Abstract
Sperm of the Pacific herring, Clupea pallasi, are unique in that they are immotile upon spawning in the environment. Herring sperm have evolved to remain motionless for up to several days after spawning, yet are still capable of fertilizing eggs. An egg chorion ligand termed “sperm motility initiation factor” (SMIF) induces motility in herring sperm and is required for fertilization. In this study, we show that SMIF induces calcium influx, sodium efflux, and a membrane depolarization in herring sperm. Sperm motility initiation by SMIF depended on decreased extracellular sodium (<350 mM) and could be induced in the absence of SMIF in very low sodium seawater. Motility initiation depended on ≥ 1 mM extracellular calcium. Calcium influx caused by SMIF involved both the opening of voltage-gated calcium channels and reverse sodium–calcium (Na+/Ca2+) exchange. Membrane depolarization was slightly inhibited by a calcium channel blocker and markedly inhibited by a Na+/Ca2+ exchange inhibitor. Sodium efflux caused by SMIF-initiated motility was observed when using both extracellular and intracellular sodium probes. A Na+/Ca2+ exchange antigen was shown to be present on the surface of the sperm, primarily over the midpiece, by using an antibody to the canine Na+/Ca2+ exchanger. This antibody recognized a 120-kDa protein that comigrated with the canine myocyte Na+/Ca2+ exchanger. Sperm of Pacific herring are now shown to use reverse Na+/Ca2+ exchange in motility initiation. This mechanism of regulation of motility initiation may have evolved for both maintenance of immotility after spawning as well as ligand-induced motility initiation.
Teleost fish sperm are quiescent within the testes and seminal plasma before spawning, but most initiate motility after dilution into the external medium (freshwater or seawater for most species) in which spawning occurs (1). In salmonids, motility initiation occurs with dilution in freshwater, specifically from a reduction in extracellular potassium that drives a membrane hyperpolarization and an increase in intracellular calcium ([Ca2+]i) (2–6). Changes in the concentrations of specific ions (Ca2+, K+, and possibly Na+ and Cl−) also have been linked to motility initiation in Atlantic croaker sperm (7). A hyperpolarization of the sperm membrane also has been documented in carp sperm, reportedly linked to the opening of voltage-gated Ca2+ channels and an increase in [Ca2+]i (8). In other freshwater teleost sperm (goldfish, zebra fish), as well as in marine teleost sperm (puffer, flounder), motility is believed to occur as a result of nonspecific hypo- or hyperosmotic changes that drive changes in intracellular ion concentrations (5, 9, 10). Thus, in all systems studied to date, a membrane hyperpolarization leads to an increase in [Ca2+]i and the initiation of motility.
Although the stimulation of sperm motility in the vicinity of eggs has been reported in some teleost fish, only herring sperm have been shown to require an egg chorion-derived ligand for initiation of motility. Upon spawning, herring sperm (Clupea pallasi) are intrinsically motionless over a wide range of salinities (11–14). This immotility may be related to the unique reproductive biology of herring in which male fish spawn first, with sperm remaining in the water column for extended periods, followed by the spawning of females (15). Herring sperm motility is initiated in the presence of sperm motility initiation factor (SMIF), a 105-kDa basic glycoprotein that is localized to the micropylar region of the herring egg (16, 17). Although nondiffusible in vivo, SMIF can be extracted in acid seawater, and soluble SMIF retains its motility-initiating properties. Both in vivo (around the chorion) and in vitro sperm motility is reduced at salinities < 8 parts per thousand (ppt) or > 24 ppt (14, 18, 19). This range of salinities correlates with the optimal salinity regime for fertilization and early development in Pacific herring (14). Previous studies have shown that extracellular Na+ ([Na+]o) and extracellular Ca2+ ([Ca2+]o) influence both sperm motility and fertilization (11–14, 18). Sperm motility can be initiated in the absence of SMIF by low-Na+ (2 mM) seawater (NaF; ref. 13); both SMIF-induced and NaF-induced motility initiation require the presence of [Ca2+]o (minimum of 1 mM). Herring sperm motility also can be initiated by herring sperm activating peptides (HSAPs) that rapidly diffuse away from the eggs at spawning (19–21).
The requirement for decreased [Na+]o and the presence of [Ca2+]o suggest that a sodium–calcium (Na+/Ca2+) exchange could occur during ligand-induced herring sperm motility initiation. In this study, we make the finding that there is an efflux of Na+ and an influx of Ca2+ during ligand-induced motility initiation in herring sperm and this movement of ions is caused by reverse-Na+/Ca2+ exchange. We present evidence for the presence of a Na+/Ca2+ exchanger on the sperm surface. We also show that voltage-sensitive Ca2+ channels participate in motility initiation.
Materials and Methods
Solutions and Animals.
