Abstract
Atherosclerosis, in its myriad incarnations the foremost killer disease in the industrialized world, is characterized by aberrant proliferation of vascular smooth muscle (VSM) cells in part as a result of the recruitment of inflammatory cells to the blood vessel wall. The epoxyeicosatrienoic acids are synthesized from arachidonic acid in a reaction catalyzed by the cytochrome P450 system and are vasoactive substances. Metabolism of these compounds by epoxide hydrolases results in the formation of compounds that affect the vasculature in a pleiotropic manner. As an outgrowth of our observations that urea inhibitors of the soluble epoxide hydrolase (sEH) reduce blood pressure in spontaneously hypertensive rats as well as the findings of other investigators that these compounds possess antiinflammatory actions, we have examined the effect of sEH inhibitors on VSM cell proliferation. We now show that the sEH inhibitor 1-cyclohexyl-3-dodecyl urea (CDU) inhibits human VSM cell proliferation in a dose-dependent manner and is associated with a decrease in the level of cyclin D1. In addition, cis-epoxyeicosatrienoic acid mimics the growth-suppressive activity of CDU; there is no evidence of cellular toxicity or apoptosis in CDU-treated cells when incubated with 20 μM CDU for up to 48 h. These results, in light of the antiinflammatory and antihypertensive properties of these compounds that have been demonstrated already, suggest that the urea class of sEH inhibitors may be useful for therapy for diseases such as hypertension and atherosclerosis characterized by exuberant VSM cell proliferation and vascular inflammation.
Eicosanoids serve both paracrine and autocrine functions in a variety of cells including those of the vasculature. The cis-epoxyeicosatrienoic acids (EETs), epoxides of arachidonic acid comprising one class of eicosanoid, consist of four regioisomers that are synthesized from arachidonic acid in a reaction catalyzed by the cytochrome P450 system (1). These compounds are synthesized by endothelial cells and are taken up rapidly by arterial vascular smooth muscle (VSM) cells (2–4). Because VSM cells function to control vascular tone and contribute to the pathogenesis of atherosclerosis, the effects of EETs and their metabolites on VSM cells is of broad clinical interest.
Epoxide hydrolases are enzymes that, broadly defined, convert epoxides to diols by the addition of water (5). Although these enzymes have been studied largely in light of their roles in degrading and detoxifying mutagenic xenobiotics, at least the soluble epoxide hydrolase (sEH) also is critical in the control of EET levels because of its ability to catalyze the degradation of the EETs into diols (6). Because of the vasoactive properties of the EETs, pharmacological attenuation of sEH activity, which causes a secondary increase in EET levels (7), has been studied with an eye toward potential clinical utility in the regulation of vascular tone and remodeling in humans.
Studies in other laboratories have established that various EET regioisomers cause either vasodilatation or vasoconstriction in a variety of vascular beds (8–10) and that they possess antiinflammatory properties (11). Based on these findings, sEH inhibition is a potentially attractive pharmacological approach to human hypertension. One such inhibitor, N,N′-dicyclohexyl urea (DCU), has been shown to lower systemic blood pressure in spontaneously hypertensive rats (7). Because of the antiinflammatory and now antihypertensive properties of sEH inhibition, we asked whether selective sEH inhibitors also decrease VSM cell proliferation, a key pathologic mechanism in the progression from systemic hypertension to the atherosclerotic state (12).
We now show that 1-cyclohexyl-3-dodecyl urea (CDU), a potent urea-based sEH inhibitor similar to DCU, significantly attenuates proliferation of human VSM cells, acting at a key distal point in the cell cycle regulatory cascade. Thus, further work may lead to the exploitation of sEH inhibitors or similar compounds as novel antihypertensive and antiatherosclerotic pharmaceuticals.
Materials and Methods
Materials.
Human recombinant platelet-derived growth factor (PDGF)-BB was obtained from Upstate Biotechnology (Lake Placid, NY). Mouse monoclonal cyclin D1 and rabbit polyclonal cyclin E antibodies were obtained from Santa Cruz Biotechnology. phospho-mitogen-activated protein kinase (MAPK) antibody was obtained from New England Biolabs. Anti-rabbit horseradish peroxidase-conjugated IgG was obtained from Bio-Rad. Methyl-EETs were synthesized by oxidation of arachidonate methyl ester with meta-chloroperbenzoic acid (13) followed by ester hydrolysis in dilute base following the procedures of Falck et al. (14). Regioisomeres were purified by a combination of open column and reverse-phase high performance liquid chromatography. Structural assignments were supported by 1H NMR, and structure and purity were evaluated by GLC MS (15). Although the 5(6)-EET acid was not recovered, the regioisomeric purity of the 8,9, 11,12, and 14,15 EETs were >98%. An equimolar solution of the three isolated isomers was prepared in dimethyl sulfoxide and stored at <−20°C under nitrogen until use. Inhibitors were prepared by reaction of the appropriate amine and isocyanate followed by recrystallization as described with structures supported by NMR and liquid chromatography MS (16). Reagents for the Enhanced Chemiluminescence system and [3H]thymidine were obtained from Amersham Pharmacia. All other reagents were from Sigma.
Cell Culture.
