Abstract
We and others previously reported that cytotoxic T lymphocyte antigen-4 (CTLA-4) regulates the severity of peptide-induced experimental autoimmune encephalomyelitis (EAE) in mouse strains that are inherently susceptible to the disease. In this report, we show that CTLA-4 engagement also controls disease susceptibility in BALB/c mice, a strain considered to be resistant to EAE induction. Although immunization of BALB/c mice with syngeneic spinal cord homogenate or an I-Ad-binding myelin peptide antigen failed to result in EAE, immunization with either antigen preparation in conjunction with anti-CTLA-4 resulted in both clinical and histological EAE. CTLA-4 blockade also resulted in a preferential increase in the frequency of antigen-specific T cells secreting IFN-γ. We conclude that CTLA-4 controls susceptibility in BALB/c mice by limiting the expansion of autoreactive T cells present in the periphery, suggesting a mechanism whereby CTLA-4 contributes to the maintenance of peripheral T cell tolerance to self antigens.
Keywords: susceptibility‖tolerance‖T helper 1 cells
Experimental autoimmune encephalomyelitis (EAE) is an autoimmune inflammatory and demyelinating disease model that shares many clinical and histological features with the human disease multiple sclerosis (MS) (1). In susceptible mouse strains, immunization with myelin protein antigens emulsified in strong adjuvant results in a CD4+ T helper 1 (Th1)-mediated inflammatory response in the central nervous system (CNS). Blockade of the inhibitory effects of CTLA-4 by administration of Abs during immunization has been shown to enhance T cell responses in many in vitro and in vivo systems (for review see ref. 2). CTLA-4 blockade has been shown to exacerbate EAE or accelerate disease relapse in susceptible strains of mice (3–5). Blockade of CTLA-4 during in vitro stimulation increased IFN-γ production leading to more effective adoptive transfer of EAE by antigen-specific T cells marked by increased disease incidence and severity (3). CTLA-4 blockade during priming in EAE appears to exacerbate disease by increasing the frequency of responding cells, especially those secreting IFN-γ (4, 6).
Susceptibility to EAE induction is governed by many genetic factors. The prototypical strain used for EAE studies is SJL/J, which expresses the H-2s major histocompatibility complex (MHC) haplotype. Although MHC is certainly an important genetic factor governing EAE susceptibility, it is not the only genetic factor. B10.S mice, which are congenic for the H-2s MHC, are resistant to induction of EAE by a variety of neural antigens. Irradiated B10.S mice reconstituted with SJL/J bone marrow are also resistant to EAE induction, whereas SJL/J chimeras reconstituted with B10.S bone marrow are susceptible (7). Backcross analysis between B10.S and SJL/J mice has uncovered multiple genetic loci that are linked to EAE susceptibility (8, 9).
The BALB/c strain of mice is considered to be resistant to EAE induction, with the BALB/cJ subline characterized as the most resistant. This resistance is at least partially controlled by MHC, as (BALB/c × SJL/J)F1 mice are highly susceptible to EAE induction induced by whole CNS tissue (10). T cell clones and lines derived from immunized (BALB/c × SJL/J)F1 mice have been extensively studied. Encephalitogenic clones and lines were predominately restricted by F1 hybrid class II MHC molecules, whereas nonencephalitogenic T cell clones and lines were restricted by the BALB/c I-Ad class II MHC molecule (11). This observation suggests that there might be differences in the peptides presented by different class II MHC molecules in these mice that may have very important implications for disease outcome. This is analogous to the association of MS susceptibility with certain HLA loci. These results underscore the importance of background genetic factors that contribute to disease susceptibility.
The resistance of BALB/c mice to EAE induction is not simply because of an inability to present or respond to myelin antigens. T cell clones can be derived from BALB/c mice that are specific for peptides derived from myelin basic protein (MBP59–76 and MBP151–160) and can adoptively transfer EAE to syngeneic animals after optimal activation in vitro (12, 13). However, active immunization of BALB/c mice with MBP or the relevant MBP peptides does not induce EAE (13), suggesting that the in vitro cloning conditions allowed the outgrowth of potentially encephalitogenic T cell clones that could not be effectively generated in intact mice. Differential cytokine production also may affect susceptibility because BALB/c mice tend to inherently produce Th2 type cytokines in many immunization protocols (14). Backcross analysis among BALB/c sublines has shown that multiple genotypic differences contribute to the disease resistance in the BALB/cJ subline, although linkage analysis has not shown an association with any of allelic differences known to distinguish the BALB/cJ substrain (15, 16).