Fluo-3 acetoxymethyl ester (AM), sodium green cell permeant (NaGi) and impermeant (NaGo), 2′,4′-dichlorobenzamil hydrochloride, 3,3′-dipropylthiacarbocyanine iodide [DiSC3(5)], 20% pluronic F-127 in DMSO, and goat anti-rabbit Alexa 488 were obtained from Molecular Probes. KB-R7943 mesylate was obtained from Tocris (Ballwin, MO). Nifedipine was obtained from Alamone Laboratories (Jerusalem, Israel). PAGE gels were obtained from Fisher Scientific. Nitrocellulose, Tris⋅HCl, glycine, and SDS were obtained from Bio-Rad. SuperSignal chemiluminescent substrate and Gel-Code blue stain reagent were obtained from Pierce. Bepridil, flunarizine, carbonyl cyanide m-chlorophenylhydrazone (CCCP), DMSO, protease inhibitor mixture, goat anti-rabbit IgG conjugated to horseradish peroxidase, and all other dry chemicals were obtained from Sigma. Rabbit anti-Na+/Ca2+ exchanger protein was obtained from Research Diagnostics, (Flanders, NJ).
Filtered seawater (FSW) was prepared by using 0.45 μm Nalgene filters and diluted with distilled water to prepare 1/2 FSW (approximately 16 ppt). Artificial seawaters were prepared according to Cavanaugh (22). Choline chloride was substituted for Na+ in NaF and 5 mM EDTA or EGTA was added to prepare Ca-free seawater (CaF), NaCa-free seawater (NaCaF), and CaMg-free seawater (CaMgF). Herring Ringer's (HR) solution (206 mM NaCl/7.2 mM KCl/2.1 mM CaCl2/3.1 mM MgCl26H2O, pH adjusted to 7.6 with 1 M NaHCO3) was prepared as per Yanagimachi (11, 12).
Mature Pacific herring were caught by otter trawl in San Francisco Bay by the California Department of Fish and Game and transported on ice to the Bodega Marine Laboratory. Testis and ovaries were dissected and stored in individual culture plates at 4°C under moist conditions (13). Some experiments were conducted at the Hokkaido National Fisheries Research Center by using animals caught at Akkeshi Lake, Hokkaido, Japan.
Isolation of SMIF.
Herring eggs were suspended and washed three to five times in 1/2 CaMgF containing 0.25% polyvinyl alcohol at 4°C. Eggs were disrupted in 1/2 CaMgF with a dounce homogenizer, the chorions were allowed to settle out of solution and were washed free of egg cytoplasm with four to five exchanges of 1/2 CaMgF, followed by washing with distilled H2O (16, 17). Chorions either were used immediately or lyophilized and stored at −70°C. SMIF was isolated by suspending the chorions in 1/2 artificial seawater (pH 3.5) at 4°C for 30 min with periodic homogenization. The homogenate was centrifuged at 12,000 × g for 15 min; the supernatant pH was adjusted to pH 7.8 and concentrated by using 10-kDa molecular mass centricon microconcentrators (Amicon). The retentate, SMIF, was used immediately or stored at −70°C. The lowest dilution that yielded >75% sperm motility (4+ motility) was used in experiments; this was typically 20–50 μg/ml protein.
Evaluation of Sperm Motility.
Sperm motility was assessed with either a 10× or 20× objective lens by using the following qualitative index: 0 = no motility, 1+ = <25% motility, 2+ = 25–50% motility, 3+ = 50–75% motility, 4+ = >75% motility (13, 14, 16). Sperm motility patterns were recorded by using NIH IMAGE v.1.61 at 20 frames/sec on a Dage-MTI CCD camera (Dage-MTI, Michigan City, IN) connected to a Scion Frame Grabber on a Macintosh computer. Frame averaging (8 frames/sec) enabled sperm tracks to be recorded as digital images.
Measurement of Intracellular Calcium.
Sperm (107 per ml) in HR were loaded with Fluo-3 AM (5 μM) for 1 h at 13°C, centrifuged at 920 × g for 5 min each through HR/10% Ficoll and HR, resuspended in fresh HR, and placed in cuvettes containing 1/2 FSW, 1/2 CaF, or 1/2 NaF. A PTI fluorescence spectrophotometer (Photon Technology International, Lawrenceville, NJ; excitation 506, emission 526, slit width 5 nm) was used for bulk measurements of [Ca2+]i. After baseline stabilization, SMIF or a comparable volume of 1/2 FSW was added to the cuvettes and fluorescence recorded. For sperm suspended in 1/2 CaF, Ca2+ (1 mM final) was added after SMIF addition. [Ca2+]i was calculated by using the equation [Ca2+]i = (F − Fmin)/(Fmax − F)Kd, where Fmax = fluorescence in a sample after addition of 50 μM digitonin, Fmin = fluorescence in 0.5 M EGTA, pH 8.5 in 1 M Tris⋅HCl, F = sample response, and Kd = dissociation constant for Fluo-3 (390 nM) (23). In some experiments, the Ca2+ channel blockers nifedipine (100 μM) or flunarizine (10 μM) or the Na+/Ca2+ exchange inhibitors bepridil (50 μM), dichlorobenzamil (10 μM), or KB-R7943 (10 μM) were preincubated (5 min) with sperm before Ca2+i measurements.
A scanning laser confocal microscope (Bio-Rad MRC-600 on an Olympus BH-2 microscope) was used to localize [Ca2+]i. Sperm loaded with Fluo-3 were placed on a Peltier-cooled stage (12°C) and viewed through a 60× fluorescence objective lens. Optical sections (0.5 μm) were collected with MRC-600 software.
Measurement of Sodium Efflux.
