Abstract
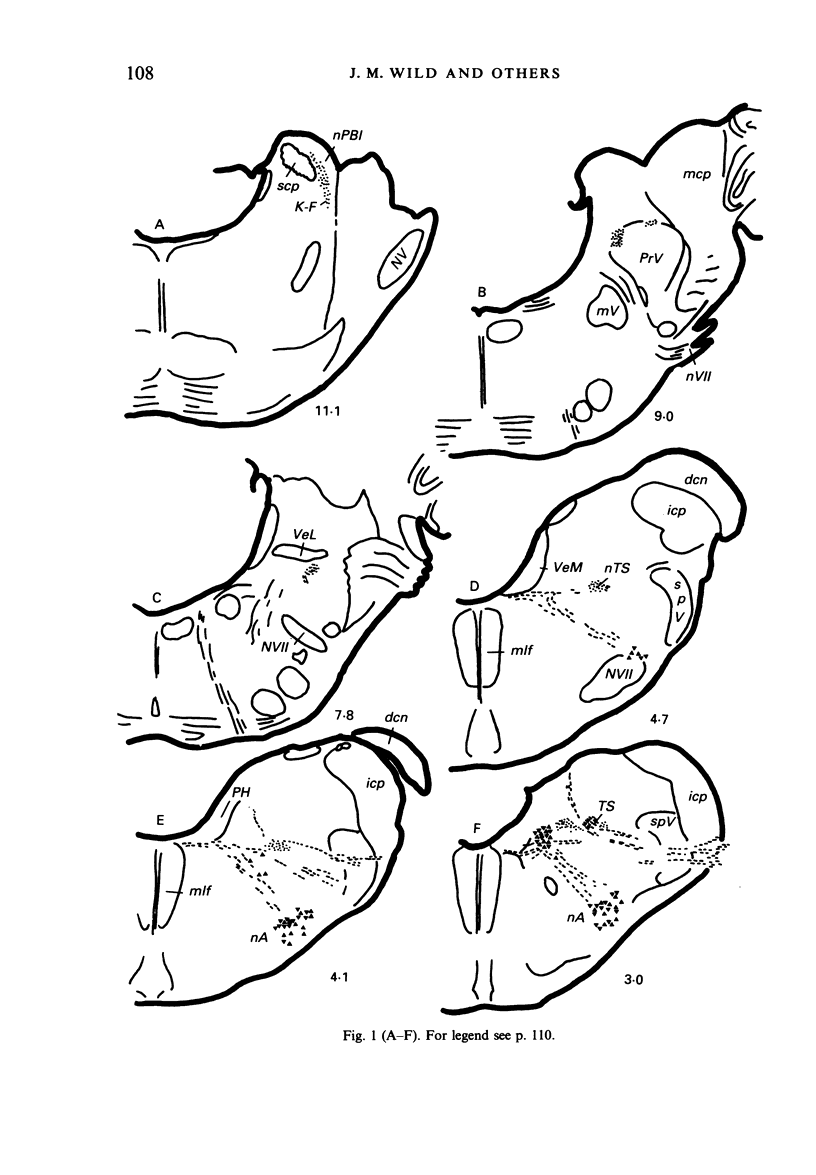
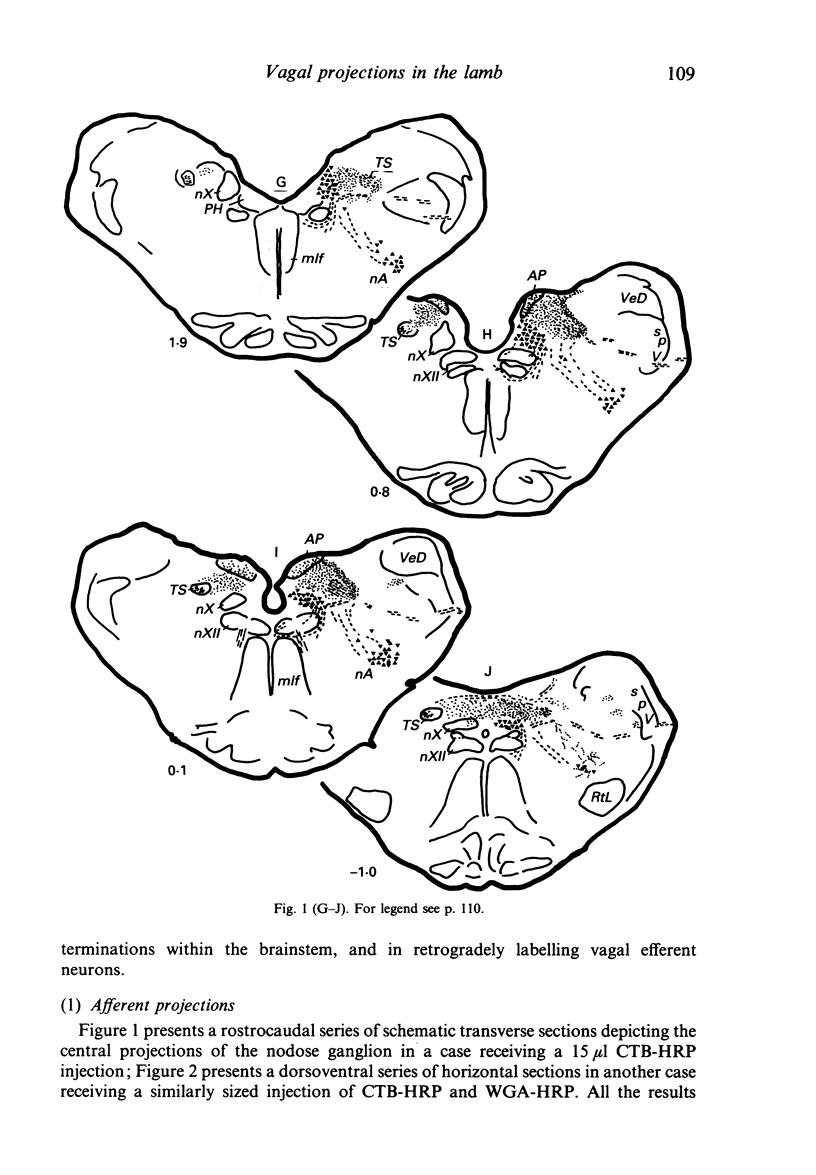
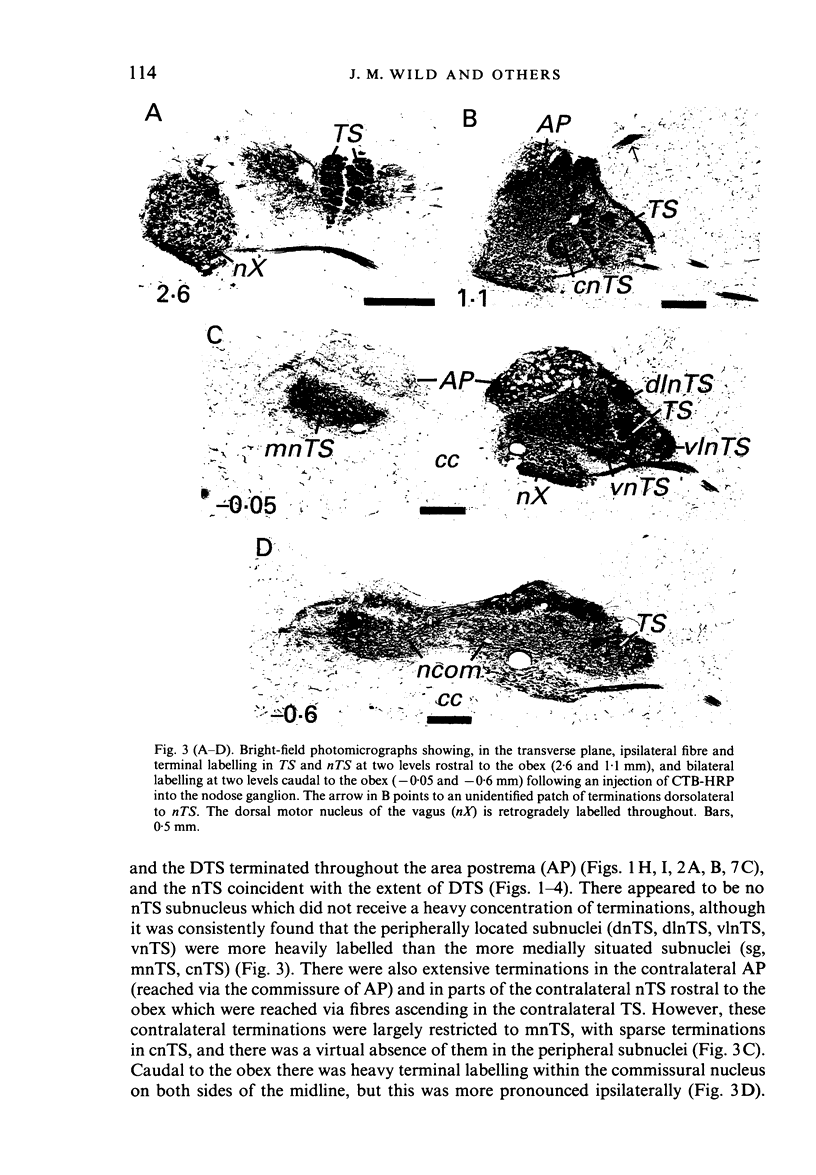
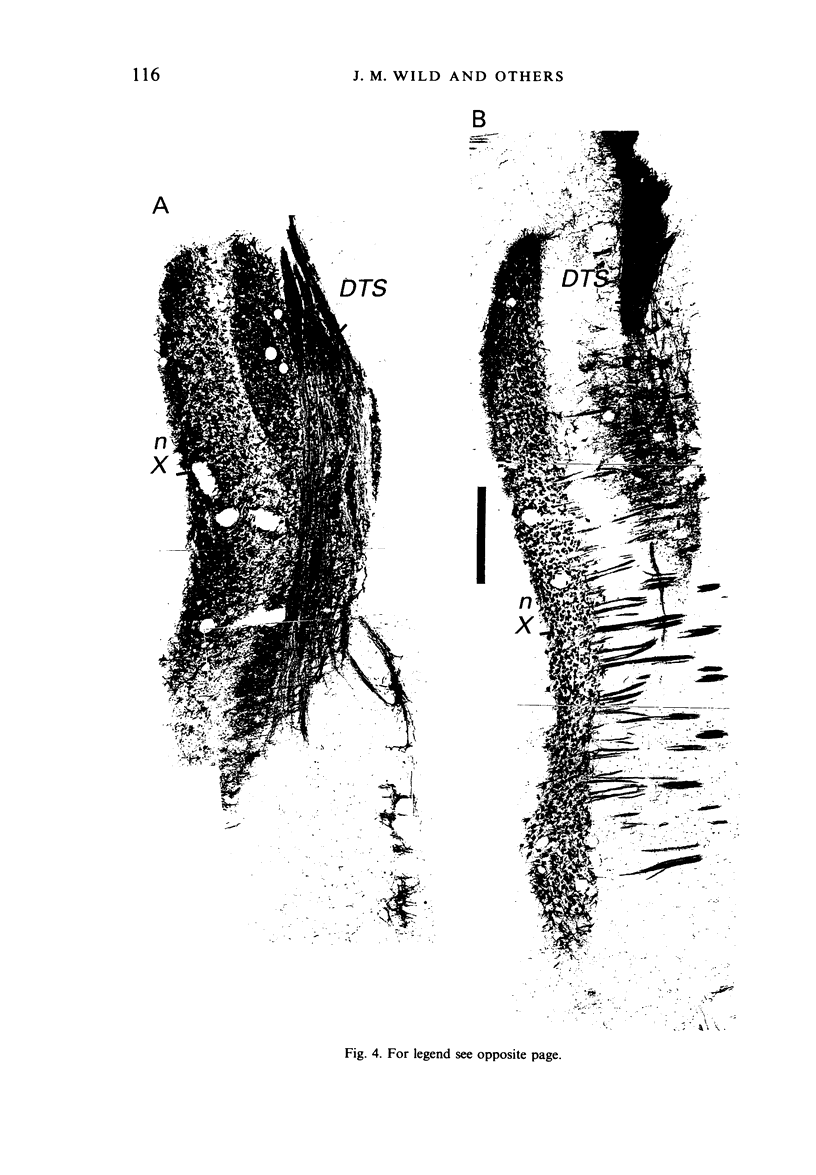
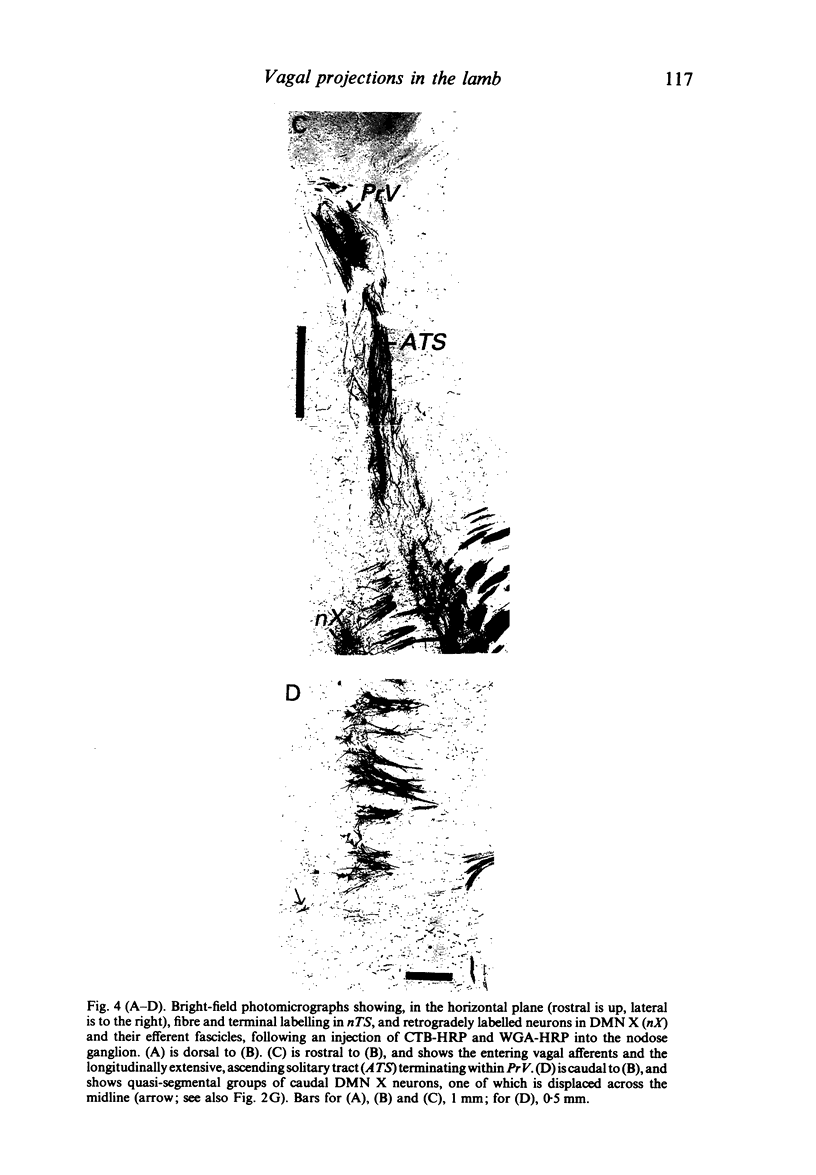
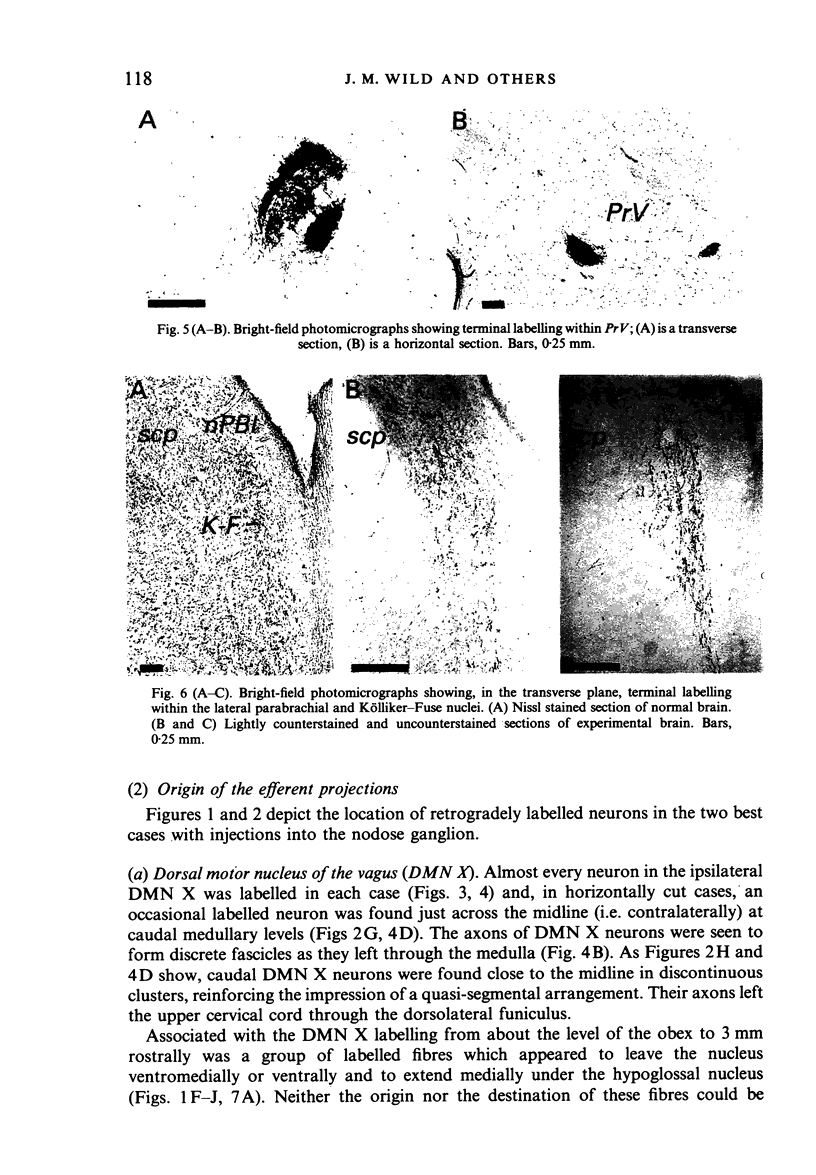
Injections of WGA-HRP and CTB-HRP were made into the cervical vagus or the nodose ganglion in a series of lambs, in order to define the sensory projections and motor origins of the vagus nerve. Injections into the nodose ganglion were much more successful than injections into the cervical vagus in effecting the desired result. The former produced labelling of both descending and ascending components of the solitary tract (TS). The descending component terminated massively in all ipsilateral and certain contralateral subnuclei of the nucleus of the solitary tract (nTS) and in the upper cervical spinal cord. Patchy terminations were also observed within the interpolated subnucleus of the nucleus of the spinal trigeminal tract, and within Lamina I of the upper cervical cord. The ascending component of TS terminated in rostral regions of the nTS, and in specific portions of the principal sensory trigeminal nucleus and the lateral parabrachial and Kölliker-Fuse nuclei. The motor origins of the vagus nerve arose almost completely ipsilaterally in the dorsal motor nucleus of the vagus, the nucleus ambiguus, and the caudal portion of the nucleus retroambiguus situated in the lateral part of the intermediate grey at upper cervical spinal levels. Labelled neurons in the nucleus dorsomedialis of the upper spinal cord were thought not to project their axons into the cervical vagus.
