Abstract
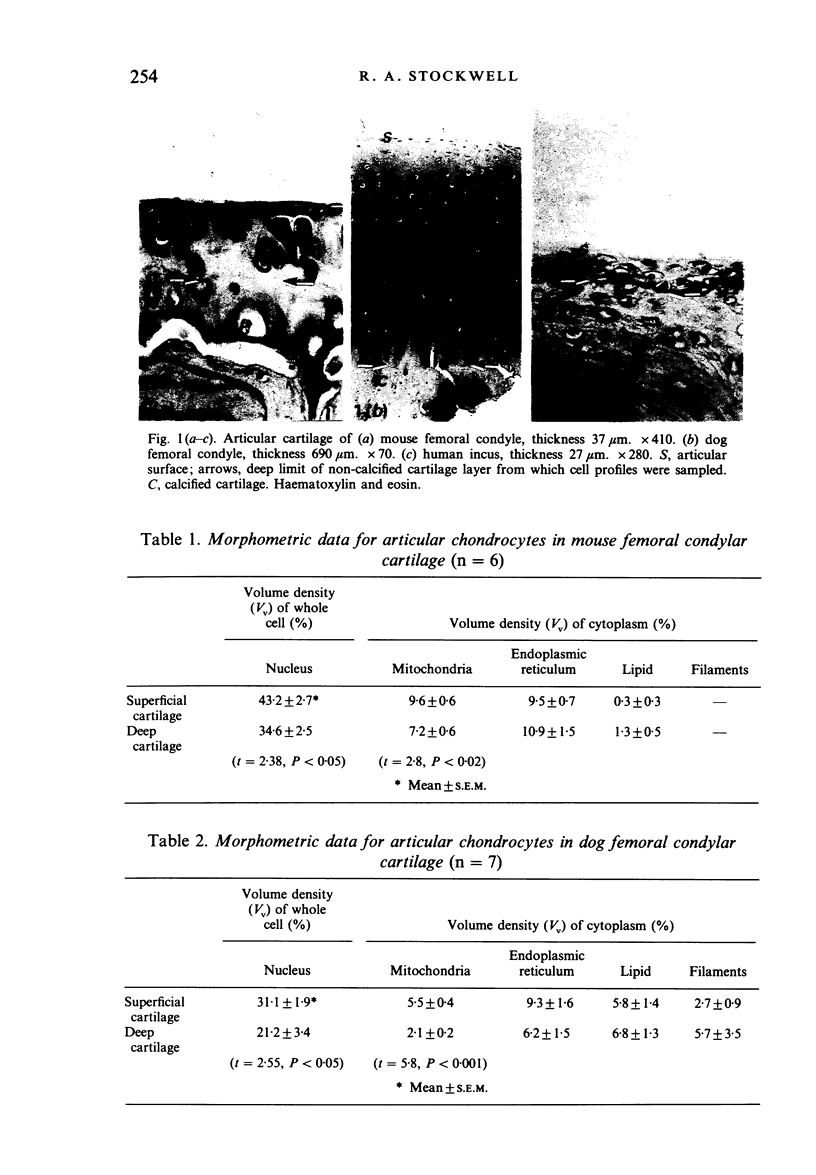
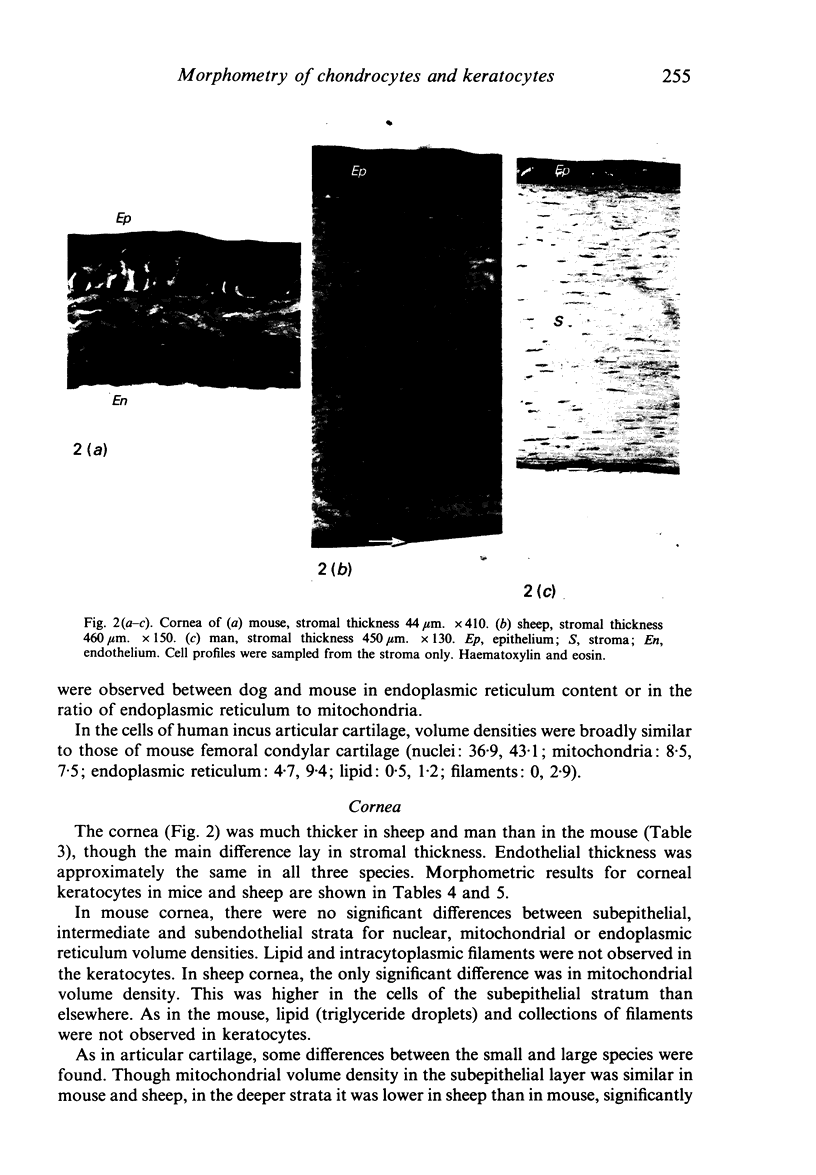
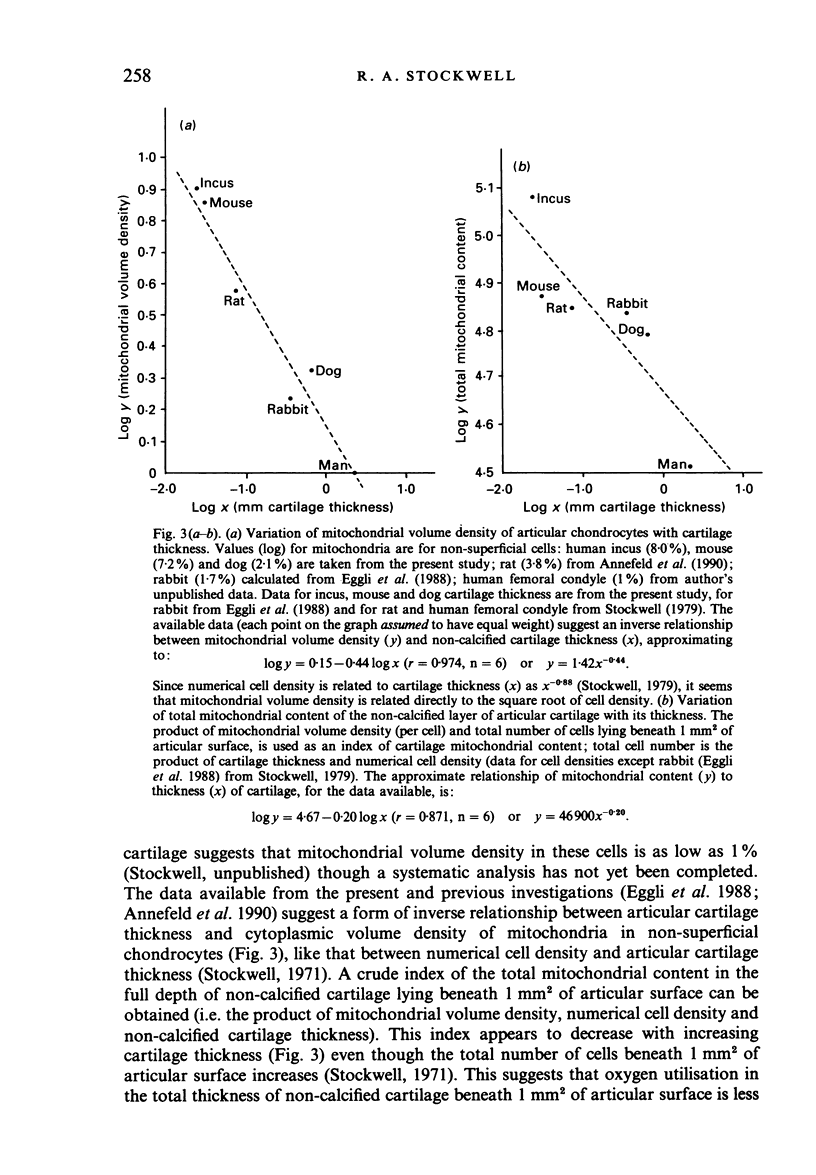
A morphometric analysis was made of nuclei and cytoplasmic structures in electron micrographs of chondrocytes in the non-calcified layer of articular cartilage of the femoral condyles in adult mouse and dog and of the human incus. Mitochondrial volume density (% cytoplasm) was lower in dog than in mouse cells or cells of the incus. It was also lower in the cells of deep zone cartilage than in superficial cells. Analysis of keratocytes of the corneal stroma in mouse and sheep gave similar findings to that in cartilage. Mitochondrial volume density was higher in mouse than in sheep keratocytes and, in sheep, higher in subepithelial (anterior) cells than in cells of the intermediate and subendothelial strata. Values in human stromal cells were similar to those in the sheep. Endoplasmic reticulum volume density was higher in mouse than in sheep keratocytes. Lipid and filaments were more abundant in dog than in mouse chondrocytes but keratocytes contained little or none. Mitochondrial volume densities correlate with diffusion distances (tissue thickness) from the sources of nutrition, for example, for oxygen, in the two tissues. The relationship to certain aspects of chondroitin sulphate and keratansulphate synthesis and topographical distribution in cartilage and cornea is discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annefeld M., Erne B., Rasser Y. Ultrastructural analysis of rat articular cartilage following treatment with dexamethasone and glycosaminoglycan-peptide complex. Clin Exp Rheumatol. 1990 Mar-Apr;8(2):151–157. [PubMed] [Google Scholar]