Efflux of Na+ was measured as a decrease in intracellular Na+ ([Na+]i) by using the permeant intracellular Na+ probe NaGi, or as an increase in [Na+]o by using cell impermeant NaGo. To measure [Na+]i, sperm (108 per ml) were loaded with 5 μM NaGi for 2 h at 13°C, centrifuged at 920 × g in HR and resuspended in fresh HR. Loaded sperm were suspended in 1/2 FSW or 1/2 FSW (final, 106 per ml) containing SMIF. [Na+]i was monitored at excitation 507 and emission 532. Calibration of the response to SMIF was not possible with NaGi because fluorescence is not linear at physiologically relevant salinities for herring sperm (i.e., 220 mM Na+o). Thus, changes in [Na+]i were represented as arbitrary fluorescence units.
Na+ efflux was measured as an increase in NaGo, at excitation 507 and emission 532. Immotile sperm (106 per ml) were suspended in 1/2 NaCaF to which 5 μM NaGo was added. After baseline stabilization, the change in fluorescence was recorded after sperm activation with the addition of Ca2+ (5 mM final). A comparable volume of 1/2 NaCaF was added to the control. In some experiments, sperm were preincubated with flunarizine (20 μM), bepridil (10 μM), or DMSO (solvent control) for 5 min before measurements. The concentration of Na+ was calculated by using a standard curve constructed from known concentrations of Na+ in 1/2 NaCaF.
Measurement of Membrane Potential.
Membrane potential was measured with DiSC3(5) (24) by using a fluorescence spectrophotometer at 620 nm excitation and 670 nm emission (slit width 5 nm) at 13°C. To reduce the contribution of mitochondrial membrane potential to the DiSC3(5) emission spectra, the mitochondrial uncoupling agent CCCP (0.5 μM) was used. Sperm (106 per ml) were suspended in 1/2 FSW with or without nifedipine (50 μM) or bepridil (20 μM), followed by the addition of 0.5 μM DiSC3(5) and CCCP. After baseline stabilization, SMIF or a comparable volume of 1/2 FSW was added to the suspensions, and the change in fluorescence was recorded.
Immunolocalization.
Live sperm were washed in HR, incubated in the IgG fraction of an anti-canine Na+/Ca2+ exchange antibody for 1 h at room temperature (RT), followed by centrifugation through PBS containing 10% (vol/vol) Ficoll (1×) and PBS (2×). Sperm then were fixed for 15 min in 3% (vol/vol) paraformaldehyde, washed two times in PBS, and resuspended in blocking solution (PBS containing 10 mg/ml BSA). Some samples were fixed before incubation with the antibody and then permeabilized by using Triton X-100 (0.5%) for 1 h at RT, followed by incubation with PBS with BSA and primary IgG. This procedure facilitated antibody access inside of cells. After primary antibody, sperm were centrifuged at 920 × g for 5 min in blocking solution, incubated in blocking solution for 30 min at RT, and then incubated in normal goat serum for 30 min. Secondary antibody (goat anti-rabbit Alexa 488) was added for 1 h followed by washing in PBS. Sperm were resuspended in mounting medium (0.04 M N-propyl gallate in 10% PBS/90% glycerol) and viewed with an Olympus BH-2 microscope equipped with a laser scanning confocal system using a 60× oil immersion fluorescence objective. Control sperm consisted of suspensions labeled with secondary antibody only.
Extraction of Sperm Samples.
Sperm (106 per ml) were washed once in HR containing a protease inhibitor mixture at 4°C, incubated in TBS containing protease inhibitor mixture for 15 min on ice, centrifuged at 920 × g, and incubated with TBS/protease inhibitor mixture/1% Triton X-100 for 1 h on ice. Sperm were centrifuged at 3,500 × g for 10 min, and the supernatant was aliquoted and stored at −70°C. For a positive control, canine cardiac tissue was homogenized in TBS/protease inhibitor mixture, extracted as for herring sperm, centrifuged, and the supernatant was aliquoted and stored as above.
Electrophoresis and Immunoblotting.
Extracts of herring sperm and canine cardiac tissue were solubilized in reducing buffer (0.3 M Tris/5% SDS/50% glycerol, pH 6.8 containing 5% βmercaptoethanol) and boiled for 3 min. Samples were electrophoresed using precast 10% Tris-glycine gels and either stained for protein using Gel-Code blue or transferred to 0.22 μm nitrocellulose membranes. The membranes were blocked overnight at 4°C in blocking buffer (10 mM Tris⋅HCl/100 mM NaCl/4% nonfat milk). The membranes were incubated with rabbit anti-Na+/Ca2+ exchange protein and washed several times with TBS containing 0.1% Tween-20 (TBST). The membranes then were incubated with goat anti-rabbit HRP, washed several times in TBST, and the proteins were visualized using chemiluminescence.
