Abstract
All members of the Wiskott–Aldrich syndrome protein (WASp) family contain a carboxyl-terminal verprolin homology, cofilin homology, and acidic region (VCA) domain that binds and activates the Arp2/3 complex, thereby linking these proteins to the induction of actin polymerization. Although the VCA domain imbues WASp and other WASp family members with the capacity to modulate cytoskeletal organization, little is known about the impact of this domain activity on lymphoid cell function. Here we demonstrate that T cell-restricted expression of VCA domain-deleted WASp (WASpΔVCA) in WAS−/− mice engenders a severe early block in T lymphopoiesis associated with impaired T cell antigen receptor αβ expression and a consequent failure to generate single-positive CD4+ and CD8+ T cells. These latter defects, which are not observed in WAS−/− mice, are associated with impaired induction of cellular actin polymerization and a failure in the terminal differentiation of double-negative thymocytes. These findings indicate that WASp family proteins play an essential role in modulating the signaling events required for early thymocyte development and reveal their capacity to subserve this role to depend on VCA domain-mediated actin polymerization.
The Wiskott–Aldrich syndrome protein (WASp) is a key regulator of lymphocyte cytoskeletal organization and has been implicated in the coupling of T cell antigen receptor (TCR) engagement to induction of both actin polymerization and transcriptional activation (1, 2). These properties of WASp are consistent with the marked alterations in T lymphocyte morphology and antigen receptor-evoked proliferation responses observed in WASp-deficient T cells (2, 3) and reflect the capacity of this multimodular cytosolic protein to act as a scaffold for linking molecules involved in the relay of activation signals to effectors of cytoskeletal rearrangement (4). WASp effects on cellular architecture are, for example, mediated by means of a C-terminal verpolin homology, cofilin homology, acidic region segment (VCA domain), which binds actin monomers and the actin-nucleating Arp2/3 complex and thereby stimulates Arp2/3-mediated actin polymerization (5–7). WASp also contains a cdc42/Rac interactive-binding (CRIB) motif that interacts with activated cdc42, an upstream, N-terminal located Ena-Vasp homology 1 (EVH1) domain that binds WASp-interacting protein (WIP), and a downstream proline-rich region, which associates with Nck, Fyn, Grb2, and other Src homology 3 domain-containing proteins (1, 4). These latter interactions facilitate the recruitment of WASp into antigen and other receptor-evoked signaling pathways and the concomitant induction of WASp relocalization to the immunologic synapse, endocytic vesicles, and other sites of dynamic actin remodeling within the cell (8, 9). WASp association with activated cdc42 also disrupts an intramolecular interaction that suppresses VCA domain induction of Arp2/3 activity and is thus critical to the capacity for WASp to evoke actin polymerization after cell stimulation (10).
The discovery and characterization of WASp also has led to the identification of several other proteins showing extensive structural and functional similarities with WASp. These other members of the WASp family include N-WASp, a ubiquitously expressed protein with a domain organization closely resembling that of haemopoetic WASp, and three WAVE proteins, in which a WAVE/Scar homology domain rather than EVH1 and CRIB domains represents the major structural module upstream of a WASp-like, proline-rich, and VCA domain-containing C-terminal segment (11–14). Like WASp, each of these proteins play integral roles in coupling extracellular stimulation to cytoskeletal rearrangement and achieve these effects by means of VCA domain-mediated induction of Arp2/3 actin nucleation activity (24). However, although a major role for WASp family members in regulating actin filament organization is supported by the defects in ligand-induced antigen receptor capping and endocytosis observed in WASp-deficient cells and by many other lines of evidence (1–3, 15), the relevance of VCA domain-stimulated actin polymerization to induction of specific biological processes such as differentiation is not well understood. To address this issue and delineate the roles of VCA domain-induced actin polymerization in modulating T cell function in vivo, we have derived mice expressing a VCA domain-deleted form of WASp on the WAS−/− background (WASpΔVCA). As described in this article, the analyses of these animals has revealed the association of WASpΔVCA expression with a severe block in thymocyte development and has thereby identified an essential role for VCA domain-mediated actin polymerization in promoting the signaling events required for the differentiation of immature T cells.
Materials and Methods
Generation of Transgenic Mouse Lines.
WASpΔVCA and WASpΔEVH1 transgenes were constructed by introducing PCR-amplified cDNA fragments representing amino acids 1–422 (WASpΔVCA) or 137–502 (WASpΔEVH1) of the WASp cDNA into an EcoRI/SalI site downstream of the CD2-promoter elements in the vector p29Δ2. All constructs derived by PCR were confirmed by DNA sequencing. The NotI transgene fragment was purified and injected into CD1 embryos, and the pups were screened by Southern blot analysis of tail DNA by using a CD2-enhancer probe. Transgene-positive founders were backcrossed to the C57BL/6 background and the pups were screened for transgene expression by immunoblotting analysis of lymphoid cell lysates with anti-WASp Ab. Selected WASpΔVCA and WASpΔEVH1 transgenic lines were than mated with previously derived WASp-deficient (WAS−/−) mice (2) to derive transgenic mice expressing WASpΔVCA and WASpΔEVH1 on the WAS−/− background. Mice carrying a lymphocytic choriomeningitis virus (LCMV)-specific TCR transgene (P14), which recognizes a peptide epitope of LCMV p33 glycoprotein in the context of H-2Db (16), were crossed with WAS−/− mice and the male WAS−/−LCMV progeny then mated with female WASpΔVCA/WAS−/− mice to obtain WAS−/− mice expressing the LCMV TCR transgene alone (WAS−/−LCMV) or with WASpΔVCA (WAS−/−ΔVCA/LCMV). LCMV TCR transgene expression was also confirmed by flow cytometric analysis of peripheral blood lymphocytes stained with phycoerythrin-conjugated anti-Thy1.2 and FITC-conjugated anti-Vα2 Abs. All mice were studied at 3–4 wk of age.
Materials.