Given that in susceptible strains CTLA-4 seems to attenuate the T cell response to myelin antigens, we hypothesized that in resistant strains CTLA-4 may regulate peripheral tolerance to myelin antigens either by preventing T cell activation to myelin antigens or by severely restraining the T cell proliferative response to myelin antigens. The present study was conducted to determine whether interruption of CTLA-4/B7 interactions would allow the induction of EAE in a resistant strain of mice. We found that blockade of CTLA-4/B7 interactions in BALB/c mice did indeed allow the induction of EAE after sensitization to either spinal cord homogenate (SCH) or a proteolipid protein (PLP)-derived IAd-binding peptide and that disease was marked by a preferential increase in the number of antigen-specific T cells producing IFN-γ.
Materials and Methods
Mice.
Female BALB/c mice aged 6–8 wk were purchased from the National Cancer Institute or Simonsen Laboratories (San Jose, CA) and used within 2 wk for experiments. Animals were housed in accordance with the Institutional Animal Care and Use Committee regulations at the University of California, Berkeley.
Antigens.
Antigens used in these studies were syngeneic SCH and PLP40–59 (TGTEKLIETYFSKNYQDYEY). All peptides were synthesized and purified at the University of California at Berkeley Cancer Research Laboratory Microchemical Facility.
EAE Induction.
For immunization with syngeneic SCH, mice received 100 μl of an emulsion consisting of 1 mg of lyophilized spinal cord in complete Freund's adjuvant (CFA) containing 400 μg of heat-killed Mycobacterium tuberculosis H37 RA (Difco) on days 0 and 7. For immunization with PLP40–59, mice received 100 μl of an emulsion consisting of 200 μg of PLP40–59 and CFA containing 400 μg of heat-killed M. tuberculosis H37 RA on day 0. Abs (100 μg) were injected i.p. on days 0, 3, and 6 for disease progression and on days 0 and 3 for ELISPOT analysis.
Assessment of Clinical and Histologic EAE.
Animals were monitored on a daily basis starting on day 8 after immunization. Disease was scored on a scale of 0–5: 1 = limp tail or an inability to grasp with hind limbs; 2 = inability to right itself from back; 3 = hindlimb paralysis; 4 = forelimb and hindlimb paralysis; and 5 = moribund. For the histological analyses, brains and spinal cords were removed and fixed in 10% formalin. Paraffin-embedded sections were stained by using standard hematoxylin and eosin techniques and examined for histopathology. Inflammatory foci were enumerated without knowledge of the treatment.
ELISA and Enzyme-Linked Immunospot (ELISPOT).
Cytokine production was analyzed by sandwich ELISA. ELISA plates (96-well, Corning) were coated with appropriate capture Abs (2 μg/ml in 50 μl of 0.1 M sodium bicarbonate buffer) overnight at 4°C. Wells were blocked with 200 μl of PBS/5% FCS at room temperature and washed three times with 0.9% NaCl/0.1% Tween 20, and then culture supernatants (50 μl) were added and incubated for 1 h at room temperature. Next, 50 μl of biotinylated secondary Abs at a concentration of 1 μg/ml were added for 45 min at room temperature, followed by a 30-min incubation with streptavidin-horseradish peroxidase. Wells were developed by using a colorimetric substrate [2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonic acid); ABTS] and read on a plate reader (Bio-Tek, Burlington, VT), and samples were analyzed by using KC4 software (Bio-Tek).
For the ELISPOT assays, draining lymph nodes were taken from five animals per group and pooled at day 7 after immunization. Then, 5 × 105 unfractionated lymph node cells were added per well to MultiScreen-hemagglutinin plates (Millipore) that had been precoated with either anti-IFN-γ (10 μg/ml) or anti-IL-4 (5 μg/ml) Abs. After a 36-h incubation, culture plates were washed extensively with PBS/0.1% Tween 20, and secondary Abs to IFN-γ (5 μg/ml) or IL-4 (5 μg/ml) were added and incubated for 5 h. at room temperature. After washing, streptavidin-horseradish peroxidase (1:1,000) was added and incubated for 1 h at room temperature. Plates were washed and developed with the peroxidase substrate 3-amino-9-ethylcarbazole (Sigma). Spots were counted under a binocular microscope (Olympus). To estimate the number of antigen-responsive T cells, the number of spots from wells containing no antigen (background) was subtracted from experimental wells.
Results
Blockade of CTLA-4 Allows Induction of EAE in BALB/c Mice Immunized with SCH.
Because myelin autoantigens are not as well defined in BALB/c mice as they are in SJL and PL mice, we first sought to determine whether unfractionated SCH might induce EAE. SCH should contain all of the potentially immunogenic CNS antigens and therefore provide the best chance of priming the pool of autoaggressive T cells. Mice were immunized with SCH emulsified in CFA on days 0 and 7. Abs were administered by (i.p.) injection on days 0, 3, and 6, and mice were monitored for clinical signs of EAE for 4 wk. Some mice were killed on day 18 for histological evaluation of inflammatory infiltration into the CNS. The remaining mice were killed at day 26 for histological examination.