Results
Herring sperm were immotile in 1/2 FSW and HR, but initiated motility in 1/2 NaF in the absence of SMIF as has been reported (13). Motility in 1/2 NaF was significantly reduced (P < 0.05) in 25 mM [Na+]o and completely inhibited in 150 mM [Na+]o (Fig 1A). SMIF-induced motility was significantly reduced by 350 mM [Na+]o (P < 0.05) and completely blocked in 500 mM [Na+]o (Fig. 1B). Both 1/2 NaF- and SMIF-induced motility depended on [Ca2+]o, requiring a minimum of 1 mM (summarized in Table 1). SMIF-induced motility was immediately blocked by the calcium channel blockers nifedipine (10 μM) and flunarizine (10 μM) and by the Na+/Ca2+ exchange inhibitors bepridil (10 μM) and dichlorobenzamil (10 μM). KB-R7943 (10 μM), a specific reverse Na+/Ca2+ exchange inhibitor (25), inhibited SMIF-induced motility and stopped all motility within 40 s when added to motile sperm in the presence of SMIF.
Figure 1.
Effect of [Na+]o on motility initiation. Motility was assessed as follows: 0, no motility; 1+, <25% motility; 2+, 25–50% motility; 3+, 50–75% motility; 4+, >75% motility. (A) Motility initiation in 1/2 NaF is inhibited with increasing [Na+]o. At >25 mM [Na+]o, motility was significantly inhibited (P < 0.05). (B) SMIF-induced motility was inhibited by increasing [Na+]o. A significant reduction in motility was observed at [Na+]o ≥ 350 mM (P < 0.05). Note that a higher concentration of Na+ was required to inhibit SMIF-induced motility vs. 1/2 NaF-induced motility.
Table 1.
Summary of the effects of extracellular ions on sperm motility initiation in the absence of SMIF
| Treatment | Motility index |
|---|---|
| 1/2 FSW | 0 |
| 1/2 NaF | 3–4 |
| 1/2 NaCaF | 0 |
| 1/2 NaCaF + 0.1–0.5 mM Ca2+ | 0 |
| 1/2 NaCaF + 1–10 mM Ca2+ | 3–4 |
| 1/2 CaF + 1–10 mM Ca2+ | 0 |
Sperm motility was initiated in 1/2 NaF, but only in the presence of at least 1 mm Ca2+. Motility index is defined as follows: 0, no motility; 1+, <25% motility; 2+, 25–50% motility; 3+, 50–75% motility; 4+, >75% motility.
SMIF and 1/2 NaF induced different motility patterns in sperm. Fig. 2 depicts these patterns captured through frame-averaging video. Sperm were immotile in 1/2 FSW (Fig. 2A), whereas a linear motility pattern was initiated in 1/2 NaF (Fig. 2B), and SMIF-induced motility was circular (Fig. 2C). The differences in trajectories corresponded to different changes in [Ca2+]i (see below).
Figure 2.
Images of herring sperm motility patterns visualized by using darkfield optics and recorded with NIH IMAGE after frame averaging (8 frames per sec) through a DSP 200 image enhancement system (Dage-MTI). (A) Sperm in 1/2 FSW were immotile as indicated by discrete foci. (B) Sperm in 1/2 NaF exhibited a linear trajectory as indicated by straight lines. (C) Sperm in 1/2 FSW containing SMIF exhibited a circular pattern of motility.
Addition of SMIF to sperm in 1/2 seawater induced both motility and an immediate increase in [Ca2+]i (Δ = 241 ± 71 nM, n = 10) (Fig. 3A). Both depended on a minimum of 1 mM [Ca2+]o. Sperm suspended in 1/2 NaF underwent a gradual increase in [Ca2+]i (Δ = 90 ± 27 nM) that never reached the maximal level attained by sperm in SMIF (Fig. 3A). Laser scanning confocal microscope images revealed that there was a global increase in fluorescence (increase in [Ca2+]i) in response to SMIF, over the midpiece and head regions of the sperm (Fig. 3B). The SMIF-induced [Ca2+]i increase was inhibited by nifedipine (90% inhibition at 100 μM), flunarizine (98% inhibition at 10 μM), bepridil (96% inhibition at 50 μM), dichlorobenzamil (97% inhibition at 10 μM), and KB-R7943 (99% inhibition at 10 μM; Fig. 4).
Figure 3.
A [Ca2+]i increase occurs at sperm motility. (A) An immediate large increase in [Ca2+]i (Δ = 241 nM) was observed in sperm suspended in 1/2 FSW to which SMIF (black arrow) was added. The SMIF-induced increase did not occur when Ca2+ was absent from the extracellular medium, but was observed upon addition of Ca2+ to the medium (white arrow). In the absence of SMIF, a gradual increase in [Ca2+]i was observed when sperm were suspended in 1/2 NaF seawater. Note that the increase in [Ca2+]i observed in 1/2 NaF (Δ = 90 nM) was lower than that for SMIF. No change in [Ca2+]i was seen when 1/2 FSW was added as a control. (B) Micrographs of Fluo-3-loaded sperm collected on a laser scanning confocal microscope on a cooled stage. (Left and Center Right) Transmitted light image. (Center Left and Right) Fluo-3-labeled sperm. Fluo-3 fluorescence was adjusted by reducing the background level of fluorescence signal of sperm in 1/2 FSW and then recording changes after SMIF addition at the same instrument settings. Sperm exhibited an increase in fluorescence (increase in [Ca2+]i) in response to SMIF, which was localized to the head and midpiece region of the sperm.
Figure 4.