Abs used included: FITC-conjugated anti-CD4, anti-CD44, anti-CD8, and anti-TCR-ζ; phycoerythrin-conjugated anti-CD4, anti-CD3ɛ, anti-B220, and anti-Thy1.2; and biotin-conjugated anti-TCR-α/β, anti-CD25 Abs, anti-Vα2, and anti-Vβ8.2; and Cy5-conjugated streptavidin, all from PharMingen. Anti-TCR-ζ was provided by L. Samelson (National Institutes of Health, Bethesda), hamster anti-mouse CD3ɛ Ab 145–2C11 was from R. Miller (Ontario, Cancer Institute, Toronto), and polyclonal rabbit anti-WASp Ab was generated as described (2). N-acetyl-l-leucinal-l-leucinal-l-norleucinal (ALLN) was purchased from Calbiochem; rabbit anti-hamster and anti-mouse IgG Abs and FITC-conjugated streptavidin were purchased from Jackson ImmunoResearch, and [35S]methionine was from New England Biolabs. A WASpΔVCA retroviral expression construct (MIEV-WASpΔVCA) was prepared by subcloning the WASpΔVCA cDNA into MIEV, a murine stem cell virus (MSCV)-based retroviral vector (from R. Hawley, American Red Cross, Rockville, MD).
Retroviral Constructs and Infection.
The MIEV-WASpΔVCA and MIEV empty vectors were lipofectin-transfected into Phoenix cells (from G. Nolan, Stanford Univ., Stanford, CA) and the cell supernatants then used to infect GP+E cells and derive stable green fluorescent protein-expressing cell lines were isolated by flow cytometry. To obtain purified lymph node T cells, single-cell suspensions of lymph node cells were subjected to repeated panning over rabbit anti-mouse Ig-coated culture plates and to selection by passage over T cell enrichment columns (R & D Systems). Purified lymph node T cells (2 × 106/ml) were stimulated with anti-CD3ɛ and anti-CD28 Abs in the presence of IL-2 (50 units/ml) for 72 h, followed by infection with viral supernatant in the presence of Polybrene (8 μg/ml) for 18 h. The cells were then cocultured for 48 h in 24-well plates seeded with stably transfected GP+E cells.
Flow Cytometric Analysis.
Lymph node T cells or thymocytes (1–2 × 105) suspended in staining buffer were incubated with the appropriate fluorochrome-conjugated Abs with or without of 0.5% saponin. For three-color staining, Cy5-conjugated streptavidin was used after staining with biotin-conjugated Abs. Stained cells were analyzed by using a FACScan with cellquest software (Becton Dickinson). To purify double-positive (DP) or double-negative (DN) thymocytes, 2 × 108 thymocytes were stained with FITC-conjugated anti-CD8 and phycoerythrin-conjugated anti-CD4 Abs and the DP or DN cells then isolated by using a FACStar Plus (Becton Dickinson). MIEV-WASpΔVCA-expressing thymocytes identified by green fluorescence were purified by using the FACStar Plus.
Immunoblotting Analysis.
Thymocytes and lymph node T cells (2 × 107) were incubated for 15 min in cold lysis buffer (2) and 1% Triton X-100, and the lysates then were boiled, electrophoresed through 10% SDS-polyacrylamide, and transferred to nitrocellulose membranes (Schleicher & Schuell). Filters were incubated at 4°C for 1 h in TBST (150 mM NaCl/10 mM Tris⋅HCl, pH 7.4/0.05% Tween 20) plus 3% gelatin and for an additional 2 h at room temperature with 1 μg/ml anti-WASp Ab. WASp was detected by chemiluminescence analysis (2). Blots were stripped and reprobed with anti-β-actin Ab to assess loading.
Reverse Transcription (RT)-PCR Analysis.
Total RNA was prepared from 0.5–1.0 × 106 FACS-sorted thymocytes by Trizol extraction (GIBCO), and cDNA then was produced by using the Ready-to-go RT kit (Pharmacia) and adjusted for equivalent amounts of template by serial dilution and amplification by using the β-actin primers 5′-ATGGATGACGATATCGCT-3′ and 5′-ATGAGGTAGTCTGTCAGGT-3′. The “normalized” cDNAs were then PCR-amplified by using the following primer pairs: for CD3ɛ, 5′-ACTGGAGCAAGAATAGGA-3′ and 5′-AAGAGAGGAAAGGAACTG-3′; for TCRα, 5′-TCAACTGGACCACAGCCTCAG-3′ and 5′-CAGAACCCAGAACCTGCTGTG-3′; for TCRβ, 5′-TAAGCGGCCGCATGCGT(AGT)(CT)TGGTA(CT)(AC)(AG)(AG)CAG-3′ and 5′-CAGCTCAGCTCCACGTGGT-3′; and for preTCR, 5′-CAGAGCCTCCTCCCCCAACAG-3′ and 5′-GCTCAGAGGGGTGGGTAAGAT-3′. PCR was performed in 25 μl of reaction containing 1× PCR buffer, 0.25 mM dNTPs, 2.5 mM MgCl2, 2.5 units Taq DNA polymerase, and appropriate dilutions of cDNA. PCR products were separated by agarose electrophoresis and visualized by using ethidium bromide.
Metabolic Labeling and Immunoprecipitation.
Thymocytes (2 × 107 cells/ml) were incubated for 1 h at 37°C in cysteine- and methionine-free culture medium and for 30 more min with 0.5 mCi/ml [35S]methionine (NEN) plus or minus 50 μg/ml ALLN. Cells were then either lysed in cold lysis buffer containing 1% Nonidet P-40 or chased by a 2-h incubation in culture medium containing nonradioactive cysteine and methionine (1 mM final concentrations) followed by cell lysis. After preclearing with protein-A-Sepharose (Amersham Pharmacia), lysates (200 μg) were incubated for 3 h at 4°C with specific Ab or rabbit preimmune serum and 25 μl of packed protein-A-Sepharose beads. The complexes were collected by centrifugation, washed in lysis buffer, fractionated over 11% SDS-polyacramide gels, and analyzed by autoradiography.
Actin Polymerization Assay.