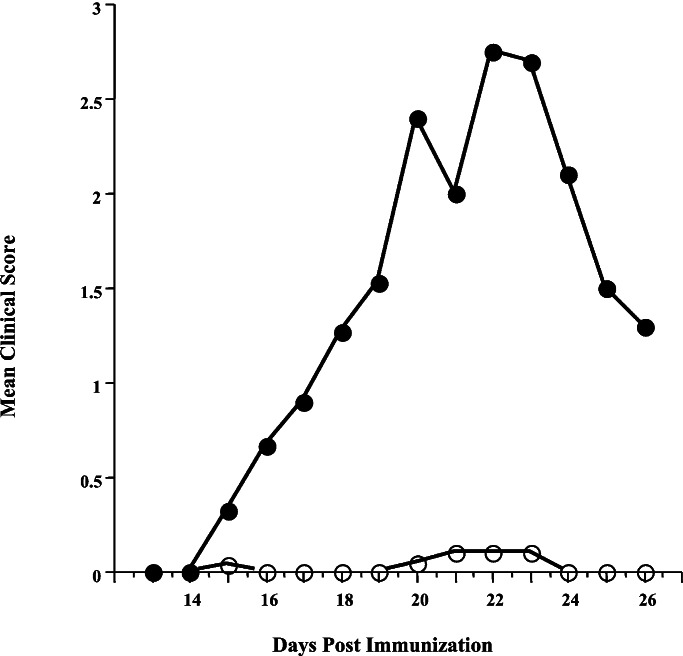
The SCH-sensitized mice treated with control Ab exhibited insignificant clinical manifestations of EAE (Fig. 1). Only 1 mouse of 15 exhibited any paralysis, and this was quite mild (Table 1). It should be noted that this mouse did not exhibit the histological lesions typically associated with EAE, which would suggest that the clinical symptomatology was not due to CNS inflammation. A small number of mice (3/14) in the control group exhibited CNS infiltration, but this was quite mild with only a few inflammatory foci.
Figure 1.
Time course of clinical EAE induction in BALB/cJ mice immunized with syngeneic SCH. Mice were immunized on days 0 and 6 with 1 mg of SCH emulsified in CFA. Control Ab (○) or anti-CTLA-4 Ab (●) was administered i.p. on days 0, 3, and 6. Animals were scored as follows: 1, limp tail; 2, weak hindlimbs; 3, hindlimb paralysis; 4, hind- and forelimb paralysis; and 5, moribund. Cumulative data from 15 mice over three experiments are shown.
Table 1.
Summary of EAE induction in BALB/cJ mice
| Antigen | Ab | Disease incidence | Mean peak clinical score | Histological incidence | Mean total inflammatory foci |
|---|---|---|---|---|---|
| SCH | Control Ig | 1 /15* | 0.07 ± 0.01* | 3 /14* | 3.9 ± 2.8* |
| Anti-CTLA-4 | 14 /15 | 2.47 ± 0.28 | 15 /15 | 110.3 ± 20.3 | |
| PLP40–59 | Control Ig | 0 /40† | 0† | 21 /46 | 9.4 ± 2.3‡ |
| Anti-CTLA-4 | 23 /40 | 0.40 ± 0.08 | 32 /46 | 37.5 ± 5.3 |
Mean peak clinical score was calculated from all animals immunized. Mean total foci was calculated from all animals tested. Statistical analysis was performed with the Mann–Whitney t test.
, P < 0.0001 compared with SCH/anti-CTLA-4;
, P < 0.0001 compared with PLP40–59/anti-CTLA-4;
, P < 0.0002 compared with PLP40–59/anti-CTLA-4.
In contrast, blockade of CTLA-4 during the initial sensitization to SCH resulted in a very severe clinical course of EAE (Fig. 1). Fourteen of 15 animals treated with anti-CTLA-4 Ab showed clinical signs of EAE. Histological analysis revealed that all 15 animals showed extensive CNS infiltration (Table 1). A significantly higher number of inflammatory foci were present as compared with control Ab-treated animals (110.3 vs. 3.9, P < 0.0001, Table 1). These results indicate that CTLA-4 plays a vital role in determining susceptibility to EAE induction after immunization with unfractionated SCH.
CTLA-4 Blockade Allows EAE Induction by a PLP Peptide in BALB/cJ Mice.