Human aortic smooth muscle cells were obtained from Clonetics (San Diego) at passage 3, and neonatal human foreskin dermal fibroblasts were a kind gift from R. Isseroff (University of California, Davis, CA). Cells were maintained in MCBD 131 medium (GIBCO) supplemented with 2.5% FBS, 5 mg/liter bovine insulin, 2 μg/liter human recombinant epidermal growth factor, 1 μg/liter human recombinant PDGF-BB, 100 units/ml of penicillin, 100 units/ml streptomycin, and 2.5 μg/ml amphotericin B as described (24). The cells were growth-arrested by placing them in quiescence medium containing MCDB 131 medium, 20 mM Hepes (pH 7.4), 5 mg/ml transferrin, 0.5 mg/ml BSA, 50 units/ml penicillin, 50 units/ml streptomycin, and 2.5 μg/ml amphotericin B. Quiescence medium was changed daily for 1–2 days before each experiment. HL-60 cells were obtained from the American Type Culture Collection or from D. Hyde (University of California, Davis, CA). HL-60 cells were cultured at cell densities between 2 × 105 and 8 × 105 cells per ml in RPMI medium 1640 (Mediatech, Washington, DC) supplemented with 10% FCS.
Proliferation Assays.
[3H]thymidine incorporation assays were performed as described (24). To evaluate proliferation of suspension cells, cells were resuspended at 2 × 105 cells per ml in culture medium, and the medium was supplemented with the compound of interest or the corresponding vehicle. At the indicated times, cell density was estimated by using light microscopy and a hemocytometer. To evaluate the proliferation of adherent cells directly, 2 × 104 cells were plated in a 35-mm culture dish and allowed to adhere overnight. The medium then was supplemented with the compound of interest or the corresponding vehicle. At the indicated times, the number of cells in the plate was calculated by subjecting the cells to trypsinization, and the cell density was quantitated by light microscopy using a hemocytometer.
Western Blots.
After treatment with appropriate compounds for the indicated times, cells were lysed, protein concentrations were determined by the Lowry method, and equal protein quantities were electrophoresed and Western-blotted as described (24). All blots were reprobed with α-actin to confirm equal protein loading of cells.
Evaluation of Nuclear Morphology.
Cells were seeded in 35-mm dishes and treated as described. At the indicated times, the medium was aspirated, and the cell culture dish was inverted over methanol for 10 min. The cells then were immersed in methanol for at least 10 min. Cells were stained in 1 μg/ml Hoechst 33258 in water with a pinch of nonfat dry milk. The nuclear morphology was evaluated visually by fluorescence microscopy.
Thymidine Uptake.
To quantify thymidine uptake, 1.73 × 104 cells were distributed per well in a 24-well plate. After ≈1 day, cells were preincubated for ≈1 h with 9 μM CDU or the corresponding vehicle. The medium then was adjusted to 40 μM [3H]methyl-thymidine [1 mCi/ml (1 Ci = 37 GBq), 25 Ci/mmol, Amersham Pharmacia]. At the indicated times, the medium was aspirated, and the cells were washed three times with ice-cold PBS and then incubated in 500 μl of 1 M NaOH for 20 min. The mixture was neutralized with 0.5 ml of 1 M HCl and diluted into scintillation fluid for liquid scintillation counting.
Evaluation of Cell Membrane Integrity and Toxicity.
To evaluate the toxicity of compounds as indicated by cellular trypan blue permeability, cells were plated in 35-mm dishes. After 2 days the medium in the dish was replaced with fresh medium supplemented with the compound of interest or vehicle. At the indicated time posttreatment, the supernatant was removed, and adherent cells were removed by using trypsin. After dislodging the adherent cells, the cell suspension was pooled with the supernatant and centrifuged. The resulting cell pellet was resuspended, and a trypan blue solution (0.4% in normal saline) was added to the aliquot. After incubating for ≈5 min, the sample was evaluated by light microscopy. To evaluate lactate dehydrogenase (LDH) release, cells were harvested and plated onto 24-well plates and serum-starved for 1–2 days. Test compounds were added, and after 65 h the medium was removed for measurement of LDH activity. LDH activity was determined by NADH oxidation using a Sigma Tox-7 in vitro toxicology kit and reported as the amount of LDH activity in the medium.
Results
CDU Inhibits Human VSM Cell Proliferation.
Blood pressure is regulated by the integration of complex systems controlling intravascular volume as well as arterial tone. Consistently elevated blood pressure can lead to atherosclerosis, a process that is at least caused partly by aberrant proliferation of arterial smooth muscle cells (12) and partly by a generalized inflammatory condition (17). Thus, medications that result in systemic antihypertensive and antiinflammatory effects as well as attenuation of VSM cell proliferation would provide a three-pronged attack on the atherosclerotic process. Urea-derived compounds showing in vitro inhibition of sEH have shown both antiinflammatory and antihypertensive effects (7). We therefore asked whether, in addition, there is a direct effect of these substances on VSM cell proliferation using early passage human aortic VSM cells.