- Brighton C. T., Kitajima T., Hunt R. M. Zonal analysis of cytoplasmic components of articular cartilage chondrocytes. Arthritis Rheum. 1984 Nov;27(11):1290–1299. doi: 10.1002/art.1780271112. [DOI] [PubMed] [Google Scholar]
- Eggli P. S., Hunziker E. B., Schenk R. K. Quantitation of structural features characterizing weight- and less-weight-bearing regions in articular cartilage: a stereological analysis of medial femoral condyles in young adult rabbits. Anat Rec. 1988 Nov;222(3):217–227. doi: 10.1002/ar.1092220302. [DOI] [PubMed] [Google Scholar]
- Freeman R. D. Oxygen consumption by the component layers of the cornea. J Physiol. 1972 Aug;225(1):15–32. doi: 10.1113/jphysiol.1972.sp009927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker E. B., Schenk R. K., Cruz-Orive L. M. Quantitation of chondrocyte performance in growth-plate cartilage during longitudinal bone growth. J Bone Joint Surg Am. 1987 Feb;69(2):162–173. [PubMed] [Google Scholar]
- Marcus R. E. The effect of low oxygen concentration on growth, glycolysis, and sulfate incorporation by articular chondrocytes in monolayer culture. Arthritis Rheum. 1973 Sep-Oct;16(5):646–656. doi: 10.1002/art.1780160509. [DOI] [PubMed] [Google Scholar]