We next determined whether CTLA-4 blockade could allow EAE induction by a single peptide antigen. We immunized mice with a peptide derived from myelin PLP. Sensitization to PLP has been demonstrated to induce EAE in several strains of mice, including some BALB/c substrains (17). Peptides derived from PLP have been characterized for MHC binding and the ability to elicit both a proliferative response and EAE disease in mice from various MHC backgrounds (18). We focused on one peptide, PLP40–59, which had been previously reported to bind to I-Ad and prime a weak T cell response in vivo but was not encephalitogenic in BALB/cJ mice (18). To test whether CTLA-4 regulates resistance to PLP40–59-induced EAE, we immunized animals with PLP40–59 emulsified in CFA under conditions of CTLA-4 blockade. Consistent with this earlier report (18), we found that PLP40–59-sensitized mice receiving control Ab showed no indications of clinical EAE. As shown in Table 1, none of 40 BALB/c mice over five separate experiments exhibited clinical manifestations of EAE, whereas 23/40 mice immunized with PLP40–59 and treated with anti-CTLA-4 Ab exhibited various degrees of paralysis (Table 1). These results demonstrate that CTLA-4 blockade converted the normally nonencephalitogenic response of BALB/c mice to this PLP-derived peptide to an encephalitogenic one (18).
Histological analysis of mice immunized with PLP40–59 and treated with control Ab revealed mild inflammation of the CNS in 21/46 animals. A greater number of mice treated with anti-CTLA-4 Ab exhibited more extensive inflammatory lesions in the CNS (32/46). More importantly, the mean number of inflammatory foci was increased 4-fold in the anti-CTLA-4-treated mice (9.4 vs. 37.5, P < 0.0002, Table 1). Together, these results indicate that immunization with PLP40–59 is sufficient to prime autoreactive T cells, but that under normal conditions the response is restrained by CTLA-4 to a level that minimizes inflammation and pathogenic destruction of CNS tissues and that blockade of the attenuating effects of CTLA-4 allows development of autoimmune disease.
CTLA-4 Blockade Results in Increased IFN-γ Secretion in Vitro.
We next sought to determine the mechanism by which CTLA-4 blockade allows induction of EAE in the PLP40–59-primed animals. Mice were immunized as described above except that they received Ab injections on days 0 and 3 only. The Ab injection on day 6 was eliminated to minimize the possibility that residual Ab would potentially confound interpretation of in vitro assays. On day 7, draining lymph nodes were harvested and assayed for proliferation and cytokine secretion in response to PLP40–59.
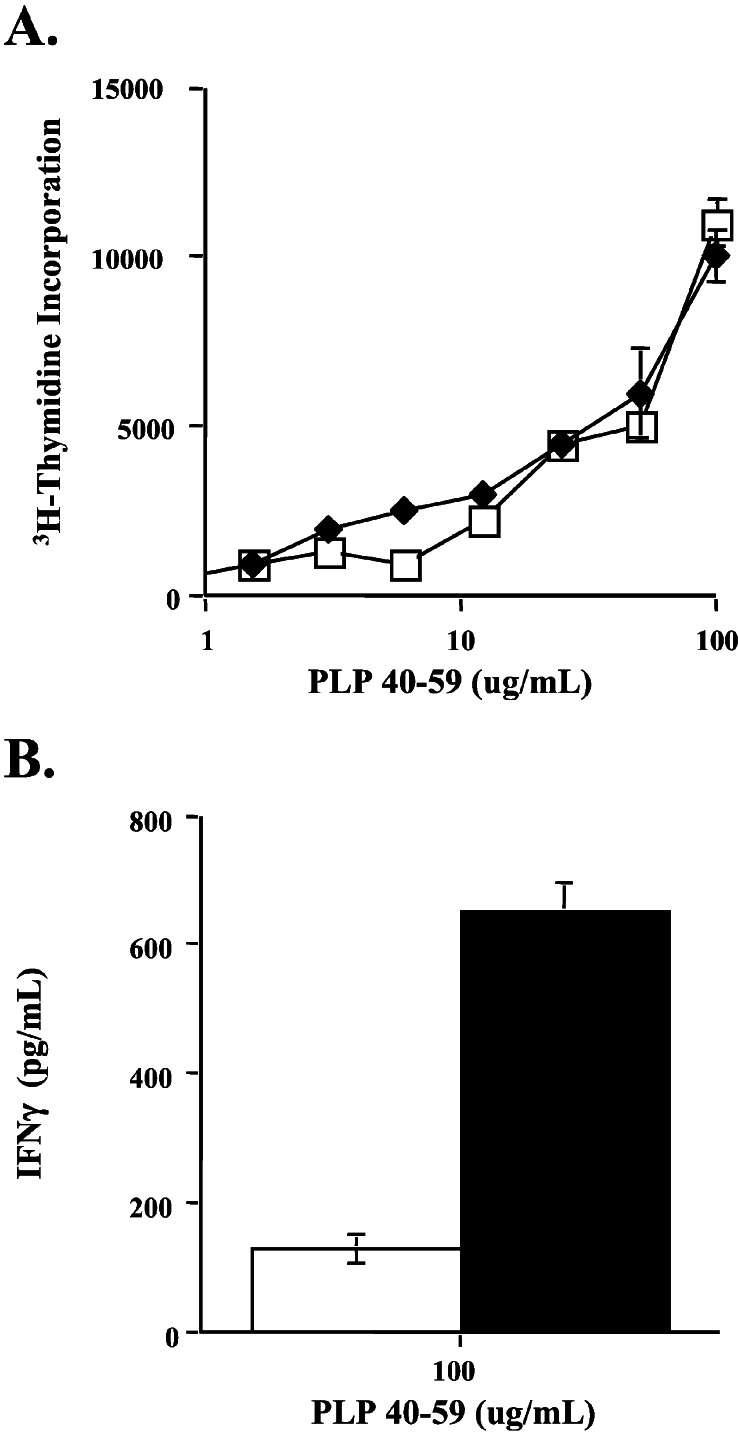
Primed lymph node cells from anti-CTLA-4-treated mice did not show any difference in proliferative response to the sensitizing antigen when compared with the response of lymph node cells from control Ab-treated mice (Fig. 2A). Although there was no significant difference between the proliferative response of control Ab- and anti-CTLA-4 Ab-treated mice to PLP40–59, it remained possible that cytokine secretion differed between the two treatment regimens.