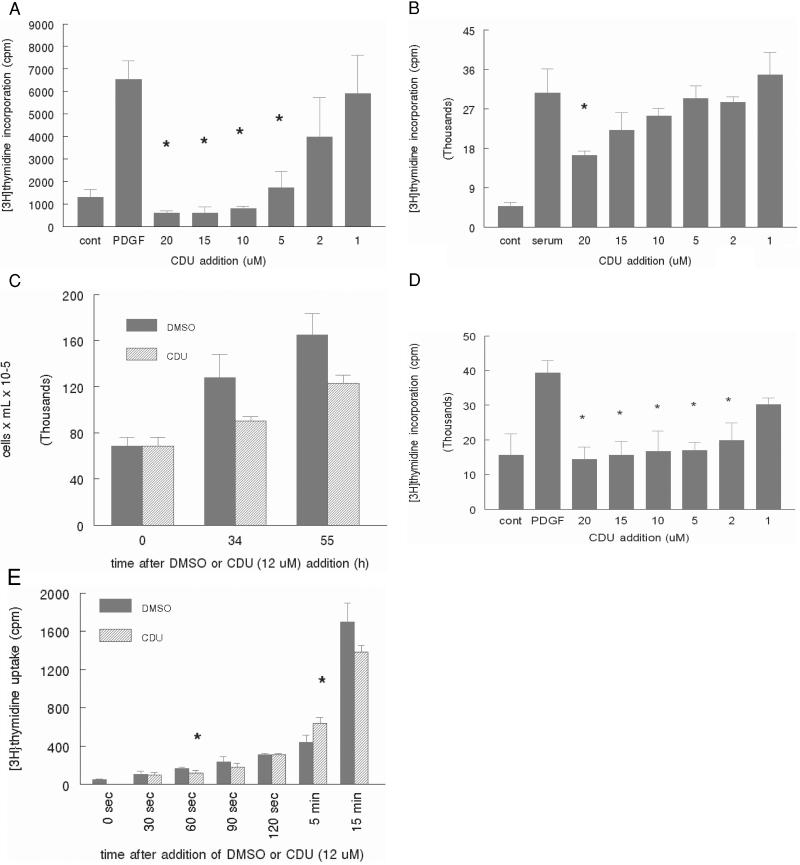
Among the most potent inhibitors of the sEH that we have developed is CDU (Ki = 20 ± 2 nM; refs. 16 and 18), which seems to act as a tight binding transition state analog of the substrate (19). When incubated with human VSM cells, CDU demonstrates a dose-dependent inhibition of DNA synthesis when the cells are stimulated to grow with either PDGF-BB (Fig. 1a) or 10% serum (Fig. 1b) at CDU concentrations from 1.0 to 20 μM. Inhibition of cell proliferation by CDU paralleled inhibition of [3H]thymidine incorporation at 12 μM as confirmed by direct counting of trypsinized cells (Fig. 1c). The differences in potency of CDU between serum and PDGF-BB-stimulated cells likely is caused by enhanced protein binding of CDU in serum-containing medium, causing it to be inaccessible to the cell replicative machinery, because the experiments using recombinant PDGF-BB are performed in the presence of significantly lower quantities of serum proteins. [3H]thymidine incorporation in human foreskin fibroblasts, which have similar characteristics to VSM cells, was inhibited also by CDU at similar concentrations to those in human VSM cells (Fig. 1d).
Figure 1.
CDU inhibits proliferation in human VSM cells. Human VSM cells were grown to 80–90% confluence and serum-starved (except where indicated) for 1 day. Immediately after PDGF-BB (a, 30 ng/ml) or 10% serum stimulation (b), CDU or DMSO vehicle was added at the concentrations indicated. After 18 h, thymidine incorporation into DNA was assessed as described in Materials and Methods. (c) CDU (12 μM) or an equal volume of DMSO vehicle was added concomitantly with 10% serum, and the cells were counted by hemocytometer after trypsinization. (d) Human foreskin fibroblasts were treated similarly to VSM cells in a, and [3H]thymidine incorporation into DNA was assessed. (e) CDU at the indicated concentrations was added to non-serum-starved cells, and uptake of thymidine into the cells was assessed at the indicated times after its addition as described in Materials and Methods. Error bars represent SD: *, P < 0.05 compared with PDGF alone (a and d), serum alone (b), or DMSO (e). The data shown are representative of at least two independent experiments.
One possible means by which CDU attenuates cellular incorporation of thymidine is through inhibition of thymidine uptake into the cell (20), an event that is not synonymous with incorporation of thymidine into DNA. This finding, if true, would eliminate the use of [3H]thymidine as an accurate measure of cell proliferation. To evaluate whether CDU inhibits thymidine uptake independently of proliferation, VSM cells were incubated with [3H]thymidine for time periods ranging from several seconds to several minutes in the presence of either 12 μM CDU or DMSO vehicle. Quantification of thymidine uptake by liquid scintillation counting revealed that cellular uptake of thymidine uptake occurs to a similar extent irrespective of CDU or DMSO vehicle treatment (Fig. 1e).
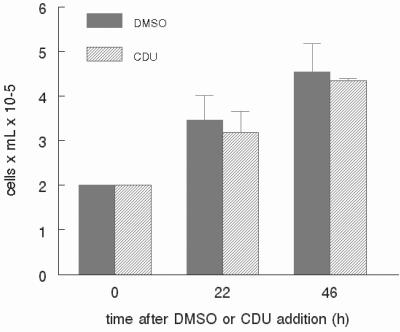
To determine whether CDU inhibits proliferation of cell types other than normal mesenchymal cells, we evaluated the influence of this compound on the proliferation of other unrelated cell lines. HL-60 cells are derived from a human promyelocytic cell line widely used as a system to model human neutrophils (reviewed in ref. 21). Whether seeded in the presence of 12 μM CDU or the corresponding vehicle, HL-60 cells proliferated to a similar extent (Fig. 2). There was a similar lack of effect of CDU on cells derived from the highly metastatic breast tumor Met-1 (22) when incubated with up to 20 μM CDU (data not shown).