Sorted green fluorescent protein-positive lymph node T cells infected with MIEV or MIEV-WASpΔVCA (1 × 106) were incubated for 30 min at 4°C with anti-CD3ɛ Ab (5 μg/ml) followed by a 5-min incubation with goat-anti-hamster IgG (5 μg/ml). Alternatively, cells were stimulated for 5 min with phorbol 12-myristate 13-acetate (10 ng/ml) plus ionomycin (100 ng/ml). Activation was terminated by addition of 4% paraformaldehyde. Cells were then washed, suspended in 0.1% PBS-Triton X-100 for 5 min at room temperature, incubated for 40 min with FITC-conjugated phalloidin, and analyzed by using a FACS Caliber (Becton Dickinson).
Results
Generation of WAS−/− Mice with T Lymphoid-Specific Expression of WASpΔVCA.
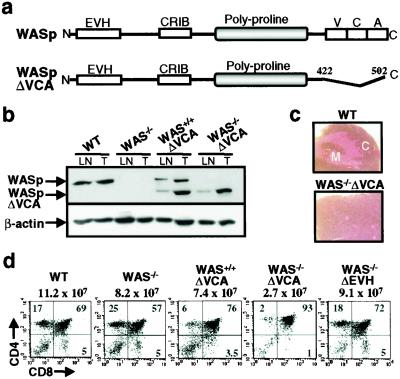
To examine the role of the VCA domain in vivo, we introduced into the WAS−/− background a transgene (WASpΔVCA) that contains a truncated WASp cDNA (amino acids 1–421) lacking the VCA domain and driven by the CD2 promoter/enhancer (Fig. 1a). This combination of promoter/enhancer element has been shown to engender lymphoid-specific expression at the early stage of lymphopoiesis (17). The integration of the transgene was screened by Southern blot analysis (data not shown). Thymocyte expression of the transgene was confirmed by immunoblotting analysis (Fig. 1b) and a selected transgenic founder, in which WASpΔVCA was expressed at a level comparable to that of endogenous WASp, was then backcrossed through multiple generations with C57BL/6 WAS−/− mice to derive WAS−/− mice with T lymphoid-specific expression of the WASpΔVCA transgene (WAS−/−ΔVCA).
Figure 1.
Characterization of WASPΔVCA mice. (a) Diagram showing the full-length WASp and the WASPΔVCA cDNA that was subcloned into the p29Δ2 vector. (b) Immunoblotting analysis with anti-WASp Ab showing WASPΔVCA transgene expression in thymocytes (THY) and lymph node T cells (LN) from WAS+/+VCA and WAS−/−ΔVCA mice. Equal loading was assessed by reprobing the blot with anti-β actin Ab. (c) Histological analysis showing the cortex (C) and medulla (M) of hematoxylin/eosin-stained thymic sections from wild-type and WASPΔVCA mice (×100). (d) Flow cytometric analysis showing CD4 and CD8 expression in thymocytes from wild-type (WT), WAS−/−, WAS+/+ΔVCA, WAS−/−ΔVCA, and WAS−/−ΔEVH1 mice. The number above each square indicates total number of thymocytes, and the numbers within each quadrant indicate percentages of total thymocytes.
Thymopoiesis Is Severely Impaired in WAS−/−ΔVCA Mice.
Previous data concerning the effects of WASp on T cell development have revealed production of mature single-positive (SP) CD4+ and CD8+ T cells to be retained in the context of WASp deficiency, although both thymic and lymph node cellularity are diminished in WAS−/− mice (2). Evaluation of the WAS−/−ΔVCA mice revealed thymic cellularity to be more adversely affected by expression of WASpΔVCA than by the absence of WASp, WASpΔVCA thymi containing 12–16% (21 ± 6.3 × 106) of the number of thymocytes present in age-matched, wild-type counterparts (126 ± 20.3 × 106) and one-quarter the number detected in WAS−/− mice (87 ± 12.1 × 106). Reduction in thymic cellularity in the WAS−/−ΔVCA animals was associated with a profound decrease in SP CD4+ and CD8+ thymocytes and a marked disruption of thymic architecture, with the medulla being essentially undetectable (Fig. 1c). This is consistent with data revealing the thymic medulla to be normally primarily composed of mature SP thymocytes (18). DP thymocytes were, however, present in the mutant mice. This thymic phenotype also was apparent in mice expressing the WASpΔVCA transgene on a wild-type background (WAS+/+ΔVCA), but was not detected in WAS−/− mice or in WAS−/− thymic populations expressing a “control” WASp transgene in which the N-terminal EVH1 rather than the C-terminal VCA domain has been deleted (Fig. 1d). These data suggest a prerequisite role for the WASp VCA domain in thymopoiesis and also imply that WASpΔVCA interferes with the function of WASp and other VCA-domain-containing proteins that normally act in concert with WASp to promote thymic differentiation. This latter possibility is supported by data revealing both N-WASp and WAVE 2 to be expressed in thymic T cells, albeit at levels somewhat lower than that of WASp (data not shown).
WASpΔVCA Expression Engenders a Block in Thymocyte Maturation.
Progression of thymocytes through the DP stage of T cell differentiation requires expression of the TCRαβ and the induction of TCR complex-mediated signals, which promote selection events and maturation to the SP stage (19). In contrast to DP thymocytes from wild-type and WAS−/− mice, however, the WAS−/−ΔVCA DP population shows negligible levels of both TCRβ and the TCR complex component CD3ɛ on the cell surface (Fig. 2a). Expression levels of the maturation marker CD69, which normally increase as DP thymocytes mature (20), are also negligible within the WAS−/−ΔVCA DP compartment as well as in the few WAS−/−ΔVCA thymocytes transiting from the DP to SP CD4+ stage (Fig. 2b). By contrast, CD25 expression, which is normally down-regulated in conjunction with pre-TCR-driven maturation of DN thymocytes (21), is present on the surface of the WAS−/−ΔVCA DP cells (Fig. 2b). Thus the DP compartment of WAS−/−ΔVCA mice is populated by thymocytes with phenotypic features (TCRlo CD69lo CD25hi) characteristic of immature thymocytes within the DN population.