Figure 2.
In vitro response of PLP40–59-primed BALB/cJ lymph node cells. Proliferation (A) and IFN-γ production (B) of unfractionated draining lymph node cells from mice treated with control Ab (open symbols) or anti-CTLA-4 Ab (filled symbols). Mice were immunized with 200 μg of PLP40–59 emulsified in CFA. Abs were administered i.p. on days 0 and 3, and draining lymph node cells were assayed on day 7 after immunization. In round-well plates, 3 × 105 cells per well were cultured with peptide at the indicated concentrations. Supernatants were collected at 72 h for ELISA, and 1 μCi (1 μCi = 37 kBq) of [3H]thymidine was added per well. Proliferation was assessed 12 h later. Results are representative of five separate experiments.
To address this possibility, supernatants from lymph node cultures stimulated with PLP40–59 were assayed for IFN-γ and IL-4. We were unable to detect IL-4 production by using ELISA. However, there were significant differences in IFN-γ production between control Ab- and anti-CTLA-4-treated groups: Antigen-stimulated lymph node cells from mice that had been treated with anti-CTLA-4 secreted greater amounts of IFN-γ than corresponding cultures from control Ab-treated animals (Fig. 2B). This result suggests that the encephalitogenic effect of CTLA-4 blockade can be attributed to an enhancement of IFN-γ production in response to PLP40–59 and that CTLA-4 engagement prevents disease induction by limiting cytokine production.
CTLA-4 Blockade Increases the Frequency of PLP40–59-Specific T cells.
The increase in IFN-γ production as a result of CTLA-4 blockade could be the consequence of induction of a higher frequency of IFN-γ-producing cells, an increased production of IFN-γ per cell, or a deviation of the immune response from a nonencephalitogenic Th2 response to an encephalitogenic Th1 response. The lack of IL-4 detected by ELISA suggested that deviation had not occurred. However, this bulk measurement would not be able to detect low levels of cytokine produced by a small number of responding T cells. To dissect more precisely the effect of CTLA-4 blockade on induction of cytokine-producing T cells, we used the more sensitive ELISPOT procedure to determine the frequencies of cells that secreted IFN-γ and IL-4 in response to PLP40–59 stimulation.
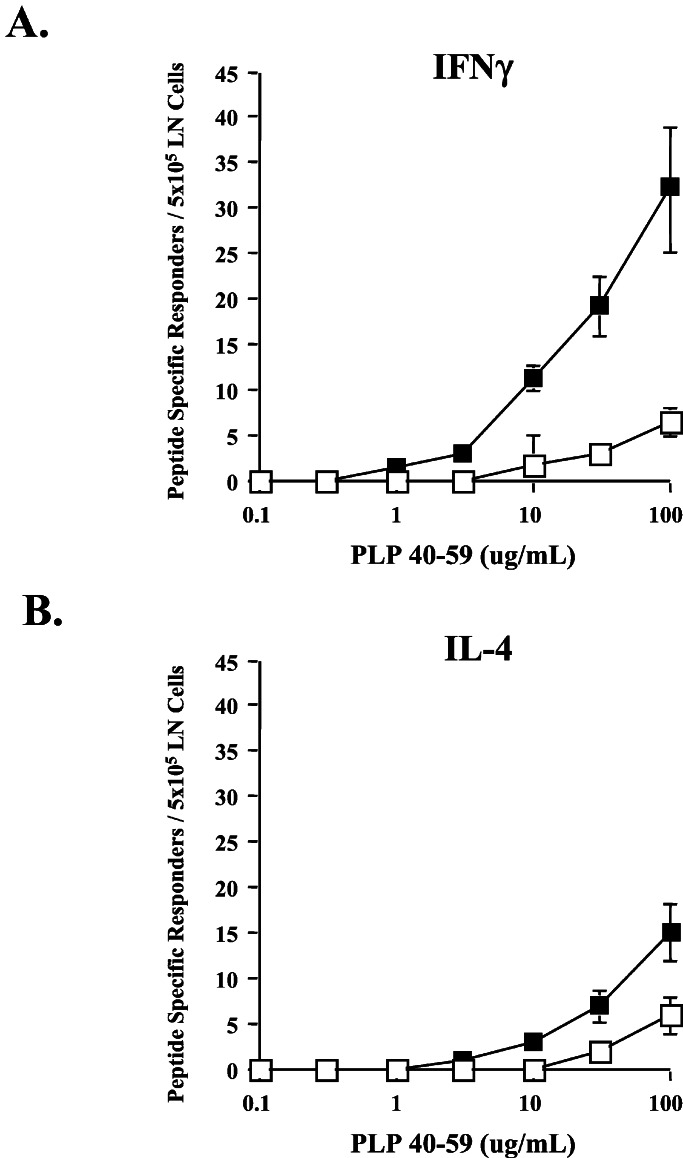
Mice were immunized in the same manner as described for the ELISA experiments. Unfractionated draining lymph node cells were analyzed by ELISPOT for IFN-γ or IL-4 production in response to PLP40–59. As shown in Fig. 3, both IL-4- and IFN-γ-secreting cells were detectable in low but comparable numbers from control Ab-treated animals. Blockade of CTLA-4 resulted in an increase in the number of both IFN-γ- and IL-4-secreting cells. The fact that the frequency of both populations increased indicates that CTLA-4 blockade does not result in overt immune deviation. However, the frequency of IFN-γ-producing cells was increased approximately 6-fold over the frequency of cells from control Ab-treated animals, whereas the frequency of IL-4-producing cells was increased only 3-fold. Also, not only was the overall frequency of peptide-specific cells increased over 4-fold by CTLA-4 blockade, but the ratio of Th1 to Th2 cytokine-secreting cells also doubled (from 1:1 to 2:1). It seems likely that this increased frequency of IFN-γ-secreting T cells is responsible for the induction of EAE after CTLA-4 blockade.
