carnitine/ALCAR (56). Feeding old rats LA significantly enhanced the effect of ALCAR, although LA alone had only a small effect on CAT activity and substrate-binding affinity.
Although extrapolation from in vitro to in vivo results should be viewed with caution, we suggest two plausible mechanisms that could account for the age-associated loss of binding affinity and activity: (i) adduction to the protein of aldehyde products of lipid peroxidation, or (ii) oxidation of the protein either directly by oxidants or by metal-catalyzed oxidation. We propose that the adduction mechanism is more likely. In vivo, brain MDA, derived from lipid peroxidation, increases with age in parallel with a decrease in CAT activity and binding affinity for substrates (Fig. 4A). We also show that MDA and HNE, another lipid peroxidation product, decrease the Vmax and binding affinity of CAT in vitro, whereas a direct oxidant, i.e., iron, does not. Lipid peroxidation may be due in part to age-associated increases in iron and copper levels. In agreement with this are our results from the ex vivo oxidation of young-rat brain with Fe(II), in which CAT Km and activity change in the same way as during aging. Aldehyde products from lipid peroxidation of membranes have been shown to react with both amino and sulfhydryl groups in protein (57), thus potentially inactivating them (53, 58). The level of MDA needed to inhibit CAT in vitro is consistent with the MDA level observed in vivo. The level of MDA required for 50% inhibition of CAT is 45 μM, which is not too far from its concentration in brain (20 pmol/mg of protein, i.e., about 4 μM in tissue) (Fig. 3). The MDA concentration in mitochondria is likely to be much higher. A fraction of MDA is bound to proteins (59). Most in vitro studies used a 10–10,000 μM range of MDA to show it toxic or mutagenic (57). MDA and HNE are only two of the many known active aldehydes formed from lipid peroxidation, many of which may contribute to enzyme inactivation.
Lowering aldehydes from lipid peroxidation does not seem to be the sole explanation for the effects of ALCAR and LA on improving CAT function and Km. Although the combination of ALCAR and LA lowered MDA levels and restored CAT function, the results with the individual compounds indicate a more complex model. In vivo, LA significantly lowered MDA levels, whereas ALCAR did not, yet ALCAR significantly restored CAT function, whereas LA did not. Extrapolating from in vitro experiments to in vivo conclusions, however, depends on physiological concentration and time, as both mitochondria and protein turn over, and a definitive conclusion as to mechanism is not yet possible. In vitro enzyme inactivation by aldehydes and the protective effect of the substrate ALCAR (Fig. 4C) are likely to explain the in vivo decrease with age of CAT-binding affinity and Vmax and their reversal by feeding ALCAR. Then why does LA not appreciably improve CAT function by itself, although it lowers the level of aldehydes? Our data show that LA enhanced the effect of ALCAR on Km, especially for CoA, therefore LA may increase binding affinity and enzyme function to a small extent. LA is synergistic or additive with ALCAR in a number of studies (Figs. 1 and 2). Thus, the most likely mechanism for our observations appears to be the interaction of aldehydes from lipid peroxidation with CAT and a protective effect of the substrate ALCAR, with the additional beneficial effect of LA's contribution in lowering mitochondrial lipid peroxidation.
The observed improvement of CAT activity and binding affinity by ALCAR and LA may depend on protein and mitochondrial turnover. Damaged proteins and mitochondria are turned over by proteasomal and lysosomal degradation, respectively (60). Oxidative damage, especially lipid peroxidation, may be responsible for some forms of proteasome dysfunction in the central nervous system, by blocking either substrate binding or protein modification (61). Inactivation of key metabolic enzymes by mixed-function oxidation reaction has been suggested in protein turnover and aging (62). Enzymes with aldehyde-inactivated SH groups can be reactivated by excess reduced glutathione and cysteine (57). It is possible LA may play a role in reactivating CAT and in preventing proteasomes from oxidative modification.