Figure 2.
Thymocyte maturation is impaired in WAS−/−ΔVCA mice. (a) Flow cytometric analysis of CD4, CD8, TCRβ, and CD3ɛ expression in wild-type, WAS−/−, and WAS−/−ΔVCA thymocytes. (Top) Percentages of total thymocytes in each quadrant are shown. (Middle and Bottom) Surface expression of TCRβ and CD3ɛ on gated DP thymocytes is shown, the numbers indicating the percentage of DP cells expressing each TCR component. (b) Surface expression of TCR, CD69, and CD25 on thymocytes from WAS−/−ΔVCA and wild-type mice. The analytical gates used to identify CD4+CD8+ (DP), CD4+CD8lo transitional (T), and CD4+CD8− (SP) thymocytes are as shown (Top) and the percentages of CD69+, TCRβ+ and CD25+ cells within the subsets defined by these gates are shown (Middle and Bottom). (c) Effect of WASpΔVCA expression on the development of peripheral T cells. Lymph node T cells from wild-type, WAS−/−, WAS+/+ΔVCA, and WAS−/−ΔVCA mice were stained with anti-CD4, anti-CD8, and anti-TCRβ Abs and the cells then subjected to flow cytometric analysis. Percentages of cells found in each quadrant are indicated. The results are representative of five independent experiments.
Although the development of CD4+ and CD8+ SP cells requires TCR-generated positive selection signals, some SP T cells are detectable in the lymph nodes of WAS−/−ΔVCA mice (Fig. 2c). Numbers of these cells are dramatically reduced in the mutant (22 ± 6.4 × 106) relative to wild-type (52 ± 11.7 × 106) lymph nodes, but in contrast to the thymocytes, some peripheral T cells from both WAS+/+ΔVCA and WAS−/−ΔVCA mice express TCRαβ on the cell surface. These cells may reflect either extrathymically derived SP cells or the few TCR-expressing WAS−/−ΔVCA thymocyte precursors that successfully transit to the SP stage. Survival and expansion of such cells in the periphery is likely favored by a down-regulation in transgene expression and consequent low level of WASpΔVCA expression in lymph node T cells (Fig. 1b).
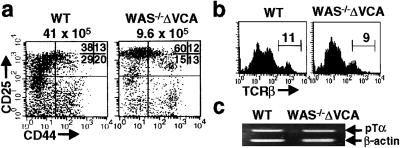
Differentiation of DN Thymocytes Is Impaired in WAS−/−ΔVCA Mice.
To further evaluate the developmental defect in WAS−/−ΔVCA thymi, expression patterns of the CD44 and CD25 maturation markers were analyzed in WAS−/−ΔVCA DN cells identified by virtue of negative staining for CD4, CD8, B220 and TCRγ/δ. CD44 and CD25 expression profiles demarcate four thymocyte subsets that represent progressive stages of maturation through the DN compartment (22). Among these subsets, the CD44−25+ population is relatively increased whereas the more mature CD44−CD25− population is diminished in WAS−/−ΔVCA compared to wild-type mice (Fig. 3a). This abnormality suggests that the maturation block conferred by WASPΔVCA expression also occurs at the DN stage despite the presence of DP and a few SP thymocytes in the mutant mice.
Figure 3.
WASpΔVCA expression impedes the terminal maturation of DN thymocytes. (a) Thymocytes were sequentially stained with biotinylated anti-CD3ɛ, anti-CD4, anti-CD8, and anti-B220 Abs, followed by Cy5-streptavidin and then phycoerythrin-anti-CD25 and FITC-anti-CD44 Abs, and flow cytometric analysis was carried out by gating to display CD25 vs. CD44 expression on Cy5-negative cells. The number above each square indicates total number of thymocytes and the numbers shown in the upper right quadrant indicate percentages of cells within each quadrant. (b) Thymocytes were stained with biotinylated anti-CD4 and anti-CD8 and FITC-labeled anti-TCRβ Abs followed by Cy5 labeling. TCRβ expression on the DN thymocytes was then analyzed by gating on Cy5-negative cells. Numbers indicate the percentages of DN cells showing medium-high expression of TCR components. (c) Total RNA was prepared from 2 × 105 DN thymocytes obtained by cell sorting and was reverse-transcribed into cDNA. The expression of the pre-TCRα chain (pTCRα) and β-actin was evaluated by RT-PCR.
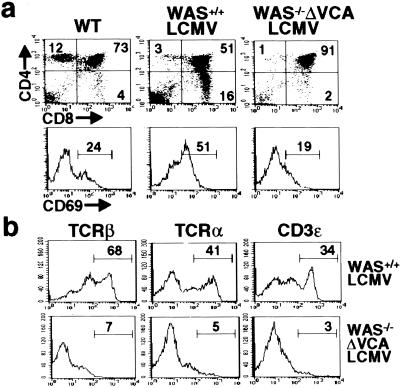
Before their transition to the DP stage, DN thymocytes must express a TCRβ chain and generate a pre-TCR complex on the cell surface capable of eliciting signals for further differentiation (30). Although DP thymocytes from WAS−/−ΔVCA mice lack TCRβ on the cell surface, both TCRβ and the preTCRα chain (pTα) are expressed on WAS−/−ΔVCA DN thymocytes, albeit at reduced levels compared to wild-type DN cells (Fig. 3 b and c). Thus WAS−/−ΔVCA thymocytes appear capable of generating a pre-TCR complex. Moreover, introduction into the WAS−/−ΔVCA mice of a transgene encoding a rearranged LCMV-specific, H-2Db restricted TCR, which normally promotes the positive selection of DP thymocytes into CD8+ SP cells (23), does not rescue thymic differentiation (Fig. 4a) and is again associated with the generation of few SP thymocytes and atypical DP cells lacking surface TCRαβ, CD3ɛ and CD69 (Fig. 4 a and b). These data are consistent with the results of a PCR-based analysis of TCRβ V(D)J rearrangements (24), which revealed the presence of both TCRβ D-J as well as V-D-J rearrangements in WAS−/−ΔVCA thymocytes (data not shown). Thus the deleterious effects of WASpΔVCA expression on thymic differentiation are not attributable to a defect in TCRβ or TCRα gene rearrangement.