Effect of inhibitors on the SMIF-induced increase in [Ca2+]i. Sperm loaded with Fluo-3 AM were preincubated with calcium channel blockers (flunarizine, 10 μM; nifedipine, 100 μM), or Na+/Ca2+ exchange inhibitors (bepridil, 50 μM; dichlorobenzamil, 10 μM; KB-R7943,10 μM). After baseline stabilization, SMIF was added to the sperm suspensions. The SMIF-induced increase in [Ca2+]i was significantly inhibited by both calcium channel blockers and Na+/Ca2+ exchange inhibitors (n = 3; P < 0.05).
The addition of SMIF to sperm preincubated with NaGi in 1/2 FSW resulted in a 12% decrease in fluorescence intensity (n = 9, P < 0.05), which translates to a decrease in [Na+]i (Fig. 5). Similar results were obtained using NaGo to measure Na+ in the extracellular medium at motility initiation, with an increase in [Na+]o (Δ = 0.59 mM), when 5 mM Ca2+ (final concentration) was added to sperm in 1/2 NaCaF, indicating an efflux of Na+ from sperm at initiation of motility in 1/2 NaF (Fig. 6). This efflux was partially inhibited by 20 μM flunarizine (51% inhibition) and completely blocked by bepridil (10 μM).
Figure 5.
Change in [Na+]i in response to SMIF. Sperm were loaded with cell permeant NaGi and were suspended in cuvettes containing 1/2 FSW; baseline fluorescence readings were taken before SMIF was added. SMIF produced a decrease in fluorescence intensity (n = 9), signifying a depolarization event. This decrease (12%) was significantly greater (P < 0.05) than that observed when 1/2 FSW alone was added to sperm suspensions (1% decrease).
Figure 6.
Efflux of Na+ from sperm in response to Ca2+. Sperm were suspended in 1/2 NaCaF containing the cell impermeant Na+ probe NaGo. After establishing a baseline fluorescence, Ca2+ (5 mM final) was added to the medium. An increase in fluorescence occurred, signifying an efflux of Na+ (Δ = 0.59 mM) from sperm (n = 3). This efflux was blocked by bepridil (100% with 10 μM) and partially inhibited by flunarazine (51% with 20 μM).
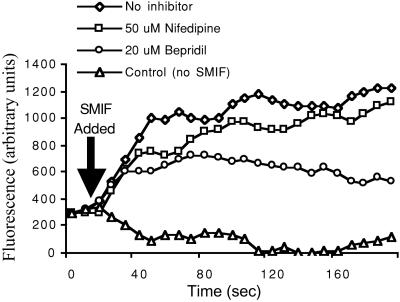
SMIF induced a depolarization of the sperm membrane (increase in fluorescence) as measured by DiSC3(5) (Fig. 7). The depolarization response was inhibited by bepridil (20 μM; 67% inhibition) and slightly reduced by nifedipine (50 μM; 6% inhibition). Sperm motility was not affected by the presence of DiSC3(5).
Figure 7.
Effect of SMIF on membrane potential. Sperm were suspended in 1/2 FSW or preincubated with 20 μM bepridil or 50 μM nifedipine for 5 min, followed by the addition of DiSC3(5) and CCCP (not shown). Upon addition of SMIF (arrow), a depolarization was observed (increase in fluorescence intensity) which was inhibited slightly by nifedipine (6%) and more so by bepridil (67%). No change in membrane potential was observed in sperm to which 1/2 FSW was added.
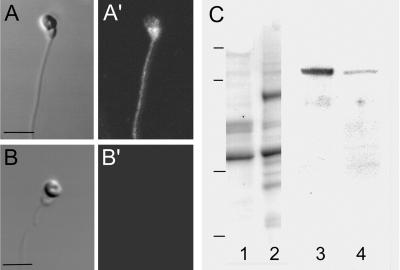
The presence of a Na+/Ca2+ exchange antigen associated with the surface of herring sperm was demonstrated using a polyclonal anti-canine Na+/Ca2+ exchange antibody. Labeling of live sperm was most intense in the midpiece region, with labeling over the head and flagellum as well (Fig. 8A′). Permeabilized (0.5% Triton X-100 after paraformaldehyde fixation) sperm exhibited the same labeling pattern, indicating there was not additional intracellular antigen. Further confirmation that labeling was surface associated rather than cytoplasmic came from collections of Z-series (0.25 μm) images (not shown). Control sperm (incubated in secondary antibody alone) exhibited no labeling (Fig. 8B′). Immunoblots of electrophoretically separated sperm polypeptides demonstrated that herring sperm possess a 120-kDa polypeptide that was recognized by the anti-Na+/Ca2+ exchanger antibody and comigrated with the exchanger from canine myocytes (Fig. 8C).
Figure 8.
Detection of the Na+/Ca2+ exchanger in whole sperm and in extracted sperm proteins. (A and A′) Live sperm that were labeled with anti-Na+/Ca2+ exchange IgG exhibited label over the entire sperm with the most intense signal over the midpiece region. (Bar = 2 μm.) (B and B′) Control sperm incubated with secondary antibody alone exhibited no fluorescence signal. (A and B) Transmitted light microscope image. (A′ and B′) Confocal fluorescent image. (C) Immunoblotting of Na+/Ca2+ exchange protein. Triton X-100 extracted sperm proteins (lanes 1, 3), and canine myocytes (lanes 2, 4) were visualized for protein (lanes 1, 2) after electrophoresis or were transferred to nitrocellulose and probed with anti Na+/Ca2+ exchange IgG and visualized using chemiluminescence detection (lanes 3, 4). A band at 120 kDa was observed for both extracts that corresponds to the known molecular mass of the exchanger in canine myocytes. Molecular mass standards: 126, 90, 43.5, 33.9 kDa.