Figure 2.
Inhibition of proliferation by CDU is not universal. Human promyelocytic HL-60 cells were incubated in serum-containing medium, and CDU (12 μM) or DMSO vehicle was added at time 0. At 24 and 48 h after CDU addition, the cells were counted by using a hemocytometer. Error bars represent SD. The data shown are representative of two independent experiments.
EETs Act with CDU to Inhibit VSM Cell Proliferation.
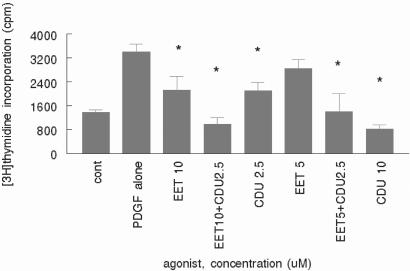
Because inhibition of sEH results in the accumulation of EETs in mice injected with DCU (7), the effect of sEH attenuation would be expected to be replicated by the addition of various EET regioisomers. A solution containing a mixture of EET-free acids (1:1:1) was added to PDGF-BB-stimulated VSM cells at concentrations from 5 to 10 μM (total EET concentration) both separately and simultaneously with CDU at 2.5 and 10 μM. The addition separately of the EETs and CDU both caused inhibition of PDGF-stimulated [3H]thymidine incorporation (Fig. 3), and there was an apparent additive affect when both compounds were added together, suggesting a common mechanism of action.
Figure 3.
EETs inhibit VSM cell proliferation. Human VSM cells were grown to 80–90% confluence and serum-starved as described for Fig. 1. PDGF-BB (30 ng/ml) was added to all but control wells, followed immediately by the addition of mixed EETs and/or CDU at the concentrations indicated of total EETs. [3H]Thymidine was added for the last 6 h of incubation, and its incorporation into DNA was assessed as described in Materials and Methods. Error bars represent SD: *, P < 0.05 compared with PDGF alone. The data shown are representative of two independent experiments.
CDU Does Not Cause Apoptosis and Is Not Toxic at the Doses Used.
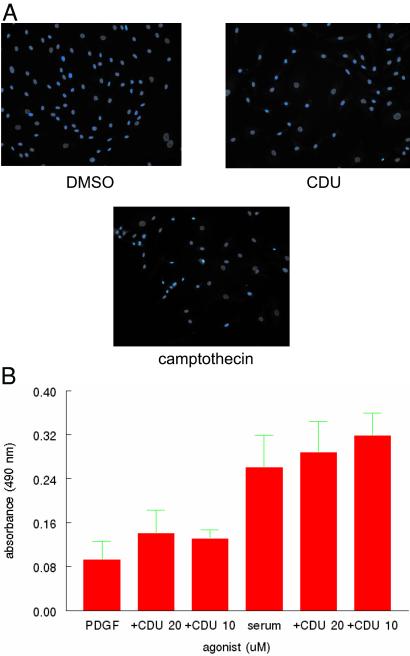
Some of the data shown above could reflect cell death as the result of exposure to CDU. To test the possibility that CDU may be toxic to human VSM cells under the conditions that we have been examining, either directly or through inhibition of sEH, we used several techniques to evaluate apoptosis as well as cell death in general. There was no change in exclusion of trypan blue in cells treated with CDU at 12 μM as compared with DMSO vehicle (3.1% of DMSO-treated cells as compared with 3.4% of CDU-treated cells stained blue at 8 h, and 3.4% of DMSO-treated cells as compared with 3.0% of CDU-treated cells stained blue at 24 h), and similar results were obtained when cells were stained with Hoechst 33258 (Fig 4a), making apoptosis an unlikely cause of the observed decrement in [3H]thymidine incorporation and cell number.
Figure 4.
CDU is not toxic to VSM cells. (a) Continuously growing human VSM cells were incubated with CDU (10 μM), DMSO vehicle for 72 h, or camptothecin (7 μg/ml as positive control) for 2 h, stained with Hoechst 33258 as described in Materials and Methods, and visualized by fluorescence microscopy (×200). (b) Human VSM cells were grown continuously in the presence of either 10% serum or PDGF-BB (30 ng/ml). After achieving confluency, the cells were incubated with CDU at the indicated concentrations, and LDH release into the medium was measured as described in Materials and Methods.
LDH is contained in living cells such that the appearance of this enzyme in the medium is an indication that cells have died and released this protein. VSM cells were treated with PDGF-BB or 10% serum in the presence or absence of CDU at 10 and 20 μM, concentrations that showed significant inhibition of proliferation after PDGF stimulation. LDH appearance in the medium was measured and found to be unchanged in cells treated with PDGF or serum when compared with these growth stimuli in the presence of CDU (Fig. 4b), further demonstrating the lack of toxicity of CDU in these cells. By using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay, there was also no toxicity observed in A549 lung cancer cells, HT-29 colon cancer cells, HTB-30 breast cancer cells, or LnCap prostate cancer cells when incubated with CDU up to 40 μM.
Time of Addition of CDU Probes the Cell Cycle.