Figure 4.
Positive selection is impaired in WAS−/−ΔVCA mice expressing an LCMV-specific TCR transgene. (a) Surface expression of CD4, CD8 (Upper) and CD69 (Lower) and TCRVα2, TCRVβ8.2 and CD3ɛ (b) is shown for the gated DP populations from wild-type (WT), WAS+/+LCMV, and WAS−/−ΔVCA/LCMV mice. Results are representative of four independent experiments.
Diminished Synthesis of TCRαβ Chains in WAS−/− ΔVCA Thymocytes.
Expression of the TCR complex on the surface of mature T cells requires the assembly of the TCRαβ, ζ and CD3ɛ, γ and δ chain subunits in the endoplasmic reticulum and subsequent transport through the transgolgi network to the plasma membrane (24). To determine whether impaired intracellular trafficking of these molecules contributes to the lack of TCRαβ on WAS−/−ΔVCA thymocytes, these cells were examined with respect to intracellular expression and synthesis of selected TCR components. Although thymocytes from both WAS−/−ΔVCA mice and WAS+/+ΔVCA mice showed negligible or markedly reduced intracellular expression of TCRβ, normal amounts of CD3ɛ and ζ were detected in these cells (Fig. 5a). Consistent with these observations, metabolic labeling studies of WAS−/−ΔVCA DN thymocytes also revealed levels of nascent TCRα and TCRβ proteins to be negligible in the WAS−/−ΔVCA compared to control thymocytes whereas levels of ζ chain and CD3γ and δ chains were comparable in mutant and wild-type cells (Fig. 5b). Because incompletely assembled TCR components are subject to rapid proteosome-dependent degradation (25), the possibility that proteosome-mediated proteolysis also contributes to the reduced TCRα and β chain levels found in WAS−/−ΔVCA thymocytes was explored by examining the effect of the proteosome inhibitor ALLN on the fate of newly synthesized TCRα. As revealed by analysis of pulse-labeled wild-type DP cells, nascent TCRα chain is subject to rapid degradation, but levels of the protein are markedly increased by ALLN treatment (Fig. 5c). TCRα levels also are stabilized in proteosome-inhibited WAS−/−ΔVCA thymocytes, but are not restored to the level present in wild-type thymocytes. These findings suggest that reduced TCRαβ expression in WAS−/−ΔVCA T cells does not reflect a defect in the degradation of TCRα and β chains, but may relate to impaired TCRα and TCRβ chain production. This possibility is supported by the results of a semiquantitative PCR analysis revealing levels of TCRα and β transcript to be markedly reduced in WAS+/+ΔVCA and essentially negligible in WAS−/−ΔVCA thymocytes whereas expression of CD3ɛ remains intact (Fig. 5d). Lack of TCRα and TCRβ transcripts in the WASpΔVCA-expressing cells cannot be ascribed to defective rearrangement of the TCRα or TCRβ genes as DN cells from the mutant mice express TCRβ on the cell surface and their failure to generate mature TCRhi thymocytes is not corrected by the introduction of a rearranged LCMV-specific TCR into these mutant cells. These findings indicate that expression of the TCRα and TCRβ genes is impaired in WAS−/−ΔVCA thymocytes and suggest an important role for WASpVCA domain activity in the relay of differentiation signals required for the up-regulation of TCRαβ gene expression that drives further thymic cell maturation.
Figure 5.
Diminished synthesis of TCRαβ in WAS−/−ΔVCA thymocytes. (a) DP thymocytes were detected by staining with anti-CD4, and anti-CD8 Abs and the cells were subjected to cytoplasmic staining with Abs to TCRβ, CD3ɛ, and CD3ζ. The intracellular expression of TCRαβ, CD3ɛ, and CD3ζ was then evaluated by flow cytometry. Numbers indicate percentages of cells showing medium-high expression of TCR components. (b) Synthesis of TCR subunits in wild-type (W) and WASpΔVCA (V) mice. DP thymocytes were 35S-methionine-labeled, lysed, and immunoprecipitated with Abs to TCRα, TCRβ, CD3ɛ, and CD3ζ. The precipitated proteins were fractionated by SDS/PAGE and visualized by autoradiography. (c) DP thymocytes (2 × 107 cells/ml) from WAS−/−ΔVCA (V) and wild-type (W) mice were preincubated without treatment or with 50 μM ALLN, labeled with 35S-methionine, and then chased for 2 h in complete medium. Cell lysates were subjected to immunoprecipitation with anti-TCRα Ab and resolved over SDS/PAGE. (d) cDNAs prepared from DP thymocytes were analyzed for β-actin expression by RT-PCR, and standardized amounts of template were then evaluated for expression of TCRα, TCRβ, and CD3ɛ by RT-PCR.
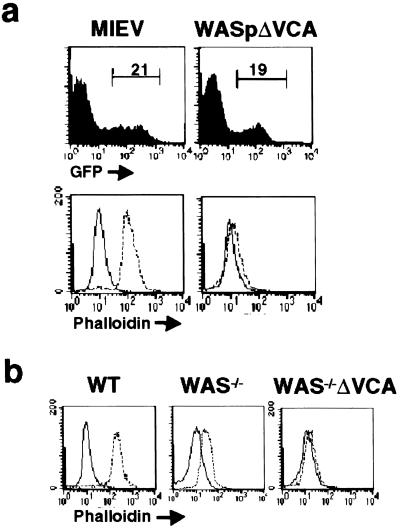
WAS−/−ΔVCA Inhibits Activation-Induced Actin Polymerization in T Cells.