MDA was more potent than HNE in affecting CAT kinetics. This may be because: (i) 4-hydroxyalkenals are highly specific reagents for SH groups, although they may also modify lysine, histidine, serine, and tyrosine; and (ii) MDA can readily modify proteins under physiological conditions, although it is less reactive with free amino acids. MDA reacts primarily with lysine residues and can then form more stable intra- and intermolecular crosslinks (57). The effect of MDA on the activity and Km of CAT was reduced greatly in TBS, presumably due to the relative stable covalent binding of MDA to the amino group of Tris. The effect of HNE, unlike that of MDA, on the activity and Km of CAT was not greatly affected by TBS. In experiments with malondialdehyde, there are two possible complications whose importance has not yet been clarified: (i) when MDA is prepared from bis-acetal, small amounts of β-ethoxy or β-methoxy acrolein, highly reactive aldehydes, are unavoidably formed during acid hydrolysis (63), and a variety of similar 2-alkenals are formed during lipid peroxidation including HNE; and (ii) MDA in solution forms reactive aldol type condensation products including dimers and trimers (64), and these condensation products may also modify proteins (57).
The enzyme dysfunction induced by lipid peroxidation products such as MDA and HNE, rather than being specific for CAT, may be a common mechanism of age-associated dysfunction of enzymes with amino and sulfhydryl groups at or near their active sites. We have shown that HNE also causes a decrease in pyruvate dehydrogenase (PDH)-binding affinity for pyruvate (data not shown), confirming a study on the loss of activity of PDH by HNE (58). MDA also causes a loss of PDH activity and a decrease in binding affinity for pyruvate (data not shown).
The brain tissue of old rats showed a significant increase in iron and copper accumulation, which can cause oxidative damage by catalyzing oxidant generation and lipid peroxidation. It should be emphasized, however, that we have assayed total iron, not free redox active iron. MDA accumulates with age (Fig. 3B) in parallel to the increase in iron and copper (see Results). Ex vivo oxidation of young-rat brain with Fe(II) induced similar reduction of enzyme activity and binding affinity; in vitro oxidation of purified CAT with Fe(II) inactivated the enzyme but did not alter the binding affinity. This increase of iron is consistent with previous studies in liver and brain using the atomic absorption technique (J.L., J.-Y. Park, Q. Jiang, L. Youngman, H. Atamna, and B.N.A., unpublished work) and spectrophotometric measurements (65). Although ALCAR and/or LA did not significantly effect transition metal accumulation in these short-term studies, the possibility of chelating the labile or “free” transition metals in the brain and consequently inhibiting oxidative damage cannot be excluded, as the accumulation of metals and oxidative damage is a lifelong process. LA, in addition to its oxidant-scavenging effect, is an efficient chelator of copper (66) and iron (67) that reduces the catalytic activity of transition metals in oxidant generation reactions. A higher dose of LA (0.5%) did in fact reduce iron in old-rat brain (J. H. Suh and T. M. Hagen, personal communication). Although iron and copper accumulation with age remains plausible as a cause of the increased lipid peroxides, further studies are warranted.
This study, as well as others on the effects of ALCAR and/or LA on cognition (33) and mitochondrial functions (5, 6, 68), and studies (B.N.A., J.L., and I. Elson-Schwab, unpublished work) of the age-associated decrease in binding affinity of other brain- and memory-related enzymes and receptors suggest that a decrease in enzyme-binding affinity by oxidative damage is an important contributor to age-associated memory decline, which may be ameliorated by feeding high doses of mitochondrial enzyme substrates and antioxidants.
Acknowledgments
We thank E. Roitman for technical assistance and Jack Kirsch, Larry Marnett, and John Nides for critical comments. This work was supported by grants to B.N.A. from the Ellison Foundation, the National Institute on Aging, the Wheeler Fund of the Dean of Biology, and the National Institute of Environmental Health Sciences Center (Grant P30-ES01896), and by a National Institutes of Health/National Institute on Aging postdoctoral training grant (5 T32 AG00266–02) to D.W.K.