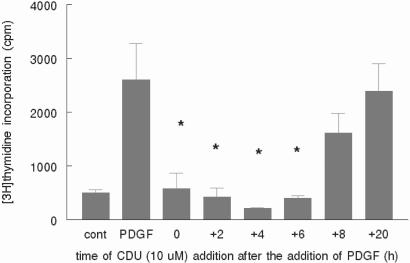
To ascertain which phase of the VSM cell cycle is being inhibited by CDU, we examined DNA synthesis as a function of time of addition of CDU relative to mitogen. CDU (10 μM) was added to human VSM cells simultaneously with PDGF-BB (time = 0) and at times ranging from 2 to 20 h after PDGF addition. In VSM cells whose cycles were synchronized by serum removal before PDGF stimulation, there was similar cell inhibition of proliferation when CDU was added simultaneously and as late as 6 h after the addition of PDGF, with the most profound inhibition occurring at 4 h (Fig. 5). These data suggest cell cycle inhibition is occurring through modulation of proteins that act in late G1 or at the G1/S phase transition (23).
Figure 5.
CDU inhibits proliferation when added up to 8 h after mitogen. Human VSM cells were grown to 80–90% confluence and serum-starved as described for Fig. 1. PDGF-BB (30 ng/ml) was added to all but control wells, and at the times indicated after PDGF addition CDU (10 μM) was added. At time = 0, CDU and PDGF were added simultaneously. [3H]Thymidine was added for the last 6 h of incubation, and its incorporation into DNA was assessed as described in Materials and Methods. Error bars represent SD: *, P < 0.05 compared with PDGF alone. The data shown are representative of three independent experiments.
CDU Attenuates Cyclin D1 but Not Phospho-MAPK Levels.
Because we have shown that CDU attenuates cell cycle transit most likely at late G1 or the G1/S transition, and this is not caused by a toxic or apoptotic process, we next asked which mitogenic signaling events are being altered by this compound. We reasoned that likely candidate signaling proteins that may be activated in late G1 after stimulation of VSM cells with mitogens such as PDGF include protein kinases in the mitogen-activated protein/extracellular signal-regulated kinase (ERK) signaling cascade as well as components of the cyclin/cyclin-dependant kinase/cyclin kinase inhibitor complex.
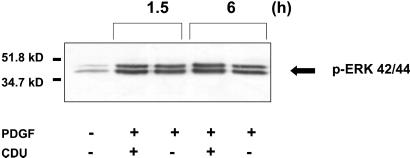
Phosphorylation of ERK1/2 occurs as a distal event in the MAPK cascade of signal transduction proteins in VSM and other cells after stimulation with both G protein-coupled and tyrosine kinase growth factors, and inhibition of its upstream kinase MAPK kinase (MEK) results in arrest of PDGF-stimulated VSM cells (24). Thus, phosphorylation of ERK serves as a readout of the integrity of the upstream signaling proteins in this pathway including but not limited to Ras, Raf, and MEK. VSM cells incubated with CDU showed no change in PDGF-stimulated ERK42/44 phosphorylation (Fig. 6), demonstrating preservation of the integrity of the PDGF receptor/ras/raf/MEK/ERK pathway in the presence of CDU despite marked inhibition of proliferation.
Figure 6.
CDU does not affect MAPK signaling in VSM cells. Human VSM cells were grown to 80–90% confluence and serum-starved as described for Fig. 1. Four hours after stimulation with PDGF-BB (30 ng/ml) in the presence or absence of CDU (10 μM), the cells were lysed, and equal protein quantities were electrophoresed and immunoblotted with the phospho-MAPK antibody. The data shown are representative of three independent experiments.
The cyclins are cell cycle regulatory proteins that activate the cyclin-dependant kinases in response to a variety of growth stimuli, resulting in subsequent transit through various cell cycle checkpoints. Levels of the cell cycle regulating cyclins are increased at different times that correspond to discrete events in the cell cycle (25); thus, examination of levels of these proteins is a useful tool to dissect out events in the cycle that are being impacted by growth inhibitors.
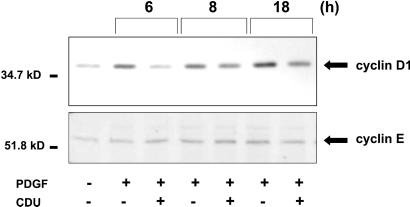
After growth stimulation, cyclin D1 is increased and remains elevated as long as growth factor is present. Consistent with its role as a positive cell cycle regulator, cyclin D1 was identified as the protooncogene PRAD1 (26). Furthermore, it has been demonstrated that overexpression of both cyclin D1 and cyclin E significantly shortens G1 phase (27) such that a decrement in these cyclins may result in lengthening G1 and the subsequent cell cycle inhibition. After the addition of growth factor, cyclin D1 is increased in late G1 and S phase, leading to phosphorylation of Rb, dissociation of Rb from the E2F group of transcription factors, and subsequent transcriptional activation of proliferation-regulating genes (25). VSM cells stimulated with PDGF-BB and incubated simultaneously with 10 μM CDU for 6–18 h demonstrated a profoundly decreased induction of cyclin D1 levels when compared with DMSO vehicle-treated cells, with minimal effect on another G1 cyclin, cyclin E (Fig. 7).
Figure 7.