Full text
PDF


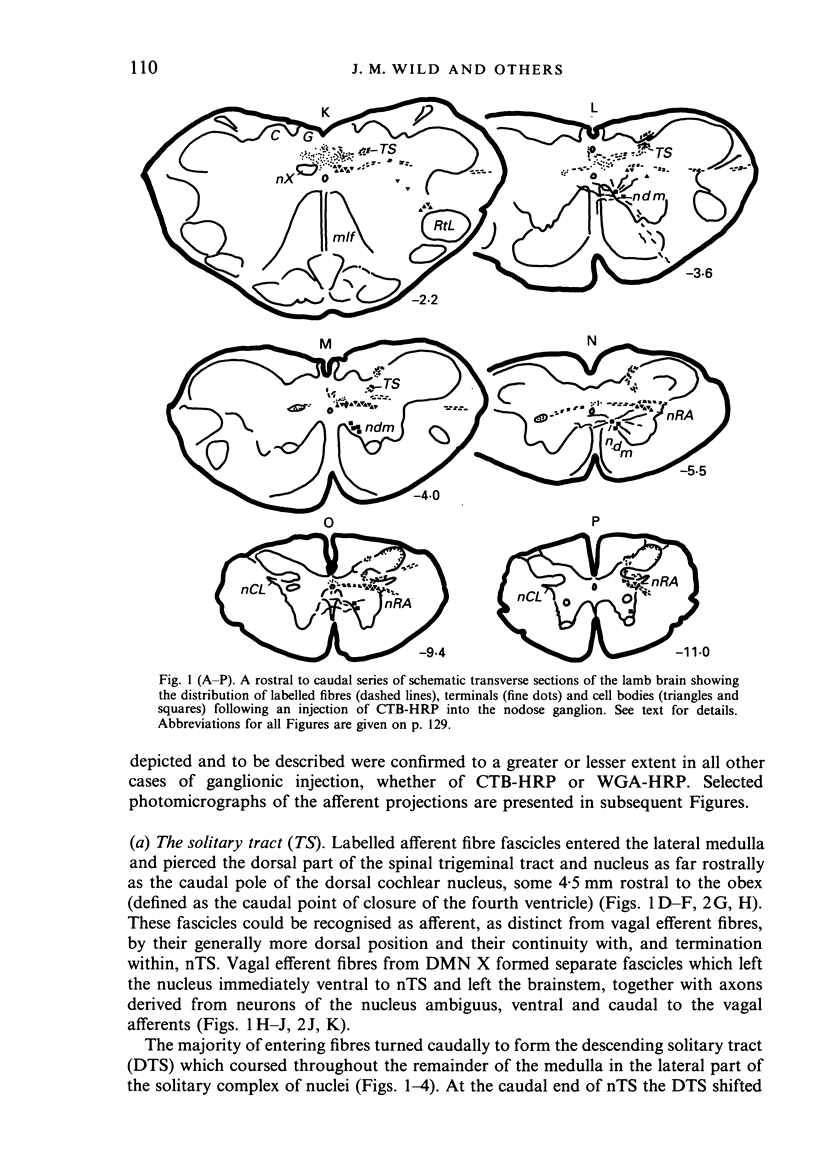
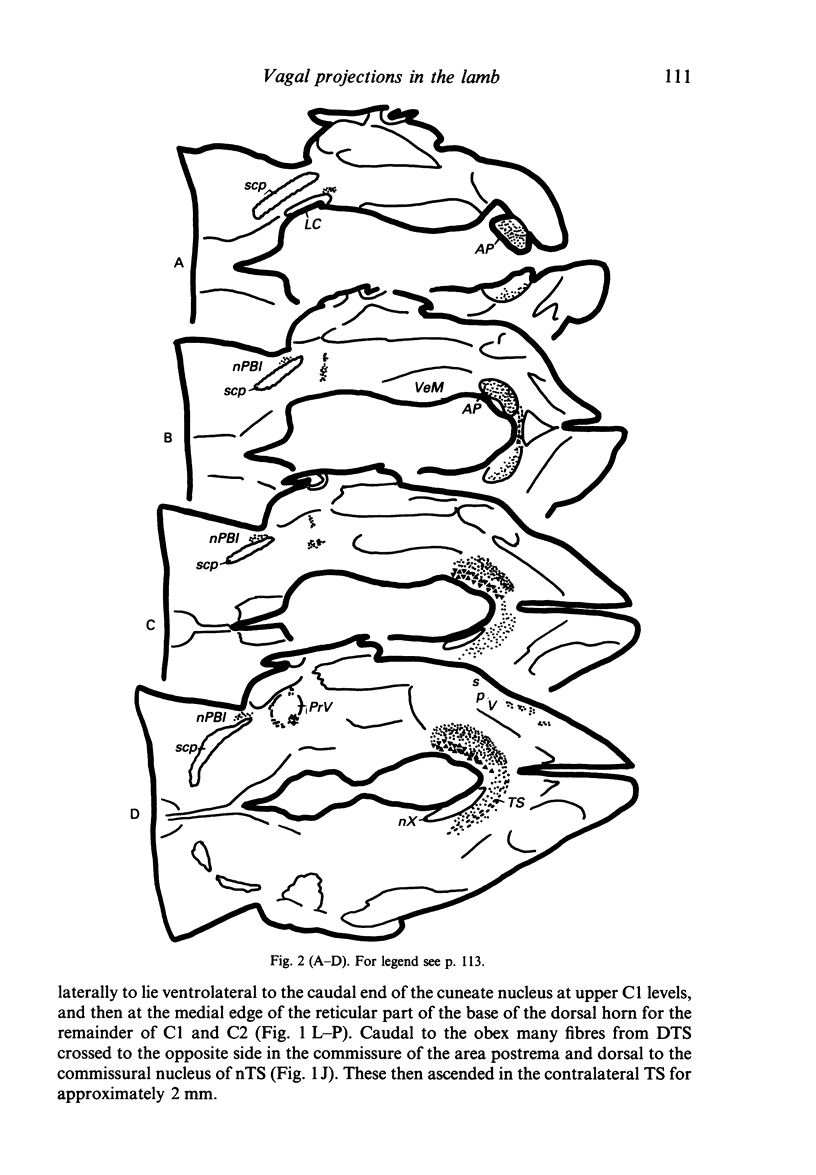
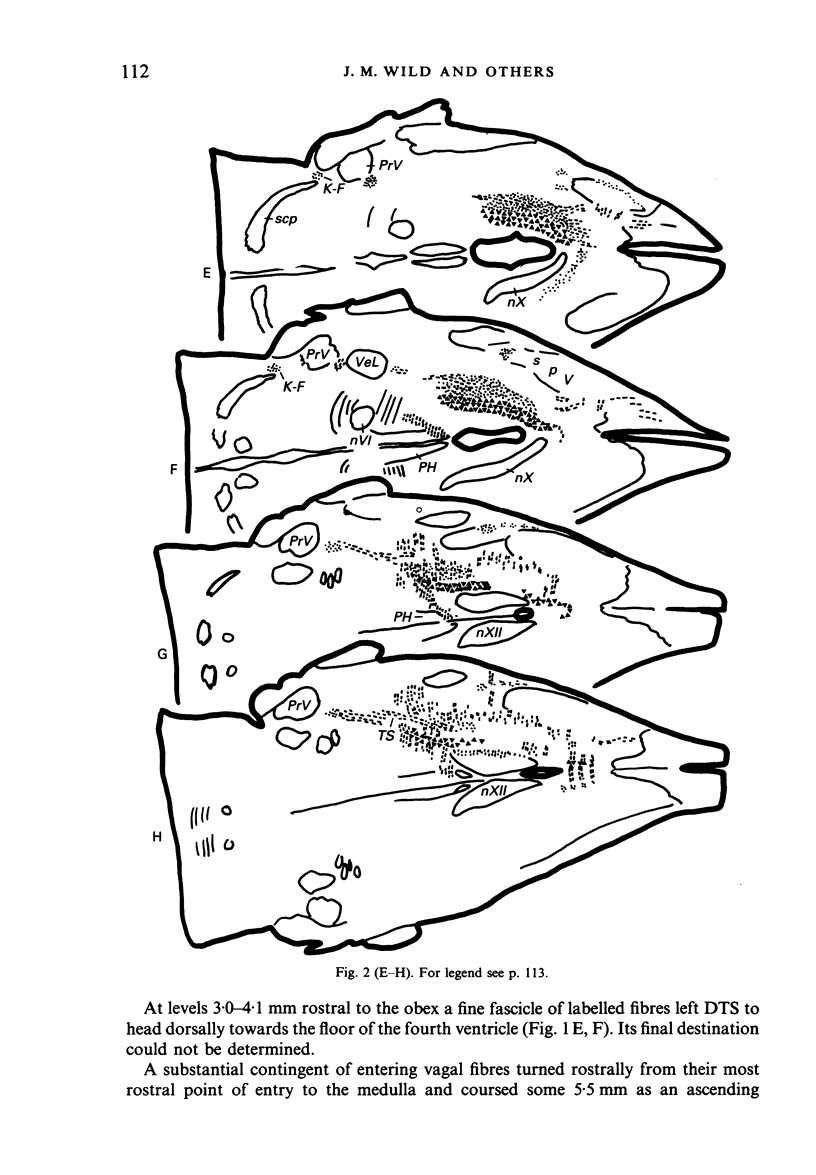
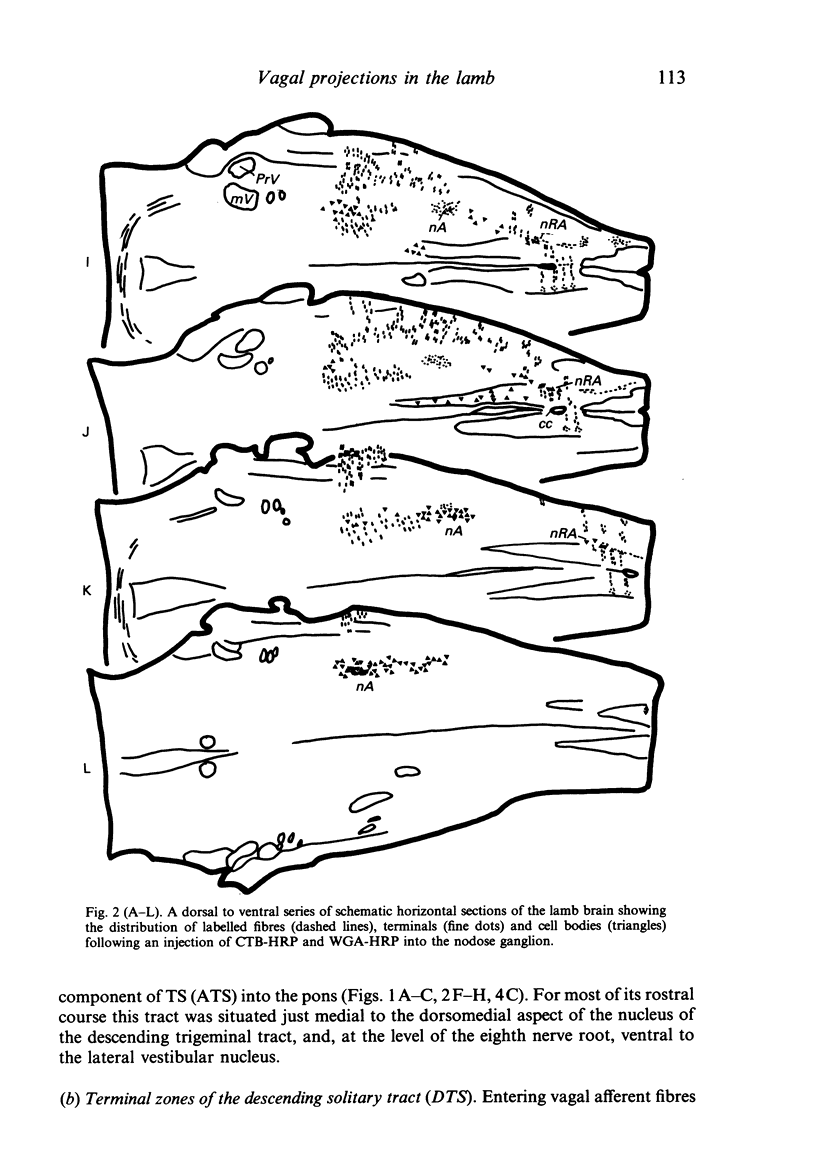










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschuler S. M., Bao X. M., Bieger D., Hopkins D. A., Miselis R. R. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol. 1989 May 8;283(2):248–268. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
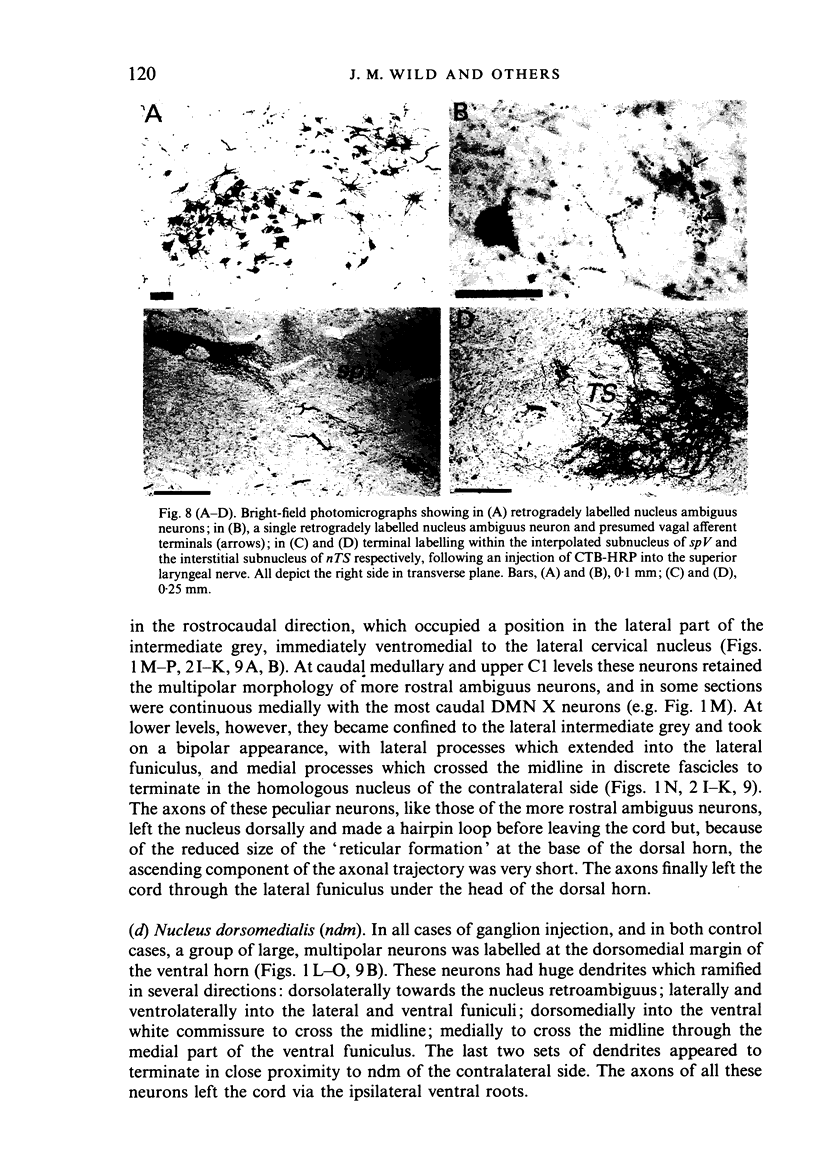
- Amri M., Car A., Jean A. Medullary control of the pontine swallowing neurones in sheep. Exp Brain Res. 1984;55(1):105–110. doi: 10.1007/BF00240503. [DOI] [PubMed] [Google Scholar]
- Amri M., Car A. Projections from the medullary swallowing center to the hypoglossal motor nucleus: a neuroanatomical and electrophysiological study in sheep. Brain Res. 1988 Feb 16;441(1-2):119–126. doi: 10.1016/0006-8993(88)91389-3. [DOI] [PubMed] [Google Scholar]
- Barbas-Henry H. A., Lohman A. H. The motor nuclei and primary projections of the IXth, Xth, XIth and XIIth cranial nerves in the monitor lizard, Varanus exanthematicus. J Comp Neurol. 1984 Jul 10;226(4):565–579. doi: 10.1002/cne.902260409. [DOI] [PubMed] [Google Scholar]
- Barry M. A. Central connections of the IXth and Xth cranial nerves in the clearnose skate, Raja eglanteria. Brain Res. 1987 Nov 3;425(1):159–166. doi: 10.1016/0006-8993(87)90494-x. [DOI] [PubMed] [Google Scholar]
- Beckstead R. M., Norgren R. An autoradiographic examination of the central distribution of the trigeminal, facial, glossopharyngeal, and vagal nerves in the monkey. J Comp Neurol. 1979 Apr 1;184(3):455–472. doi: 10.1002/cne.901840303. [DOI] [PubMed] [Google Scholar]
- Bieger D., Hopkins D. A. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J Comp Neurol. 1987 Aug 22;262(4):546–562. doi: 10.1002/cne.902620408. [DOI] [PubMed] [Google Scholar]
- Callister R. J., Brichta A. M., Peterson E. H. Quantitative analysis of cervical musculature in rats: histochemical composition and motor pool organization. II. Deep dorsal muscles. J Comp Neurol. 1987 Jan 15;255(3):369–385. doi: 10.1002/cne.902550305. [DOI] [PubMed] [Google Scholar]
- Car A., Amri M. Activity of neurons located in the region of the hypoglossal motor nucleus during swallowing in sheep. Exp Brain Res. 1987;69(1):175–182. doi: 10.1007/BF00247040. [DOI] [PubMed] [Google Scholar]
- Car A., Jean A., Roman C. A pontine primary relay for ascending projections of the superior laryngeal nerve,. Exp Brain Res. 1975;22(2):197–210. doi: 10.1007/BF00237689. [DOI] [PubMed] [Google Scholar]
- Chernicky C. L., Barnes K. L., Ferrario C. M., Conomy J. P. Afferent projections of the cervical vagus and nodose ganglion in the dog. Brain Res Bull. 1984 Sep;13(3):401–411. doi: 10.1016/0361-9230(84)90090-x. [DOI] [PubMed] [Google Scholar]