Discussion
In herring sperm, the initiation of motility is in response to contact with a glycoprotein component of the egg chorion, SMIF. Ionic changes that accompany motility include an increase in [Ca2+]i and a decrease in [Na+]i. This study has shown that the movements of these ions are associated with a depolarization of the sperm membrane and the opening of voltage-sensitive Ca2+ channels, as well as the activity of a Na+/Ca2+ exchanger operating in reverse (the Ca2+-influx mode). We suggest that both mechanisms operate to elevate [Ca2+]i and thus initiate sperm motility in herring. Na+/Ca2+-exchange mechanisms have been extensively investigated in a number of species and in a variety of tissue types (26). In most systems, the exchanger operates to pump Ca2+ out of the cell. Reverse Na+/Ca2+ exchange, however, has been described in mammalian smooth muscle, cardiac myocytes, canine and ferret red blood cells, pancreatic B cells, barnacle muscle cells, and squid axons (26–29). Although the presence of a Na+/Ca2+ exchange mechanism in bovine sperm plasma membrane vesicles has been suggested (30), the present study demonstrates the presence of such an exchanger on the surface of intact sperm. Not only does the Na+/Ca2+ exchanger in herring sperm comigrate with the canine myocyte polypeptide at 120 kDa, a 70-kDa band was recognized by the antibody in some preparations, which corresponds to a proteolytic degradation product or subunit of the exchanger (31, 32). Interestingly, the Na+/Ca2+ exchanger localization studies produced similar binding patterns as a study (17) that localized SMIF-binding sites over the entire sperm surface, with the majority of label over the midpiece region.
A membrane depolarization was associated with herring sperm motility initiation. In barnacle muscle fibers a membrane depolarization seems to favor Ca2+ influx by means of reverse Na+/Ca2+ exchange (33). Elevated [Na+]o inhibits reverse Na+/Ca2+ exchange in squid axons and some mammalian tissues (26, 34, 35), observations consistent with what was observed in herring sperm.
Ion deletion experiments and studies using Ca2+ channel inhibitors point to the second mechanism, a role for voltage-sensitive Ca2+ channels in herring sperm motility initiation. Krasznai et al. (8) demonstrated that the activation of voltage-gated calcium channels is required in carp sperm for motility initiation; this activation depends upon membrane hyperpolarization through opening of K+ channels and a K+ efflux. In other teleosts, there has been debate over the source of the Ca2+ and thus the need for Ca2+ channels during the [Ca2+]i increase at motility initiation (36). Although some studies have suggested that Ca2+ from intracellular stores may be sufficient for motility initiation (36) or may participate in the initiation of motility as has been described in puffer fish (9), most studies have suggested that it is the influx of external Ca2+ after membrane hyperpolarization that is required for motility (5, 8). In herring sperm, it is clear that SMIF-induced depolarization activates voltage sensitive Ca2+ channels, and that external Ca2+ (≥ 1 mM) is required. Although it is possible that herring sperm motility initiation, maintenance, and the accompanying increase in [Ca2+]i, could involve store-operated Ca2+ channels, the presence of such channels in teleost fish sperm has not been investigated. Store-operated Ca2+ channels have been shown to function in the sperm acrosome reaction in mammals and sea urchins (37, 38), but teleost fish sperm lack acrosomes and it is unclear whether such channels would be involved in motility.
A link between increased [Ca2+]i through voltage-gated Ca2+ channels and enhancement of the Na+/Ca2+ exchange-associated increase in [Ca2+]i also has been observed in squid giant axons (34, 35), and inhibition of calcium channels may indirectly impact the overall activity of the Na+/Ca2+ exchanger in this system (34). This also may be the case for herring sperm, because influx of Ca2+ and efflux of Na+ are inhibited by voltage-gated calcium channel inhibitors as well as Na+/Ca2+ exchange inhibitors. The patterns of motility in herring sperm are a function of the induction method and are related to the different levels of [Ca2+]i. The increase in [Ca2+]i under SMIF induction, which elicits circular motility, was 75% greater than that observed with 1/2 NaF induction, which results in more linear motility. Differences in motility patterns are the result of [Ca2+]i-associated flagellar bending, which becomes more asymmetric (circular motility) as [Ca2+]i increases (7, 36, 39, 40).
We have observed another egg-derived inducer of motility in herring sperm, HSAPs, which induce a linear motility pattern (19). Thus, two inducers, two motility patterns, and two mechanisms for Ca2+i increase exist in herring sperm. A change from a linear to a more circular trajectory near the micropyle vestibule (where SMIF is localized) would increase the likelihood of a sperm entering the micropylar canal to fertilize the egg. The duration of sperm motility, once initiated, suggests another role for the Na+/Ca2+ exchanger. Herring sperm can remain motile in the presence of SMIF or an egg micropyle for more than 60 min in 1/2 FSW (13). Because Na+/Ca2+ exchangers can change direction depending on intracellular ionic environments (26), it seems plausible that motility initiation by means of reverse Na+/Ca2+ exchange is followed by regulation of [Ca2+]i via alternation of forward and reverse Na+/Ca2+. Because high [Ca2+]i inhibits axonemal bending (41), this would provide a mechanism for maintaining [Ca2+]i at levels that permit motility for extended periods.