The maturation defects found in WAS−/−ΔVCA mice identify a critical role for WASp/WASp-related proteins in promoting early thymic development and suggest that their capacity to do so absolutely depends on the presence of the VCA domain. Because a link between the VCA domain and inducible actin polymerization has not been directly established in lymphocytes, the effect of retrovirally mediated WASpΔVCA expression on TCR-evoked lymphocyte actin polymerization was also examined. As shown by immunofluorescence analysis, TCR engagement induces rapid F-actin formation in empty vector-infected phalloidin-stained lymphocytes from wild-type mice, but is essentially undetectable in WASpΔVCA-expressing cells (Fig. 6a). Similarly, PMA-evoked actin polymerization is markedly reduced in WAS−/−ΔVCA DP thymocytes compared to both wild-type and WAS−/− cells (Fig. 6b). These data confirm the association of the WASpΔVCA-mediated block in thymocyte maturation with impaired induction of actin polymerization and thus support the conclusion of an obligate role for WASp-evoked actin polymerization in coupling upstream stimulatory signal to thymocyte maturation.
Figure 6.
Effect of WASpΔVCA expression on inducible actin polymerization. (a) Lymph node T cells from C57BL/6 mice were infected with control retrovirus or retrovirus-encoding WASPΔVCA protein. The green fluorescent protein-expressing cells detected by flow cytometry (Upper) were purified by cell sorting and stimulated with anti-CD3 Ab, and their F-actin content was quantitated by FITC-phalloidin staining and flow cytometric analysis (Lower). (b) After stimulation with PMA/ionomycin, thymocytes from wild-type, WAS−/−, and WAS−/−ΔVCA mice were stained with FITC-phalloidin, and their F-actin content was measured by flow cytometry.
Discussion
Cytoskeletally mediated processes such as cellular polarization, increased adhesiveness, and immunologic synapse formation are now recognized as essential steps in the activation of mature T cells (26). Although actin polymerization and the proteins, such as WASp, which modulate actin remodeling, have emerged as central elements in the T cell response to antigen stimulation, the role of actin cytoskeletal rearrangement in directing T cell differentiation is not well established. In the current study, we demonstrate that the WASp VCA domain, a region that couples WASp to Arp2/3 complex-mediated actin polymerization, is absolutely required for the generation of mature CD4+ and CD8+ SP thymocytes and for the induction of actin polymerization after TCR engagement. The data reveal expression of VCA domain-deleted WASp on the WAS−/− background to be associated with a severe reduction in thymocyte numbers and with the production of DP thymocytes that fail to express TCRs on the cell surface consequent to impaired expression of the TCRα and TCRβ chain genes. These results indicate that WASp-mediated actin polymerization promotes the differentiation of immature thymocytes and identify a critical role for the VCA domain in enabling WASp to subserve this role. Thus the induction of actin polymerization is essential to the differentiative and proliferative events that drive the early stages of T lymphopoiesis and the subsequent production of mature SP T cells.
Although thymocyte numbers are reduced in WAS−/− mice, these animals produce mature CD4+ and CD8+ SP cells, and their thymopoietic defect is thus substantively less severe than that conferred by WASpΔVCA expression (2). These observations suggest that WASp functions in T cells can be mediated at least in part by other proteins, the effector activities of which are impeded by WASpΔVCA expression. This possibility is supported by genetic data suggesting that the lymphocyte dysfunction conferred by WAS gene mutation is subject to modifier effects (27, 28) and also by the presence in T lineage cells of N-WASp and WAVE 2, proteins showing considerable functional redundancy with WASp (11–15). Involvement of these VCA domain-containing proteins in the regulation of actin remodeling in lymphoid cells is implied by their demonstrated capacity to couple extracellular stimuli to actin cytoskeletal rearrangement in other cell types (15, 29) and by data showing TCR-evoked actin polymerization in Jurkat T cells to be inhibited by ectopic expression of the WAVE VCA domain (30). These observations suggest that WASpΔVCA acts in dominant negative fashion to compete with VCA domain-containing member(s) of WASp family proteins for binding to upstream effectors that provide stimulatory signals required to induce VCA domain-mediated actin polymerization. Similarly, actin comet formation in fibroblasts, a process implicated in vesicular trafficking, is inhibited by overexpression of VCA domain-deleted WASp and consequent disruption of the WASp-Arp2/3 pathway (31). The relevance of the VCA domain specifically to thymocyte development is also supported by the lack of effect of an EVH1-deleted WASp transgene on thymic differentiation. Together these data suggest that WASp family proteins act in concert to generate the VCA domain-dependent actin polymerization required for the differentiation of T cells and likely for induction of many other biological responses.
During normal thymic development, immature DN thymocytes progress through distinct stages of differentiation that lead to TCRβ chain gene rearrangement and the generation of a pre-TCR complex that provides proliferative and differentiative signals required for transition to the DP stage (21). Expression of the pre-TCR and the ensuing relay of pre-TCR-derived differentiation signals define a developmental checkpoint, β-selection, which occurs during the terminal stage of DN maturation and promotes transition from the CD44−CD25+ to the CD44−CD25− stage (32). Thus the present results, which reveal progression of DN thymocytes from the CD44−CD25+ to CD44−CD25− stages to be severely impaired in WAS−/−ΔVCA thymi, suggest a pivotal role for WASp in promoting the late DN and early DP stages of development. In this regard, the thymic phenotype of WAS−/−ΔVCA mice closely resembles that of mice lacking the ZAP-70 protein tyrosine kinase, a signaling effector that plays a central role in modulating both TCR and pre-TCR signaling function (33, 34). This observation, together with the current data revealing TCRβ and pTα to be expressed in WAS−/−ΔVCA DN thymocytes, suggest that WASpΔVCA expression does not impair formation of the pre-TCR, but instead compromises downstream delivery of pre-TCR signals required for proliferative expansion and maturation of late-stage DN thymocytes. This conclusion is consistent with the failure of a rearranged TCR transgene to mitigate the thymopoietic defects of WAS−/−ΔVCA mice and also with the arrest of thymic cell maturation at a later stage in WAS−/−ΔVCA mice than in mice in which TCRβ, pre-TCR, or RAG deficiency abrogate pre-TCR formation (35–37). Pre-TCR signaling is not, however, required for expression of CD4 and CD8 (35) and thus the disruption of pre-TCR signal delivery does not prevent the genesis of a DP thymocyte population in WAS−/−ΔVCA mice. Interestingly, the Rho GTPase, another effector of actin cytoskeletal rearrangement (38), is also thought to mediate its effects on the terminal differentiation of DN thymocytes by means of modulation of pTα-initiated signals required for proliferation and cell survival (39). Although this stage of thymocyte development is also impaired in WAS−/− mice, in these latter animals, induction of TCRαβ expression and, by inference, the level of pre-TCR signal, is sufficient to drive the development of a sizeable TCRhi DP and SP T cell population. Thus it appears likely that pre-TCR/TCR signaling is modulated by one or more WASp-related proteins that overlap with WASp in relation to their interactions with upstream signaling effectors as well as their use of a VCA domain to couple stimulatory signals to thymocyte differentiation. Together these data suggest that WASp family proteins play key roles in driving the terminal maturation of DN thymocytes and identify an essential role for VCA domain-mediated actin polymerization in mediating this critical stage in thymocyte differentiation.