Figure 3.
Frequency of PLP40–59-specific T cells in primed BALB/cJ mice. ELISPOT analysis of antigen-specific IFN-γ (A) and IL-4 (B) production by draining lymph node cells from control Ab- (□) or anti-CTLA-4 Ab- (■) treated mice. Mice were immunized with 200 μg of PLP40–59 emulsified in CFA. Abs were administered i.p. on days 0 and 3, and draining lymph node cells were assayed on day 7 after immunization. Results are representative of three independent experiments.
Discussion
There is a growing body of evidence indicating that CTLA-4 is pivotal in controlling autoaggressive T cells. The importance of CTLA-4 in peripheral T cell regulation is perhaps best demonstrated by the CTLA-4−/− mouse, which shows massive lymphoproliferation and death at an early age (reviewed in ref. 2). An important role for CTLA-4 in the regulation of immune responses to self antigens also is demonstrated by the fact that blockade of CTLA-4, in combination with a melanoma cell-based vaccine, resulted in potent anti-tumor responses and the development of autoimmune depigmentation as a consequence of destruction of normal melanocytes (19, 20).
Several human autoimmune disorders have been associated with potential deficits in CTLA-4 function or expression. A search for non-HLA-linked genes associated with Grave's disease concluded that a polymorphism in the CTLA-4 gene might be correlated with susceptibility to Grave's disease (21). Also, T cells from patients suffering from Chediak–Higashi syndrome were shown to be defective in cycling CTLA-4 to the cell surface after stimulation. This defect was postulated to result in the lymphoproliferative disorder seen in Chediak–Higashi syndrome patients (22). Another study found auto-Abs to CTLA-4 present in the serum of patients suffering from a number of autoimmune disorders, including rheumatoid arthritis, Behcet's disease, systemic lupus erythematosus, and Sjögren's syndrome (23). This finding led to the suggestion that auto-Abs to CTLA-4 may interfere with normal CTLA-4 function and amplify T cell responses in these patients, resulting in more severe autoimmune responses (23). Additionally, recent studies have shown a potential linkage between CTLA-4 gene polymorphisms and MS (24–26).
We found that blockade of CTLA-4/B7 interactions during immunization of BALB/cJ mice with SCH allowed the induction of severe EAE, indistinguishable from that of EAE in other susceptible strains. Similarly, CTLA-4 blockade during immunization of BALB/cJ mice with an I-Ad-binding peptide derived from PLP, PLP40–59, allowed induction of EAE. However, disease in mice immunized with the peptide was much milder than that in mice sensitized to the SCH. This result may simply reflect that fact that the mice were immunized with a single PLP epitope rather than the array of antigens found in unfractionated SCH.