This study identifies a sperm-surface Na+/Ca2+ exchange mechanism that regulates motility initiation. It is likely that this mechanism has evolved in herring sperm for regulation of motility initiation so sperm can remain immotile (up to several days in the water column) until contact with the egg ligand. Because fertilization in herring occurs in a range near half-strength seawater in the environment (14), the requirement for a decrease in extracellular Na+ can be directly related to the efflux of Na+ against the Na+ gradient at motility initiation. This efflux of Na+ is probably energy-dependent, as has been shown in other systems where reverse Na+/Ca2+ exchange occurs (26). We suggest that immotility in herring sperm is maintained by the Na+/Ca2+ exchanger under salinity extremes. Even when salinity in the environment is very low, where extracellular Na+ would be low enough for motility initiation to occur in the absence of an inducer, herring sperm probably remain immotile because the extracellular Ca2+ would be below the required level of 1 mM. Thus, reverse Na+/Ca2+ exchange in herring sperm functions as a regulator of immotility as well as of ligand-induced motility initiation.
Acknowledgments
We thank Ken Oda, Diana Watters, Eric Larson, and Tom Moore of the California Department of Fish and Game for providing experimental animals and Dr. Takahiro Matsubara and the staff of the National Fisheries Research Institute, Kushiro, Hokkaido, Japan for providing animals and laboratory space for some investigations. This research was supported by National Science Foundation Grant IBN-9904711 and by a National Science Foundation International Travel Supplement. Partial support came from the University of California Toxic Substances Research and Teaching Program (to C.A.V.) and from the Bodega Marine Laboratory's Distinguished Research Fellow Program (to M. M.). This paper is Bodega Marine Laboratory Contribution No. 2156.
Abbreviations
- [Ca2+]i
intracellular calcium
- SMIF
sperm motility initiation factor
- ppt
parts per thousand
- Na+/Ca2+
sodium–calcium exchange
- [Na+]o
extracellular sodium
- [Ca2+]o
extracellular calcium
- NaF
low sodium seawater
- NaGi
sodium green cell permeant
- NaGo
sodium green impermeant
- DiSC3(5)
3,3′-dipropylthiacarbocyanine iodide
- FSW
filtered seawater
- HR
herring Ringer's solution
- CaF
calcium-free seawater
- NaCaF
sodium-calcium free sweater
- [Na+]i
intracellular sodium
References
- 1.Morisawa M. Zool Sci. 1994;11:647–662. [PubMed] [Google Scholar]
- 2.Gatti J-L, Billard R, Christen R. J Cell Physiol. 1990;143:546–554. doi: 10.1002/jcp.1041430320. [DOI] [PubMed] [Google Scholar]
- 3.Boitano S, Omoto C K. J Cell Sci. 1991;98:343–349. doi: 10.1242/jcs.98.3.343. [DOI] [PubMed] [Google Scholar]
- 4.Tanimoto S, Kudo Y, Nakazawa T, Morisawa M. Mol Reprod Dev. 1994;39:409–414. doi: 10.1002/mrd.1080390409. [DOI] [PubMed] [Google Scholar]
- 5.Darszon A, Labarca P, Nishigaki T, Espinosa F. Physiol Rev. 1999;79:481–510. doi: 10.1152/physrev.1999.79.2.481. [DOI] [PubMed] [Google Scholar]
- 6.Kho K H, Tanimoto S, Inaba K, Oka Y, Morisawa M. Zool Sci. 2001;18:919–928. [Google Scholar]
- 7.Detweiler C, Thomas P. J Exp Zool. 1998;281:139–148. [PubMed] [Google Scholar]
- 8.Krasznai Z, Marian T, Izumi H, Damjanovich S, Balkay L, Tron L, Morisawa M. Proc Nat Acad Sci. USA. 2000;97:2052–2057. doi: 10.1073/pnas.040558097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oda S, Morisawa M. Cell Motil Cytoskeleton. 1993;25:171–178. doi: 10.1002/cm.970250206. [DOI] [PubMed] [Google Scholar]
- 10.Takai H, Morisawa M. J Cell Sci. 1995;108:1175–1181. doi: 10.1242/jcs.108.3.1175. [DOI] [PubMed] [Google Scholar]
- 11.Yanagimachi R. Annot Zool (Japan) 1957a;30:114–119. [Google Scholar]
- 12.Yanagimachi R. Zool Mag (Japan) 1957b;66:222–225. [Google Scholar]