Our data indicating that WASp VCA domain-mediated actin polymerization is required for maturation of pre-TCR-expressing DN thymocytes is consistent with recent data revealing a prerequisite role for the Drosophila WASp homolog in the cell-fate decisions mediated by Notch signaling (40). The Notch signaling cascade also has been implicated in the regulation of thymopoiesis (41), and the possibility that WASp effects on T cell development involve its intersection with this pathway thus merits further investigation. Similarly, the mechanisms whereby actin cytoskeletal rearrangement modulate thymopoiesis are also undefined, although these are likely to include the modulation of fundamental dynamic cellular processes such as vesicular trafficking and antigen receptor endocytosis (42–44). Actin microfilament reorganization also endows the cell with a structural scaffold that is likely required for assembling and stabilizing the molecular complexes, which connect pre-TCR/TCR signals to T cell differentiation (26). Resolution of the extent to which these and other actin-dependent processes underlie WASp effects on lymphocyte development should lay the groundwork for elucidating the molecular interactions whereby stimulatory signals are structurally and transcriptionally integrated so as to evoke the appropriate biological outcomes.
Acknowledgments
We thank Drs. Paul Love and Pam Ohashi for providing the p29Δ2 vector and P14 TCR transgenic mice, respectively. This work was supported by a grant from the Canadian Institutes for Health Research. K.A.S. holds Senior Scientist awards from the Canadian Institutes for Health Research and the Arthritis Society of Canada; J.Z. holds a fellowship award from the Canadian Arthritis Network; M.M. holds a fellowship award from the University of Toronto Department of Medicine.
Abbreviations
- VCA
verprolin homology, cofilin homology, and acidic region
- TCR
T cell antigen receptor
- EVH1
Ena-Vasp homology 1
- ALLN
N-acetyl-l-leucinal-l-leucinal-l-norleucinal
- RT
reverse transcription
- SP
single-positive
- DP
double-positive
- DN
double-negative
- LCMV
lymphocytic choriomeningitis virus
- MIEV
murine stem cell virus-based internal ribosome entry site-EGFP virus
References
- 1.Snapper S B, Rosen F S. Annu Rev Immunol. 1999;17:905–929. doi: 10.1146/annurev.immunol.17.1.905. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Shehabeldin A, da Cruz L A, Butler J, Somani A K, McGavin M, Kozieradzki I, dos Santos A O, Nagy A, Grinstein S, et al. J Exp Med. 1999;190:1329–1342. doi: 10.1084/jem.190.9.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molina I J, Sancho J, Terhorst C, Rosen F S, Remold-O'Donnell E. J Immunol. 1993;151:4384–4390. [PubMed] [Google Scholar]
- 4.Fawcett J, Pawson T. Science. 2000;290:725–726. doi: 10.1126/science.290.5492.725. [DOI] [PubMed] [Google Scholar]
- 5.Machesky L M, Insall R H. Curr Biol. 1998;8:1347–1356. doi: 10.1016/s0960-9822(98)00015-3. [DOI] [PubMed] [Google Scholar]
- 6.Rohatgi R, Ma L, Miki H, Lopez M, Kirchhausen T, Takenawa T, Kirschner M W. Cell. 1999;97:221–231. doi: 10.1016/s0092-8674(00)80732-1. [DOI] [PubMed] [Google Scholar]
- 7.Marchand J-B, Kaiser D, Pollard T, Higgs H N. Nat Cell Biol. 2001;3:76–82. doi: 10.1038/35050590. [DOI] [PubMed] [Google Scholar]
- 8.Cannon J L, Labno C M, Bosco G, Seth A, McGavin M H K, Siminovitch K A, Rosen M K, Burkhardt J K. Immunity. 2001;15:249–259. doi: 10.1016/s1074-7613(01)00178-9. [DOI] [PubMed] [Google Scholar]
- 9.McGavin M K H, Badour K, Hardy L A, Kubiseski T J, Zhang J, Siminovitch K A. J Exp Med. 2001;194:1–12. doi: 10.1084/jem.194.12.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prehoda K E, Scott J A, Dyche Mullins R, Lim W A. Science. 2000;290:801–806. doi: 10.1126/science.290.5492.801. [DOI] [PubMed] [Google Scholar]
- 11.Miki H, Miura K, Takenawa T. EMBO J. 1996;15:5326–5335. [PMC free article] [PubMed] [Google Scholar]
- 12.Bear J E, Rawls J F, Saxe C L., III J Cell Biol. 1998;142:1325–1335. doi: 10.1083/jcb.142.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miki H, Suetsugu S, Takenawa T. EMBO J. 1998;17:6932–6941. doi: 10.1093/emboj/17.23.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suetsugu S, Miki H, Takenawa T. Biochem Biophys Res Commun. 1999;260:296–302. doi: 10.1006/bbrc.1999.0894. [DOI] [PubMed] [Google Scholar]
- 15.Takenawa T, Miki H. J Cell Sci. 2001;114:1801–1809. doi: 10.1242/jcs.114.10.1801. [DOI] [PubMed] [Google Scholar]