- Paukkonen K., Helminen H. J. Rough endoplasmic reticulum and fine intracytoplasmic filaments in articular cartilage chondrocytes of young rabbits; a stereological morphometric study using transmission electron microscopy. J Anat. 1987 Jun;152:47–54. [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Haigh M. Keratan sulphate and the ultrastructure of cornea and cartilage: a 'stand-in' for chondroitin sulphate in conditions of oxygen lack? J Anat. 1988 Jun;158:95–108. [PMC free article] [PubMed] [Google Scholar]
- Scott J. E., Stockwell R. A., Balduini C., De Luca G. Keratan sulphate: a functional substitute for chondroitin sulphate in O2 deficient tissues? Pathol Biol (Paris) 1989 Jun;37(6):742–745. [PubMed] [Google Scholar]
- Shore R. C., Moxham B. J., Berkovitz B. K. A quantitative comparison of the ultrastructure of the periodontal ligaments of impeded and unimpeded rat incisors. Arch Oral Biol. 1982;27(5):423–430. doi: 10.1016/0003-9969(82)90153-4. [DOI] [PubMed] [Google Scholar]
- Stockwell R. A., Scott J. E. Distribution of acid glycosaminoglycans in human articular cartilage. Nature. 1967 Sep 23;215(5108):1376–1378. doi: 10.1038/2151376a0. [DOI] [PubMed] [Google Scholar]
- Stockwell R. A., Scott J. E. Observations on the acid glycosaminoglycan (mucopolysaccharide) content of the matrix of aging cartilage. Ann Rheum Dis. 1965 Jul;24(4):341–350. doi: 10.1136/ard.24.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell R. A. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J Anat. 1971 Sep;109(Pt 3):411–421. [PMC free article] [PubMed] [Google Scholar]