- 13.Yanagimachi R, Cherr G N, Pillai M C, Baldwin J D. Dev Growth Differ. 1992;34:447–461. doi: 10.1111/j.1440-169X.1992.00447.x. [DOI] [PubMed] [Google Scholar]
- 14.Griffin F J, Pillai M C, Vines C A, Kaaria J, Hibbard-Robbins T, Yanagimachi R, Cherr G N. Biol Bull. 1998;194:25–35. doi: 10.2307/1542510. [DOI] [PubMed] [Google Scholar]
- 15.Hay D-E. Can J Fish Aquat Sci. 1985;42,Suppl.:111–126. [Google Scholar]
- 16.Pillai M C, Shields T S, Yanagimachi R, Cherr GN. J Exp Zool. 1993;265:336–342. [Google Scholar]
- 17.Griffin F J, Vines C A, Pillai M C, Yanagimachi R, Cherr G N. Dev Growth Differ. 1996;38:193–202. doi: 10.1046/j.1440-169X.1996.t01-1-00009.x. [DOI] [PubMed] [Google Scholar]
- 18.Yanagimachi R, Kanoh Y. J Fac Sci Hokkaido Univ Ser IV Zool (Japan) 1953;11:487–494. [Google Scholar]
- 19.Morisawa M, Tanimoto S, Ohtake H. J Exp Zool. 1992;264:225–230. [Google Scholar]
- 20.Oda S, Igarashi Y, Ohtake H, Sakai K, Shimizu N, Morisawa M. Dev Growth Differ. 1995;37:257–261. doi: 10.1046/j.1440-169X.1995.t01-2-00003.x. [DOI] [PubMed] [Google Scholar]
- 21.Oda S, Igarashi Y, Manaka K, Koibuchi N, Sakai-Sawada M, Sakai K, Morisawa M, Ohtake H, Shimizu N. Dev Biol. 1998;204:55–63. doi: 10.1006/dbio.1998.9056. [DOI] [PubMed] [Google Scholar]
- 22.Cavanaugh G-M, editor. Formulae and Methods of the Marine Biological Laboratory Chemical Room. Woods Hole, MA: Marine Biological Laboratory; 1975. pp. 67–69. [Google Scholar]
- 23.Grynkiewicz G, Poenie M, Tsien R Y. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 24.Plasek J, Sigler K. J Photochem Photobiol B. 1996;33:101–124. doi: 10.1016/1011-1344(96)07283-1. [DOI] [PubMed] [Google Scholar]
- 25.Iwamoto T, Watano T, Shigekawa M. J Biol Chem. 1996;271:22391–22397. doi: 10.1074/jbc.271.37.22391. [DOI] [PubMed] [Google Scholar]
- 26.Blaustein M P, Lederer W J. Physiol Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- 27.Bittar E E, Nwoga J. J Physiol. 1990;424:263–282. doi: 10.1113/jphysiol.1990.sp018066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasgado-Flores H, DeSantiago J, Espinosa-Tanguma R. Ann NY Acad Sci. 1991;639:22–33. doi: 10.1111/j.1749-6632.1991.tb17286.x. [DOI] [PubMed] [Google Scholar]
- 29.Herchuelz A, Plasman P-O. Ann NY Acad Sci. 1991;639:642–656. doi: 10.1111/j.1749-6632.1991.tb17361.x. [DOI] [PubMed] [Google Scholar]
- 30.Rufo G A, Schoff P K, Lardy H A. J Biol Chem. 1984;259:2547–2552. [PubMed] [Google Scholar]
- 31.Saba R I, Bollen A, Herchuelz A. Biochem J. 1999;338:139–145. [PMC free article] [PubMed] [Google Scholar]
- 32.Saba R I, Goormaghtigh E, Ruysschaert J-M, Herchuelz A. Biochemistry. 2001;40:3324–3332. doi: 10.1021/bi0010672. [DOI] [PubMed] [Google Scholar]
- 33.Blaustein M P, Goldman W F, Fontana G, Krueger B K, Santiago E M, Steele T D, Weiss D N, Yarowsky P J. Ann NY Acad Sci. 1991;639:254–273. doi: 10.1111/j.1749-6632.1991.tb17315.x. [DOI] [PubMed] [Google Scholar]
- 34.DiPolo R, Rojas H, Beauge L. Cell Calcium. 1982;3:19–41. doi: 10.1016/0143-4160(82)90035-5. [DOI] [PubMed] [Google Scholar]
- 35.DiPolo R, Beauge L. Biochem Biophys Acta. 1986;854:298–306. [Google Scholar]
- 36.Boitano S, Omoto C K. Cell Motil Cytoskeleton. 1992;21:74–82. [Google Scholar]
- 37.O'Toole M B, Arnoult C, Darszon A, Steinhardt R A, Florman H M. Mol Biol Cell. 2000;11:1571–1584. doi: 10.1091/mbc.11.5.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonzalez-Martinez M T, Galindo B E, De La Torre L D, Zapata O, Rodriguez E, Florman H M, Darszon A. Dev Biol. 2001;236:220–229. doi: 10.1006/dbio.2001.0323. [DOI] [PubMed] [Google Scholar]
- 39.Brokaw C J. J Cell Biochem. 1987;35:175–184. doi: 10.1002/jcb.240350302. [DOI] [PubMed] [Google Scholar]
- 40.Cook S P, Brokaw C J, Muller C H, Babcock D F. Dev Biol. 1994;165:10–19. doi: 10.1006/dbio.1994.1229. [DOI] [PubMed] [Google Scholar]
- 41.Okuno M, Morisawa M. Cell Motil Cytoskeleton. 1989;14:194–200. [Google Scholar]