- 16.Pirchner H, Burki K, Lang R, Hengartner H, Zinkernagel R M. Nature (London) 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 17.DeJarnette J B, Sommers C L, Huang K, Woodside K J, Emmons R, Katz K, Shores E W, Love P E. Proc Natl Acad Sci USA. 1998;95:14909–14914. doi: 10.1073/pnas.95.25.14909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Surh C D, Ernst B, Sprent J. J Exp Med. 1992;176:611–616. doi: 10.1084/jem.176.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shinkai Y, Koyasu S, Nakayama K, Murphy K M, Loh D Y, Reinherz E L, Alt F W. Science. 1993;259:822–825. doi: 10.1126/science.8430336. [DOI] [PubMed] [Google Scholar]
- 20.Swat W, Dessing M, von Boehmer H, Kisielow P. Eur J Immunol. 1993;23:739–726. doi: 10.1002/eji.1830230326. [DOI] [PubMed] [Google Scholar]
- 21.von Boehmer H, Aifantis I, Feinberg J, Lechner O, Saint-Ruf C, Walter U, Buer J, Azogui O. Curr Opin Immunol. 1999;11:135–142. doi: 10.1016/s0952-7915(99)80024-7. [DOI] [PubMed] [Google Scholar]
- 22.Godfrey D I, Kennedy J, Suda T, Zlotnik A. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- 23.Gu Y, Seidl K J, Rathbun G A, Zhu C, Manis J P, van der Stoep N, Davidson L, Cheng H L, Sekiguchi J M, Frank K, et al. Immunity. 1997;7:653–665. doi: 10.1016/s1074-7613(00)80386-6. [DOI] [PubMed] [Google Scholar]
- 24.Klausner R D, Lippincott-Schwartz J, Bonifacino J S. Annu Rev Cell Biol. 1990;6:403–431. doi: 10.1146/annurev.cb.06.110190.002155. [DOI] [PubMed] [Google Scholar]
- 25.Yu H, Kaung G, Kobayashi S, Kopito R R. J Biol Chem. 1997;272:20800–20804. doi: 10.1074/jbc.272.33.20800. [DOI] [PubMed] [Google Scholar]
- 26.Dustin M L, Cooper J A. Nat Immunol. 2000;1:23–29. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- 27.Greer W L, Shehabeldin A, Schulman J, Junker A, Siminovitch K A. Hum Genet. 1996;98:685–690. doi: 10.1007/s004390050285. [DOI] [PubMed] [Google Scholar]
- 28.Schindelhauer D, Weiss M, Hellebrand H, Golla A, Hergersberg M, Seger R, Belohradsky B H, Meindl A. Hum Genet. 1996;98:68–76. doi: 10.1007/s004390050162. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi H, Miki H, Suetsugu S, Ma L, Kirschner M W, Takenawa T. Proc Natl Acad Sci USA. 2000;97:12631–12636. doi: 10.1073/pnas.190351397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krause M, Sechi A S, Kondradt M, Monner D, Gertler F B, Wehland J. J Cell Biol. 2000;149:181–194. doi: 10.1083/jcb.149.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rozelle A L, Machesky L M, Yamamoto M, Driessens M H, Insall R H, Roth M G, Luby-Phelps K, Marriott G, Hall A, Yin H L. Curr Biol. 2000;10:311–320. doi: 10.1016/s0960-9822(00)00384-5. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman E S, Passoni L, Crompton T, Leu T M, Schatz D G, Koff A, Owen M J, Hayday A C. Genes Dev. 1996;10:948–962. doi: 10.1101/gad.10.8.948. [DOI] [PubMed] [Google Scholar]
- 33.Negishi I, Motoyama N, Nakayama K, Nakayama K, Senju S, Hatakeyama S, Zhang Q, Chan A C, Loh D Y. Nature (London) 1995;376:435–438. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 34.Saint-Ruf C, Panigada M, Azogui O, Debey P, von Boehmer H, Grassi F. Nature (London) 2000;406:524–527. doi: 10.1038/35020093. [DOI] [PubMed] [Google Scholar]
- 35.Mombaerts P, Clarke A R, Rudnicki M A, Iacomini J, Itohara S, Lafaille J J, Wang L, Ichikawa Y, Jaenisch R, Hooper M L, et al. Nature (London) 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 36.Fehling H J, Krotkova A, Saint-Ruf C, von Boehmer H. Nature (London) 1995;375:795–798. doi: 10.1038/375795a0. [DOI] [PubMed] [Google Scholar]
- 37.Mombaerts P, Iacomini J, Johnson R S, Herrup K, Tonegawa S, Papaioannou V E. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 38.Nobes C, Hall A. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 39.Henning S W, Galandrini R, Hall A, Cantrell D A. EMBO J. 1997;16:2397–2407. doi: 10.1093/emboj/16.9.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben-Yaacov S, Le Borgne R, Abramson I, Schwisguth F, Schejter E D. J Cell Biol. 2001;152:1–13. doi: 10.1083/jcb.152.1.1-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robey E, Chang D, Itano A, Cado D, Alexander H, Lans D, Weinmaster G, Salmon P. Cell. 1996;87:483–492. doi: 10.1016/s0092-8674(00)81368-9. [DOI] [PubMed] [Google Scholar]
- 42.Lamaze C, Fujimoto L M, Yin H L, Schmid S L. J Biol Chem. 1997;272:20332–20335. doi: 10.1074/jbc.272.33.20332. [DOI] [PubMed] [Google Scholar]
- 43.Hsu V W, Peter P J. Adv Immunol. 1998;70:369–415. doi: 10.1016/s0065-2776(08)60391-0. [DOI] [PubMed] [Google Scholar]
- 44.Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. J Exp Med. 1995;181:577–584. doi: 10.1084/jem.181.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]