CDU attenuates cyclin D1 levels. Human VSM cells were grown to 80–90% confluence and serum-starved as described for Fig. 1. Four hours after stimulation with PDGF-BB (30 ng/ml) in the presence or absence of CDU (10 μM), the cells were lysed, and equal protein quantities of the same lysate were electrophoresed and immunoblotted with the antibody to either cyclin D1 or cyclin E. The data shown are representative of at least two independent experiments.
Discussion
Eicosanoids function as potent regulators of vascular tone and have been implicated in blood pressure control (7) as well as in modulation of the inflammatory state (11). Because atherosclerosis, which is the foremost killer disease in the western world, is both a consequence of chronic hypertension and an inflammatory disease (28), the metabolic enzymes of the cytochrome P450 epoxygenase-derived eicosanoids represent an ideal pharmacological target for the treatment of both hypertension and atherosclerosis. The EETs, at physiologic concentrations, decrease cytokine-induced endothelial cell adhesion-molecule expression as well as leukocyte adhesion to the vascular wall (11), both processes intimately connected to atherosclerotic progression. Our findings that exogenous EETs as well as the addition of in vitro inhibitors of sEH (which also may increase cellular EET levels) decrease VSM cell proliferation suggest that this metabolic pathway is promising as a drug-therapy target.
The sEH functions in vivo to metabolize EETs to their corresponding dihydroxy derivatives (29). This enzyme, which represents a single known and highly conserved gene product with over 90% homology between rodent and human (30), can be inhibited selectively and competitively in vitro by a variety of urea, carbamate, and amide derivatives (16, 18). We have shown that injection of one such inhibitor, DCU, into spontaneously hypertensive rats resulted in a lowering of blood pressure. In addition, there was an increase in urinary 14,15-EET and a decrease in urinary dihydroxy derivative in these animals, which is consistent with an effect of DCU occurring on the sEH in this in vivo setting. Whether the effects of CDU, a similar urea-based sEH inhibitor, are attributable to an increase in EET levels in our tissue culture model or to modulation of a non-sEH enzyme pathway is unknown.
Because VSM cell proliferation is an integral process in the pathophysiology of atherosclerosis, our finding that CDU decreases proliferation of this cell type in vitro with no evidence of toxicity makes this compound a promising and novel approach to human hypertension, which has the potential to impact the morbidity of this disease on multiple fronts. Our results are in contrast to other studies in which EETs have been shown to be growth-stimulatory in porcine renal epithelial and aortic cell lines. In porcine aortic VSM cells, the addition of 2 μM exogenous 14,15-EET was reported to increase PDGF-mediated DNA synthesis, and the potent in vitro inhibitor of epoxide hydrolases, 4-phenylchalcone oxide (31), results in an additive increase in mitogenesis in porcine aortic VSM cells when incubated with PDGF and exogenous commercial EETs (32). Although potent inhibitors of the sEH in vitro, the chalcone oxides actually are substrates for the sEHs that are turned over slowly. This turn over and their metabolism by glutathione S-transferases and reaction with glutathione makes the inhibition caused by the chalcone oxides transient (33) and may partially explain the differences between these and our experiments. Another group showed stimulation of LLCPK cell mitogenesis with various EETs and their sulfonimide derivatives, although in that study the 14,15-EET sulfonimide derivative tested showed an inhibitory effect after 2 days of incubation in these cells (34). These disparities compared with our results may be caused by species differences in metabolism of EETs or differences in purity of the exogenous EET stereoisomers used. Alternatively, our use of a mixture of free acid EETs as opposed to the use of specific regioisoforms also may explain the observed differences. It is conceivable also that the growth-inhibitory function of CDU in vivo may be independent of its in vitro inhibitory effect on the sEH.
The lipid solubility of the various sEH inhibitors may be playing some role in their effects both in vivo and in vitro as well as in the bioavailability of these inhibitors in future animal and human trials. The bioavailability of a particular drug is in large part a function of its diffusibility across cell membranes and its binding to serum proteins. This diffusibility may explain the decreased magnitude of inhibition by CDU in cells stimulated by serum as compared with PDGF-BB (Fig. 1 a and b). It is possible that increasing the water solubility of the extant sEH inhibitors would make it bioavailable through per-oral administration. Should these compounds be engineered such that they are available orally, the VSM cell antiproliferative action of the sEH inhibitors may prove useful in a variety of vascular diseases. Reminiscent of the hydroxymethyl glutaryl (HMG)-CoA reductase inhibitors, which also inhibit VSM cell proliferation (35), the sEH inhibitors also may prove useful in the treatment of transplant vasculopathy (36) and restenosis after angioplasty (37), both processes that are characterized by aberrant proliferation of VSM cells. Safety of these compounds in an in vivo setting is supported by our findings of relative specificity to mesenchymal cells, because proliferation in both HL-60 promyelocytic and Met-1 breast tumor cells is not affected by CDU.