- Ciriello J., Hrycyshyn A. W., Calaresu F. R. Glossopharyngeal and vagal afferent projections to the brain stem of the cat: a horseradish peroxidase study. J Auton Nerv Syst. 1981 Jun;4(1):63–79. doi: 10.1016/0165-1838(81)90007-2. [DOI] [PubMed] [Google Scholar]
- Contreras R. J., Beckstead R. M., Norgren R. The central projections of the trigeminal, facial, glossopharyngeal and vagus nerves: an autoradiographic study in the rat. J Auton Nerv Syst. 1982 Nov;6(3):303–322. doi: 10.1016/0165-1838(82)90003-0. [DOI] [PubMed] [Google Scholar]
- Culberson J. L., Kimmel D. L. Central distribution of primary afferent fibers of the glossopharyngeal and vagal nerves in the opossum, Didelphis virginiana. Brain Res. 1972 Sep 29;44(2):325–335. doi: 10.1016/0006-8993(72)90306-x. [DOI] [PubMed] [Google Scholar]
- Cunningham E. T., Jr, Sawchenko P. E. A circumscribed projection from the nucleus of the solitary tract to the nucleus ambiguus in the rat: anatomical evidence for somatostatin-28-immunoreactive interneurons subserving reflex control of esophageal motility. J Neurosci. 1989 May;9(5):1668–1682. doi: 10.1523/JNEUROSCI.09-05-01668.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. O., Kalia M. Carotid sinus nerve projections to the brain stem in the cat. Brain Res Bull. 1981 Jun;6(6):531–541. doi: 10.1016/s0361-9230(81)80028-7. [DOI] [PubMed] [Google Scholar]
- Dubbeldam J. L., Brus E. R., Menken S. B., Zeilstra S. The central projections of the glossopharyngeal and vagus ganglia in the mallard, Anas platyrhynchos L. J Comp Neurol. 1979 Jan 1;183(1):149–168. doi: 10.1002/cne.901830111. [DOI] [PubMed] [Google Scholar]
- Fitzakerley J. L., Lucier G. E. Connections of a vagal communicating branch in the ferret. I. Pathways and cell body location. Brain Res Bull. 1988 Feb;20(2):189–196. doi: 10.1016/0361-9230(88)90178-5. [DOI] [PubMed] [Google Scholar]
- Fox E. A., Powley T. L. Longitudinal columnar organization within the dorsal motor nucleus represents separate branches of the abdominal vagus. Brain Res. 1985 Aug 26;341(2):269–282. doi: 10.1016/0006-8993(85)91066-2. [DOI] [PubMed] [Google Scholar]
- Fryscak T., Zenker W., Kantner D. Afferent and efferent innervation of the rat esophagus. A tracing study with horseradish peroxidase and nuclear yellow. Anat Embryol (Berl) 1984;170(1):63–70. doi: 10.1007/BF00319459. [DOI] [PubMed] [Google Scholar]
- Gluckman P. D., Johnston B. M. Lesions in the upper lateral pons abolish the hypoxic depression of breathing in unanaesthetized fetal lambs in utero. J Physiol. 1987 Jan;382:373–383. doi: 10.1113/jphysiol.1987.sp016372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwyn D. G., Leslie R. A., Hopkins D. A. Observations on the afferent and efferent organization of the vagus nerve and the innervation of the stomach in the squirrel monkey. J Comp Neurol. 1985 Sep 8;239(2):163–175. doi: 10.1002/cne.902390204. [DOI] [PubMed] [Google Scholar]