Abbreviations
- ALCAR
acetyl-l-carnitine
- CAT
carnitine acetyltransferase
- HNE
4hydroxynonenal
- LA
R-α-lipoic acid
- MDA
malondialdehyde
- TBS
Tris-buffered saline
References
- 1.Harman D. J Am Geriatr Soc. 1972;20:145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- 2.Harman D. Proc Natl Acad Sci USA. 1981;78:7124–7128. doi: 10.1073/pnas.78.11.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ames B N, Shigenaga M K, Hagen T M. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shigenaga M K, Hagen T M, Ames B N. Proc Natl Acad Sci USA. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hagen T M, Ingersoll R T, Lykkesfeldt J, Liu J, Wehr C M, Vinarsky V, Bartholomew J C, Ames A B. FASEB J. 1999;13:411–418. doi: 10.1096/fasebj.13.2.411. [DOI] [PubMed] [Google Scholar]
- 6.Hagen T M, Ingersoll R T, Wehr C M, Lykkesfeldt J, Vinarsky V, Bartholomew J C, Song M H, Ames B N. Proc Natl Acad Sci USA. 1998;95:9562–9566. doi: 10.1073/pnas.95.16.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagen T M, Vinarsky V, Wehr C M, Ames B N. Antioxid Redox Signal. 2000;2:473–483. doi: 10.1089/15230860050192251. [DOI] [PubMed] [Google Scholar]
- 8.Hagen T M, Yowe D L, Bartholomew J C, Wehr C M, Do K L, Park J Y, Ames B N. Proc Natl Acad Sci USA. 1997;94:3064–3069. doi: 10.1073/pnas.94.7.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hagen T M, Wehr C M, Ames B N. Ann NY Acad Sci. 1998;854:214–223. doi: 10.1111/j.1749-6632.1998.tb09904.x. [DOI] [PubMed] [Google Scholar]
- 10.Beckman K B, Ames B N. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 11.Feuers R J. Ann NY Acad Sci. 1998;854:192–201. doi: 10.1111/j.1749-6632.1998.tb09902.x. [DOI] [PubMed] [Google Scholar]
- 12.Paradies G, Ruggiero F M. Biochim Biophys Acta. 1990;1016:207–212. doi: 10.1016/0005-2728(90)90060-h. [DOI] [PubMed] [Google Scholar]
- 13.Paradies G, Ruggiero F M. Arch Biochem Biophys. 1991;284:332–337. doi: 10.1016/0003-9861(91)90304-2. [DOI] [PubMed] [Google Scholar]
- 14.Paradies G, Ruggiero F M, Dinoi P. Int J Biochem. 1992;24:783–787. doi: 10.1016/0020-711x(92)90012-p. [DOI] [PubMed] [Google Scholar]
- 15.Cox T C, Bottomley S S, Wiley J S, Bawden M J, Matthews C S, May B K. N Engl J Med. 1994;330:675–679. doi: 10.1056/NEJM199403103301004. [DOI] [PubMed] [Google Scholar]
- 16.Fenton W A, Rosenberg L E. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C, editor. II. New York: McGraw–Hill; 1995. pp. 3129–3149. [Google Scholar]
- 17.Mudd S H, Skovby F, Levy H L, Pettigrew K D, Wilcken B, Pyeritz R E, Andria G, Boers G H, Bromberg I L, Cerone R, et al. Am J Hum Genet. 1985;37:1–31. [PMC free article] [PubMed] [Google Scholar]
- 18.Ames, B. N., Elson-Schwab, I. & Silver, E. (2002) Am. J. Clin. Nutr., in press. [DOI] [PubMed]
- 19.Ames B N. Toxicol Lett. 1998;102–103:5–18. doi: 10.1016/s0378-4274(98)00269-0. [DOI] [PubMed] [Google Scholar]
- 20.Liu, J., Atamna, H., Kuratsune, H. & Ames, B. N. (2002) Ann. N.Y. Acad. Sci.959, in press. [DOI] [PubMed]
- 21.Lykkesfeldt J, Hagen T M, Vinarsky V, Ames B N. FASEB J. 1998;12:1183–1189. doi: 10.1096/fasebj.12.12.1183. [DOI] [PubMed] [Google Scholar]