Induction of EAE by CTLA-4 blockade corresponded with an increase in IFN-γ production in response to the priming antigen. This finding is consistent with data from studies using the susceptible SJL/J strain (4). The increase in IFN-γ production corresponded to a 6-fold increase in the frequency of IFN-γ-producing cells and a 3-fold increase in the number of neuroantigen-specific, IL-4-producing cells. This observation demonstrates that both Th1 and Th2 populations had increased as a result of CTLA-4 blockade and agrees with our previous studies in the SJL/J strain, which found a similar increase in the ratio of Th1 to Th2 populations and attributed the exacerbation of EAE by CTLA-4 blockade to the preferential increase in IFN-γ-secreting T cells (6).
In B10.S mice, it was demonstrated that resistance to EAE induction might be due to ineffective signaling through the IL-12/CD40 costimulatory axis. Adoptive transfer of T cells activated in vitro with IL-12 was found to be sufficient to restore susceptibility to MBP-induced EAE in B10.S mice (27). The defect in IL-12 expression was attributable to a defect in CD40L expression (28). These findings again reflect an observation that similar signaling defects are observed in MS patients (29). Our findings do not preclude the possibility that CTLA-4 blockade may bypass a defect in CD40L-induced IL-12 production.
The findings presented in this report directly implicate CTLA-4 as a critical factor regulating susceptibility to autoimmune disease. The fact that a weak response is detected in mice immunized to PLP40–59 alone suggests that CTLA-4 blockade is not acting by the reversal of T cell anergy. Thus the resistance of BALB/cJ mice to EAE induction is not due to an inherent inability to respond to myelin antigens but is due to the fact that CTLA-4 restricts the magnitude of the specific T cell response and inhibits the induction of EAE predominately by limiting the induction of a Th1 response.
There are at least two possible explanations for the results presented here. One is that CTLA-4 blockade lowers an activation threshold and allows the recruitment of a broader population of T cells, which would not have otherwise responded. In this scenario, autopathogenic T cells that are normally poorly responsive to autoantigen, perhaps because of expression of low-affinity T cell antigen receptors, become activated when CTLA-4-mediated inhibitory signals are removed. Alternatively, CTLA-4 blockade may selectively permit expansion of strongly reactive T cells. In this scenario, T cells that respond in the presence of CTLA-4 blockade might have a quantitative and qualitative advantage for expansion and development of an autopathogenic response when CTLA-4 function is abrogated. Based on results we recently published that suggest preferential CTLA-4-mediated inhibition of T cells receiving strong T cell antigen receptor signals, we favor the latter possibility (2, 6, 30).
Viewed either way, it is clear that the induction of an autopathogenic response is a quantitative effect whereby induction of autoimmunity is the result of the T cell response reaching and exceeding a “critical mass.” Thus, CTLA-4 may restrict the expansion of autoreactive T cells and regulate autoimmune responses. This observation has important implications as to the function of CTLA-4 in the regulation of T cell responses and to the way in which this regulation is manipulated in immunotherapy of human diseases such as MS and cancer.
Acknowledgments
A.A.H. is a Young Investigator of the Association for the Cure of Cancer of the Prostate (CaP CURE) and is supported in part by the National Multiple Sclerosis Society. J.P.A. is an Investigator of the Howard Hughes Medical Institute. This work was supported in part by National Institutes of Health Grant CA40041 to J.P.A.
Abbreviations
- CTLA-4
cytotoxic T lymphocyte antigen-4
- EAE
experimental autoimmune encephalomyelitis
- MS
multiple sclerosis
- CFA
complete Freund's adjuvant
- CNS
central nervous system
- PLP
proteolipid protein
- MBP
myelin basic protein
- SCH
spinal cord homogenate
- ELISPOT
enzyme-linked immunospot
- Th1 and Th2
T helper 1 and 2
References
- 1.Raine C. Lab Invest. 1984;50:608–635. [PubMed] [Google Scholar]