- Hamilton R. B., Norgren R. Central projections of gustatory nerves in the rat. J Comp Neurol. 1984 Feb 1;222(4):560–577. doi: 10.1002/cne.902220408. [DOI] [PubMed] [Google Scholar]
- Hamilton R. B., Pritchard T. C., Norgren R. Central distribution of the cervical vagus nerve in Old and New World primates. J Auton Nerv Syst. 1987 May;19(2):153–169. doi: 10.1016/0165-1838(87)90008-7. [DOI] [PubMed] [Google Scholar]
- Harding R., Leek B. F. Central projections of gastric afferent vagal inputs. J Physiol. 1973 Jan;228(1):73–90. doi: 10.1113/jphysiol.1973.sp010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert H., Moga M. M., Saper C. B. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990 Mar 22;293(4):540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Jansen A. H., Chernick V. Development of respiratory control. Physiol Rev. 1983 Apr;63(2):437–483. doi: 10.1152/physrev.1983.63.2.437. [DOI] [PubMed] [Google Scholar]
- Jean A., Amri M., Calas A. Connections between the ventral medullary swallowing area and the trigeminal motor nucleus of the sheep studied by tracing techniques. J Auton Nerv Syst. 1983 Feb;7(2):87–96. doi: 10.1016/0165-1838(83)90038-3. [DOI] [PubMed] [Google Scholar]
- Jean A. Brainstem organization of the swallowing network. Brain Behav Evol. 1984;25(2-3):109–116. doi: 10.1159/000118856. [DOI] [PubMed] [Google Scholar]
- Jean A., Car A. Inputs to the swallowing medullary neurons from the peripheral afferent fibers and the swallowing cortical area. Brain Res. 1979 Dec 14;178(2-3):567–572. doi: 10.1016/0006-8993(79)90715-7. [DOI] [PubMed] [Google Scholar]
- Jean A., Car A., Roman C. Comparison of activity in pontine versus medullary neurones during swallowing. Exp Brain Res. 1975;22(2):211–220. doi: 10.1007/BF00237690. [DOI] [PubMed] [Google Scholar]
- Jean A. Control of the central swallowing program by inputs from the peripheral receptors. A review. J Auton Nerv Syst. 1984 May-Jun;10(3-4):225–233. doi: 10.1016/0165-1838(84)90017-1. [DOI] [PubMed] [Google Scholar]
- KERR F. W. Facial, vagal and glossopharyngeal nerves in the cat. Afferent connections. Arch Neurol. 1962 Apr;6:264–281. doi: 10.1001/archneur.1962.00450220006003. [DOI] [PubMed] [Google Scholar]
- Kalia M., Mesulam M. M. Brain stem projections of sensory and motor components of the vagus complex in the cat: I. The cervical vagus and nodose ganglion. J Comp Neurol. 1980 Sep 15;193(2):435–465. doi: 10.1002/cne.901930210. [DOI] [PubMed] [Google Scholar]
- Kalia M., Mesulam M. M. Brain stem projections of sensory and motor components of the vagus complex in the cat: II. Laryngeal, tracheobronchial, pulmonary, cardiac, and gastrointestinal branches. J Comp Neurol. 1980 Sep 15;193(2):467–508. doi: 10.1002/cne.901930211. [DOI] [PubMed] [Google Scholar]