- 22.Suh J H, Shigeno E T, Morrow J D, Cox B, Rocha A E, Frei B, Hagen T M. FASEB J. 2001;15:700–706. doi: 10.1096/fj.00-0176com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rebouche C J. FASEB J. 1992;6:3379–3386. [PubMed] [Google Scholar]
- 24.Maccari F, Arseni A, Chiodi P, Ramacci M T, Angelucci L. Exp Gerontol. 1990;25:127–134. doi: 10.1016/0531-5565(90)90043-2. [DOI] [PubMed] [Google Scholar]
- 25.Paradies G, Petrosillo G, Gadaleta M N, Ruggiero F M. FEBS Lett. 1999;454:207–209. doi: 10.1016/s0014-5793(99)00809-1. [DOI] [PubMed] [Google Scholar]
- 26.Paradies G, Petrosillo G, Ruggiero F M. Biochim Biophys Acta. 1997;1319:5–8. doi: 10.1016/s0005-2728(97)00012-1. [DOI] [PubMed] [Google Scholar]
- 27.Paradies G, Ruggiero F M, Petrosillo G, Gadaleta M N, Quagliariello E. FEBS Lett. 1994;350:213–215. doi: 10.1016/0014-5793(94)00763-2. [DOI] [PubMed] [Google Scholar]
- 28.Paradies G, Ruggiero F M, Petrosillo G, Gadaleta M N, Quagliariello E. Ann NY Acad Sci. 1994;717:233–243. doi: 10.1111/j.1749-6632.1994.tb12093.x. [DOI] [PubMed] [Google Scholar]
- 29.Packer L, Tritschler H J, Wessel K. Free Radical Biol Med. 1997;22:359–378. doi: 10.1016/s0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- 30.Packer L, Roy S, Sen C K. Adv Pharmacol. 1997;38:79–101. doi: 10.1016/s1054-3589(08)60980-1. [DOI] [PubMed] [Google Scholar]
- 31.McGahon B M, Martin D S, Horrobin D F, Lynch M A. Neurobiol Aging. 1999;20:655–664. doi: 10.1016/s0197-4580(99)00050-0. [DOI] [PubMed] [Google Scholar]
- 32.Hagen T M, Liu J, Lykkesfeldt J, Wehr C M, Ingersoll R T, Vinarsky V, Bartholomew J C, Ames B N. Proc Natl Acad Sci USA. 2002;99:1870–1875. doi: 10.1073/pnas.261708898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Head E, Gharib A M, Yuan W, Ingersoll R T, Hagen T M, Cotman C W, Ames B N. Proc Natl Acad Sci USA. 2002;99:2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zammit V A. Prog Lipid Res. 1999;38:199–224. doi: 10.1016/s0163-7827(99)00002-8. [DOI] [PubMed] [Google Scholar]
- 35.Bieber L L. Annu Rev Biochem. 1988;57:261–283. doi: 10.1146/annurev.bi.57.070188.001401. [DOI] [PubMed] [Google Scholar]
- 36.McCaman R E, McCaman M W, Stafford M L. J Biol Chem. 1966;241:930–934. [PubMed] [Google Scholar]
- 37.Hansford R G. Biochem J. 1978;170:285–295. doi: 10.1042/bj1700285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansford R G, Castro F. Mech Ageing Dev. 1982;19:191–200. doi: 10.1016/0047-6374(82)90010-0. [DOI] [PubMed] [Google Scholar]
- 39.Sung S C, Sandberg P R, McGeer E G. Neurochem Res. 1978;3:815–820. doi: 10.1007/BF00966003. [DOI] [PubMed] [Google Scholar]
- 40.Moret C, Pastrie I, Briley M. Neurobiol Aging. 1990;11:57–59. doi: 10.1016/0197-4580(90)90063-6. [DOI] [PubMed] [Google Scholar]
- 41.Kalaria R N, Harik S I. Ann Neurol. 1992;32:583–586. doi: 10.1002/ana.410320417. [DOI] [PubMed] [Google Scholar]
- 42.Makar T K, Cooper A J, Tofel-Grehl B, Thaler H T, Blass J P. Neurochem Res. 1995;20:705–711. doi: 10.1007/BF01705539. [DOI] [PubMed] [Google Scholar]
- 43.Maurer I, Zierz S, Moller H J. Alzheimer Dis Assoc Disord. 1998;12:71–76. doi: 10.1097/00002093-199806000-00003. [DOI] [PubMed] [Google Scholar]