- 2.Chambers C A, Kuhns M S, Egen J G, Allison J P. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 3.Karandikar N J, Vanderlugt C L, Walunas T L, Miller S D, Bluestone J A. J Exp Med. 1996;184:783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrin P J, Maldonado J H, Davis T A, June C H, Racke M K. J Immunol. 1996;157:1333–1336. [PubMed] [Google Scholar]
- 5.Hurwitz A A, Sullivan T J, Krummel M F, Sobel R A, Allison J P. J Neuroimmunol. 1997;73:57–62. doi: 10.1016/s0165-5728(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 6.Kuhns M S, Epshteyn V, Sobel R A, Allison J P. Proc Natl Acad Sci USA. 2000;97:12711–12716. doi: 10.1073/pnas.220423597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korngold R, Feldman A, Rorke L B, Lublin F D, Doherty P C. Immunogenetics. 1986;24:309–315. doi: 10.1007/BF00395536. [DOI] [PubMed] [Google Scholar]
- 8.Encinas J A, Lees M B, Sobel R A, Symonowicz C, Greer J M, Shovlin C L, Weiner H L, Seidman C E, Seidman J G, Kuchroo V K. J Immunol. 1996;157:2186–2192. [PubMed] [Google Scholar]
- 9.Butterfield R J, Sudweeks J D, Blankenhorn E P, Korngold R, Marini J C, Todd J A, Roper R J, Teuscher C. J Immunol. 1998;161:1860–1867. [PubMed] [Google Scholar]
- 10.Bernard C. J Immunogenet. 1976;1:352. doi: 10.1111/j.1744-313x.1976.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 11.Trotter J, Zamvil S S, Steinman L. J Immunol. 1987;139:1834–1839. [PubMed] [Google Scholar]
- 12.Abromson-Leeman S, Hayashi M, Martin C, Sobel R, al-Sabbagh A, Weiner H, Dorf M E. J Neuroimmunol. 1993;45:89–101. doi: 10.1016/0165-5728(93)90168-x. [DOI] [PubMed] [Google Scholar]
- 13.Abromson-Leeman S, Alexander J, Bronson R, Carroll J, Southwood S, Dorf M. J Immunol. 1995;154:388–398. [PubMed] [Google Scholar]
- 14.Charles P C, Weber K S, Cipriani B, Brosnan C F. J Neuroimmunol. 1999;100:64–73. doi: 10.1016/s0165-5728(99)00189-7. [DOI] [PubMed] [Google Scholar]
- 15.Teuscher C, Blankenhorn E P, Hickey W F. Cell Immunol. 1987;110:294–304. doi: 10.1016/0008-8749(87)90124-9. [DOI] [PubMed] [Google Scholar]
- 16.Teuscher C, Blankenhorn E P, Hickey W F. Curr Top Microbiol Immunol. 1988;137:233–239. doi: 10.1007/978-3-642-50059-6_35. [DOI] [PubMed] [Google Scholar]
- 17.Tuohy V K, Sobel R A, Lees M B. J Immunol. 1988;140:1868–1873. [PubMed] [Google Scholar]
- 18.Greer J M, Sobel R A, Sette A, Southwood S, Lees M B, Kuchroo V K. J Immunol. 1996;156:371–379. [PubMed] [Google Scholar]
- 19.van Elsas A, Hurwitz A A, Allison J P. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Elsas A, Sutmuller R P M, Hurwitz A A, Ziskin J, Villasenor J, Medema J-P, Overwijk W W, Restifo N P, Melief C J M, Offringa R, et al. J Exp Med. 2001;194:481–489. doi: 10.1084/jem.194.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yanagawa T, Hidaka Y, Guimaraes V, Soliman M, DeGroot L J. J Clin Endocrinol Metab. 1995;80:41–45. doi: 10.1210/jcem.80.1.7829637. [DOI] [PubMed] [Google Scholar]
- 22.Barrat F J, Le Deist F, Benkerrou M, Bousso P, Feldmann J, Fischer A, de Saint Basile G. Proc Natl Acad Sci USA. 1999;96:8645–8650. doi: 10.1073/pnas.96.15.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsui T, Kurokawa M, Kobata T, Oki S, Azuma M, Tohma S, Inoue T, Yamamoto K, Nishioka K, Kato T. J Immunol. 1999;162:4328–4335. [PubMed] [Google Scholar]
- 24.Fukazawa T, Yanagawa T, Kikuchi S, Yabe I, Sasaki H, Hamada T, Miyasaka K, Gomi K, Tashiro K. J Neurol Sci. 1999;171:49–55. doi: 10.1016/s0022-510x(99)00251-8. [DOI] [PubMed] [Google Scholar]
- 25.Ligers A, Xu C, Saarinen S, Hillert J, Olerup O. J Neuroimmunol. 1999;97:182–190. doi: 10.1016/s0165-5728(99)00072-7. [DOI] [PubMed] [Google Scholar]
- 26.Harbo H F, Celius E G, Vartdal F, Spurkland A. Tissue Antigens. 1999;53:106–110. doi: 10.1034/j.1399-0039.1999.530112.x. [DOI] [PubMed] [Google Scholar]
- 27.Segal B M, Shevach E M. J Exp Med. 1996;184:771–775. doi: 10.1084/jem.184.2.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang J T, Shevach E M, Segal B M. J Exp Med. 1999;189:969–978. doi: 10.1084/jem.189.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balashov K E, Smith D R, Khoury S J, Hafler D A, Weiner H L. Proc Natl Acad Sci USA. 1997;94:599–603. doi: 10.1073/pnas.94.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egen J G, Allison J P. Immunity. 2002;16:23–35. doi: 10.1016/s1074-7613(01)00259-x. [DOI] [PubMed] [Google Scholar]