- Kalia M., Sullivan J. M. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol. 1982 Nov 1;211(3):248–265. doi: 10.1002/cne.902110304. [DOI] [PubMed] [Google Scholar]
- Kalia M., Welles R. V. Brain stem projections of the aortic nerve in the cat: a study using tetramethyl benzidine as the substrate for horseradish peroxidase. Brain Res. 1980 Apr 21;188(1):23–32. doi: 10.1016/0006-8993(80)90553-3. [DOI] [PubMed] [Google Scholar]
- Kanwal J. S., Caprio J. Central projections of the glossopharyngeal and vagal nerves in the channel catfish, Ictalurus punctatus: clues to differential processing of visceral inputs. J Comp Neurol. 1987 Oct 8;264(2):216–230. doi: 10.1002/cne.902640207. [DOI] [PubMed] [Google Scholar]
- Katz D. M., Karten H. J. The discrete anatomical localization of vagal aortic afferents within a catecholamine-containing cell group in the nucleus solitarius. Brain Res. 1979 Aug 3;171(2):187–195. doi: 10.1016/0006-8993(79)90326-3. [DOI] [PubMed] [Google Scholar]
- Katz D. M., Karten H. J. Topographic representation of visceral target organs within the dorsal motor nucleus of the vagus nerve of the pigeon Columba livia. J Comp Neurol. 1985 Dec 15;242(3):397–414. doi: 10.1002/cne.902420308. [DOI] [PubMed] [Google Scholar]
- Katz D. M., Karten H. J. Visceral representation within the nucleus of the tractus solitarius in the pigeon, Columba livia. J Comp Neurol. 1983 Jul 20;218(1):42–73. doi: 10.1002/cne.902180104. [DOI] [PubMed] [Google Scholar]
- Kumada M., Nakajima H. Field potentials evoked in rabbit brainstem by stimulation of the aortic nerve. Am J Physiol. 1972 Sep;223(3):575–582. doi: 10.1152/ajplegacy.1972.223.3.575. [DOI] [PubMed] [Google Scholar]
- Leslie R. A., Gwyn D. G., Hopkins D. A. The central distribution of the cervical vagus nerve and gastric afferent and efferent projections in the rat. Brain Res Bull. 1982 Jan;8(1):37–43. doi: 10.1016/0361-9230(82)90025-9. [DOI] [PubMed] [Google Scholar]
- McIlhinney R. A., Bacon S. J., Smith A. D. A simple and rapid method for the production of cholera B-chain coupled to horseradish peroxidase for neuronal tracing. J Neurosci Methods. 1988 Jan;22(3):189–194. doi: 10.1016/0165-0270(88)90039-8. [DOI] [PubMed] [Google Scholar]
- Mesulam M. M. Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem. 1978 Feb;26(2):106–117. doi: 10.1177/26.2.24068. [DOI] [PubMed] [Google Scholar]
- Miceli M. O., Malsbury C. W. Brainstem origins and projections of the cervical and abdominal vagus in the golden hamster: a horseradish peroxidase study. J Comp Neurol. 1985 Jul 1;237(1):65–76. doi: 10.1002/cne.902370105. [DOI] [PubMed] [Google Scholar]
- Nomura S., Mizuno N. Central distribution of efferent and afferent components of the cervical branches of the vagus nerve. A HRP study in the cat. Anat Embryol (Berl) 1983;166(1):1–18. doi: 10.1007/BF00317941. [DOI] [PubMed] [Google Scholar]