- 44.DiDonato S, Rimoldi M, Moise A, Bertagnoglio B, Uziel G. Neurology. 1979;29:1578–1583. doi: 10.1212/wnl.29.12.1578. [DOI] [PubMed] [Google Scholar]
- 45.Melegh B, Seress L, Bedekovics T, Kispal G, Sumegi B, Trombitas K, Mehes K. J Inherit Metab Dis. 1999;22:827–838. doi: 10.1023/a:1005562209034. [DOI] [PubMed] [Google Scholar]
- 46.Brevetti G, Angelini C, Rosa M, Carrozzo R, Perna S, Corsi M, Matarazzo A, Marcialis A. Circulation. 1991;84:1490–1495. doi: 10.1161/01.cir.84.4.1490. [DOI] [PubMed] [Google Scholar]
- 47.Fritz I B, Schultz S K. J Biol Chem. 1965;240:2188–2192. [PubMed] [Google Scholar]
- 48.Chase J F A. Methods Enzymol. 1969;13:387–393. [Google Scholar]
- 49.Chase J F A, Tubbs P K. Biochem J. 1966;99:32–40. doi: 10.1042/bj0990032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cornish-Bowden A, Wharton C W. Enzyme Kinetics. Oxford: IRL; 1988. [Google Scholar]
- 51.Liu J, Yeo H C, Doniger S J, Ames B N. Anal Biochem. 1997;245:161–166. doi: 10.1006/abio.1996.9990. [DOI] [PubMed] [Google Scholar]
- 52.Yeo H C, Liu J, Helbock H J, Ames B N. Methods Enzymol. 1999;300:70–78. doi: 10.1016/s0076-6879(99)00115-9. [DOI] [PubMed] [Google Scholar]
- 53.Humphries K M, Yoo Y, Szweda L I. Biochemistry. 1998;37:552–557. doi: 10.1021/bi971958i. [DOI] [PubMed] [Google Scholar]
- 54.Killilea D W, Armstrong G, Ames B N. Free Radical Biol Med. 2001;31:S33. [Google Scholar]
- 55.Verbanac D, Milin C, Domitrovic R, Giacometti J, Pantovic R, Ciganj Z. Biol Trace Elem Res. 1997;57:91–96. doi: 10.1007/BF02803873. [DOI] [PubMed] [Google Scholar]
- 56.Alhomida A S. Biochem Mol Biol Int. 1996;39:923–931. doi: 10.1080/15216549600201072. [DOI] [PubMed] [Google Scholar]
- 57.Esterbauer H, Schaur R J, Zollner H. Free Radical Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 58.Humphries K M, Szweda L I. Biochemistry. 1998;37:15835–15841. doi: 10.1021/bi981512h. [DOI] [PubMed] [Google Scholar]
- 59.Yeo H C, Helbock H J, Chyu D W, Ames B N. Anal Biochem. 1994;220:391–396. doi: 10.1006/abio.1994.1355. [DOI] [PubMed] [Google Scholar]
- 60.Terman A. Redox Rep. 2001;6:15–26. doi: 10.1179/135100001101535996. [DOI] [PubMed] [Google Scholar]
- 61.Ding Q, Keller J N. Free Radical Biol Med. 2001;31:574–584. doi: 10.1016/s0891-5849(01)00635-9. [DOI] [PubMed] [Google Scholar]
- 62.Fucci L, Oliver C N, Coon M J, Stadtman E R. Proc Natl Acad Sci USA. 1983;80:1521–1525. doi: 10.1073/pnas.80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marnett L J, Tuttle M A. Cancer Res. 1980;40:276–282. [PubMed] [Google Scholar]
- 64.Golding B T, Patel N, Watson W P. J Chem Soc Perkin Trans. 1989;1:668–669. [Google Scholar]
- 65.Cook C I, Yu B P. Mech Ageing Dev. 1998;102:1–13. doi: 10.1016/s0047-6374(98)00005-0. [DOI] [PubMed] [Google Scholar]
- 66.Ou P, Tritschler H J, Wolff S P. Biochem Pharmacol. 1995;50:123–126. doi: 10.1016/0006-2952(95)00116-h. [DOI] [PubMed] [Google Scholar]
- 67.Persson H L, Svensson A I, Brunk U T. Redox Report. 2001;6:327–334. doi: 10.1179/135100001101536472. [DOI] [PubMed] [Google Scholar]
- 68.Paradies G, Ruggiero F M, Gadaleta M N, Quagliariello E. Biochim Biophys Acta. 1992;1103:324–326. doi: 10.1016/0005-2736(92)90103-s. [DOI] [PubMed] [Google Scholar]