Our finding that CDU decreases VSM cell proliferation independent of MAP/ERK phosphorylation indicates that this sEH inhibitor is affecting an event downstream of this signaling molecule. Cyclin D1 is regulated positively by p42/p44 MAPK (38), and our findings suggest that this may be the target of CDU. The cyclin molecules, by regulating the activity of their partner cyclin-dependant kinases, intimately control phase transitions in the cell cycle (25). Thus, these proteins have been explored extensively as targets for the treatment of diseases, particularly cancer, characterized by cellular proliferation (39). Our finding that the level of cyclin D1 but not cyclin E is attenuated by CDU is entirely consistent with the role of this cyclin as a G1 → S phase modulator. We have found also that cyclin D1 protein abundance is not being regulated by CDU at the level of transcription (B.B.D. and R.H.W., unpublished observations), suggesting, as would be expected, that it is an alteration of stability that is controlling cellular levels of this protein. These data further suggest that these compounds in some manner may prove useful in proliferative diseases other than atherosclerosis, although our initial foray using CDU in cancer cells (by looking at the proliferation of highly metastatic mouse breast cancer cells) did not show effectiveness. It is possible that under other conditions these compounds may show antineoplastic activity.
Our findings open up avenues of exploration for the possible use of urea sEH inhibitors as antihypertension and antiatherosclerotic pharmaceuticals. Whether the VSM cell growth-inhibitory effect of these inhibitors is caused by sEH inhibition or is an effect of the ureas on other cellular substrates has not been established. However, because of the striking effects of the urea sEH inhibitors on inflammation, hypertension, and now VSM cell proliferation, it is clear that these compounds offer an approach to treatment of disease that results in atherosclerosis.
Acknowledgments
We thank Marjorie Tano and John Newman for technical assistance. R.H.W. and B.B.D. are supported by the Research Service of the U.S. Department of Veterans Affairs, grants from the National Kidney Foundation, American Society of Nephrology, and the University of California at Davis Health System, and a gift from Dialysis Clinics, Inc. B.D.H. and C.M. are supported by National Institute on Environmental Health Sciences (NIEHS) Grant R01 ES02710, NIEHS Superfund Basic Research Program Grant P42 ES04699, NIEHS Center for Children's Environmental Health and Disease Prevention 1 Grant P01 ES11269, and NIEHS Center Grant P30 ES05707. D.A.T. is supported by Interdisciplinary Training in Comparative Lung Biology and Medicine Grant T32 HL07013.
Abbreviations
- EET
cis-epoxyeicosatrienoic acid
- VSM
vascular smooth muscle
- sEH
soluble epoxide hydrolase
- DCU
N,N′-dicyclohexyl urea
- CDU
1-cyclohexyl-3-dodecyl urea
- PDGF
platelet-derived growth factor
- MAP
mitogen-activated protein
- MAPK
MAP kinase
- LDH
lactate dehydrogenase
- ERK
extracellular signal-regulated kinase
Footnotes
Present address: Fresno Pacific University, 1717 South Chestnut Avenue, Fresno, CA 93702.
References
- 1.Capdevila J H, Falck J R, Estabrook R W. FASEB J. 1992;6:731–736. doi: 10.1096/fasebj.6.2.1537463. [DOI] [PubMed] [Google Scholar]
- 2.Fang X, Kaduce T L, Weintraub N L, Spector A A. Prostaglandins Leukotrienes Essent Fatty Acids. 1997;57:367–371. doi: 10.1016/s0952-3278(97)90412-9. [DOI] [PubMed] [Google Scholar]
- 3.Fang X, Kaduce T L, Weintraub N L, VanRollins M, Spector A A. Circ Res. 1996;79:784–793. doi: 10.1161/01.res.79.4.784. [DOI] [PubMed] [Google Scholar]
- 4.Rosolowsky M, Campbell W B. Biochim Biophys Acta. 1996;1299:267–277. doi: 10.1016/0005-2760(95)00216-2. [DOI] [PubMed] [Google Scholar]
- 5.Fretland A J, Omiecinski C J. Chem Biol Interact. 2000;129:41–59. doi: 10.1016/s0009-2797(00)00197-6. [DOI] [PubMed] [Google Scholar]
- 6.Chacos N, Capdevila J, Falck J R, Manna S, Martin-Wixtrom C, Gill S S, Hammock B D, Estabrook R W. Arch Biochem Biophys. 1983;223:639–648. doi: 10.1016/0003-9861(83)90628-8. [DOI] [PubMed] [Google Scholar]
- 7.Yu Z, Xu F, Huse L M, Morisseau C, Draper A J, Newman J W, Parker C, Graham L, Engler M M, Hammock B D, et al. Circ Res. 2000;87:992–998. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 8.Katoh T, Takahashi K, Capdevila J, Karara A, Falck J R, Jacobson H R, Badr K F. Am J Physiol. 1991;261:F578–F586. doi: 10.1152/ajprenal.1991.261.4.F578. [DOI] [PubMed] [Google Scholar]