- Norgren R., Smith G. P. Central distribution of subdiaphragmatic vagal branches in the rat. J Comp Neurol. 1988 Jul 8;273(2):207–223. doi: 10.1002/cne.902730206. [DOI] [PubMed] [Google Scholar]
- Norman P., Bower A. J. An autoradiographic study of the brainstem projections of vagal visceral afferent fibres in the domestic hen. J Anat. 1982 May;134(Pt 3):583–589. [PMC free article] [PubMed] [Google Scholar]
- Odekunle A., Bower A. J. Brainstem connections of vagal afferent nerves in the ferret: an autoradiographic study. J Anat. 1985 May;140(Pt 3):461–469. [PMC free article] [PubMed] [Google Scholar]
- Rhoton A. L., Jr, O'Leary J. L., Ferguson J. P. The trigeminal, facial, vagal, and glossopharyngeal nerves in the monkey. Afferent connections. Arch Neurol. 1966 May;14(5):530–540. doi: 10.1001/archneur.1966.00470110074010. [DOI] [PubMed] [Google Scholar]
- Rinaman L., Miselis R. R. The organization of vagal innervation of rat pancreas using cholera toxin-horseradish peroxidase conjugate. J Auton Nerv Syst. 1987 Dec;21(2-3):109–125. doi: 10.1016/0165-1838(87)90014-2. [DOI] [PubMed] [Google Scholar]
- Roman C. Contrôle nerveux de la déglutition et de la motricité oesophagienne chez les mammifères. J Physiol (Paris) 1986;81(2):118–131. [PubMed] [Google Scholar]
- Rubinson K., Friedman B. Vagal afferent projections in Rana pipiens, Rana catesbeiana, and Xenopus mülleri. With a note on lateral line and VIII nerve projection zones. Brain Behav Evol. 1977;14(5):368–380. doi: 10.1159/000125686. [DOI] [PubMed] [Google Scholar]
- Scharoun S. L., Barone F. C., Wayner M. J., Jones S. M. Vagal and gastric connections to the central nervous system determined by the transport of horseradish peroxidase. Brain Res Bull. 1984 Oct;13(4):573–583. doi: 10.1016/0361-9230(84)90040-6. [DOI] [PubMed] [Google Scholar]
- Shapiro R. E., Miselis R. R. The central organization of the vagus nerve innervating the stomach of the rat. J Comp Neurol. 1985 Aug 22;238(4):473–488. doi: 10.1002/cne.902380411. [DOI] [PubMed] [Google Scholar]
- Störmer R., Goller H. Zur Feinstruktur des Nucleus tractus solitarii von Schaf und Ziege. J Hirnforsch. 1988;29(6):633–641. [PubMed] [Google Scholar]
- Sweazey R. D., Bradley R. M. Central connections of the lingual-tonsillar branch of the glossopharyngeal nerve and the superior laryngeal nerve in lamb. J Comp Neurol. 1986 Mar 22;245(4):471–482. doi: 10.1002/cne.902450404. [DOI] [PubMed] [Google Scholar]
- TABER E. The cytoarchitecture of the brain stem of the cat. I. Brain stem nuclei of cat. J Comp Neurol. 1961 Feb;116:27–69. doi: 10.1002/cne.901160104. [DOI] [PubMed] [Google Scholar]
- Wild J. M. Identification and localization of the motor nuclei and sensory projections of the glossopharyngeal, vagus, and hypoglossal nerves of the cockatoo (Cacatua roseicapilla), Cacatuidae. J Comp Neurol. 1981 Dec 10;203(3):351–377. doi: 10.1002/cne.902030304. [DOI] [PubMed] [Google Scholar]