- 9.Lin W K, Falck J R, Wong P Y. Biochem Biophys Res Commun. 1990;167:977–981. doi: 10.1016/0006-291x(90)90619-x. [DOI] [PubMed] [Google Scholar]
- 10.Imig J D, Navar L G, Roman R J, Reddy K K, Falck J R. J Am Soc Nephrol. 1996;7:2364–2370. doi: 10.1681/ASN.V7112364. [DOI] [PubMed] [Google Scholar]
- 11.Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin D C, Liao J K. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross R. Nature (London) 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 13.Gill S S, Hammock B D. Biochem Biophys Res Commun. 1979;89:965–971. doi: 10.1016/0006-291x(79)91872-2. [DOI] [PubMed] [Google Scholar]
- 14.Falck J R, Yadagiri P, Capdevila J. Methods Enzymol. 1990;187:357–364. doi: 10.1016/0076-6879(90)87042-2. [DOI] [PubMed] [Google Scholar]
- 15.Newman J W, Hammock B D. J Chromatogr A. 2001;925:223–240. doi: 10.1016/s0021-9673(01)00998-0. [DOI] [PubMed] [Google Scholar]
- 16.Morisseau, C., Goodrow, M. H., Newman, J. W., Wheelock, C. E. & Hammock, B. D. (2001) Biochem. Pharmacol., in press. [DOI] [PubMed]
- 17.Ross R. Am Heart J. 1999;138:S419–S420. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 18.Morisseau C, Goodrow M H, Dowdy D, Zheng J, Greene J F, Sanborn J R, Hammock B D. Proc Natl Acad Sci USA. 1999;96:8849–8854. doi: 10.1073/pnas.96.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Argiriadi M A, Morisseau C, Hammock B D, Christianson D W. Proc Natl Acad Sci USA. 1999;96:10637–10642. doi: 10.1073/pnas.96.19.10637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griner R D, Bollag W B. J Pharmacol Exp Ther. 2000;294:1219–1224. [PubMed] [Google Scholar]
- 21.Collins S J. Blood. 1987;70:1233–1244. [PubMed] [Google Scholar]
- 22.Cheung A T W, Young L J T, Chen P C Y, Chao C Y, Ndoye A, Barry P A, Muller W J, Cardiff R D. Int J Oncology. 1997;11:69–77. [PubMed] [Google Scholar]
- 23.NicAmhlaoibh R, Heenan M, Cleary I, Touhey S, O'Loughlin C, Daly C, Nunez G, Scanlon K J, Clynes M. Int J Cancer. 1999;82:368–376. doi: 10.1002/(sici)1097-0215(19990730)82:3<368::aid-ijc10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 24.Weiss R H, Maga E A, Ramirez A. Am J Physiol. 1998;274:C1521–C1529. doi: 10.1152/ajpcell.1998.274.6.C1521. [DOI] [PubMed] [Google Scholar]
- 25.Arellano M, Moreno S. Int J Biochem Cell Biol. 1997;29:559–573. doi: 10.1016/s1357-2725(96)00178-1. [DOI] [PubMed] [Google Scholar]
- 26.Motokura T, Bloom T, Kim H G, Juppner H, Ruderman J V, Kronenberg H M, Arnold A. Nature (London) 1991;350:512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- 27.Resnitzky D, Gossen M, Bujard H, Reed S I. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor W R, DePrimo S E, Agarwal A, Agarwal M L, Schonthal A H, Katula K S, Stark G R. Mol Biol Cell. 1999;10:3607–3622. doi: 10.1091/mbc.10.11.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang X, Kaduce T L, Weintraub N L, Harmon S, Teesch L M, Morisseau C, Thompson D A, Hammock B D, Spector A A. J Biol Chem. 2001;276:14867–14874. doi: 10.1074/jbc.M011761200. [DOI] [PubMed] [Google Scholar]
- 30.Arand M, Grant D F, Beetham J K, Friedberg T, Oesch F, Hammock B D. FEBS Lett. 1994;338:251–256. doi: 10.1016/0014-5793(94)80278-5. [DOI] [PubMed] [Google Scholar]
- 31.Mullin C A, Hammock B D. Arch Biochem Biophys. 1982;216:423–439. doi: 10.1016/0003-9861(82)90231-4. [DOI] [PubMed] [Google Scholar]
- 32.Fang X, Moore S A, Stoll L L, Rich G, Kaduce T L, Weintraub N L, Spector A A. Am J Physiol. 1998;275:H2113–H2121. doi: 10.1152/ajpheart.1998.275.6.H2113. [DOI] [PubMed] [Google Scholar]
- 33.Morisseau C, Du G, Newman J W, Hammock B D. Arch Biochem Biophys. 1998;356:214–228. doi: 10.1006/abbi.1998.0756. [DOI] [PubMed] [Google Scholar]
- 34.Chen J K, Falck J R, Reddy K M, Capdevila J, Harris R C. J Biol Chem. 1998;273:29254–29261. doi: 10.1074/jbc.273.44.29254. [DOI] [PubMed] [Google Scholar]
- 35.Weiss R H, Ramirez A, Joo A. J Am Soc Nephrol. 1999;9:1880–1890. doi: 10.1681/ASN.V1091880. [DOI] [PubMed] [Google Scholar]
- 36.Katznelson S, Wilkinson A H, Kobashigawa J A, Wang X M, Chia D, Ozawa M, Zhong H P, Hirata M, Cohen A H, Teraski P I. Transplantation. 1996;61:1469–1474. doi: 10.1097/00007890-199605270-00010. [DOI] [PubMed] [Google Scholar]
- 37.Kobashigawa J A, Katznelson S, Laks H, Johnson J A, Yeatman L, Wang X M, Chia D, Terasaki P I, Sabad A, Cogert G A. N Engl J Med. 1995;333:621–627. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 38.Lavoie J N, Rivard N, L'Allemain G, Pouyssegur J. Prog Cell Cycle Res. 1996;2:49–58. doi: 10.1007/978-1-4615-5873-6_5. [DOI] [PubMed] [Google Scholar]
- 39.Yu Q, Geng Y, Sicinski P. Nature (London) 2001;411:1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]