Abstract
1. The effect of electrically induced `exercise' on the respiratory oscillation of arterial pH was studied in chloralose-anaesthetized dogs with spinal cord transection at T8/9 (dermatome level T6/7).
2. Respiratory oscillations of arterial pH (presumed to be due to oscillations of arterial PCO2) were sensed with a fast-responding electrode in one carotid artery. Breath-by-breath estimates of the maximum rate of change of pH of the downstroke of the pH oscillation (dpH/dt↓max) were obtained by differentiating the pH signal.
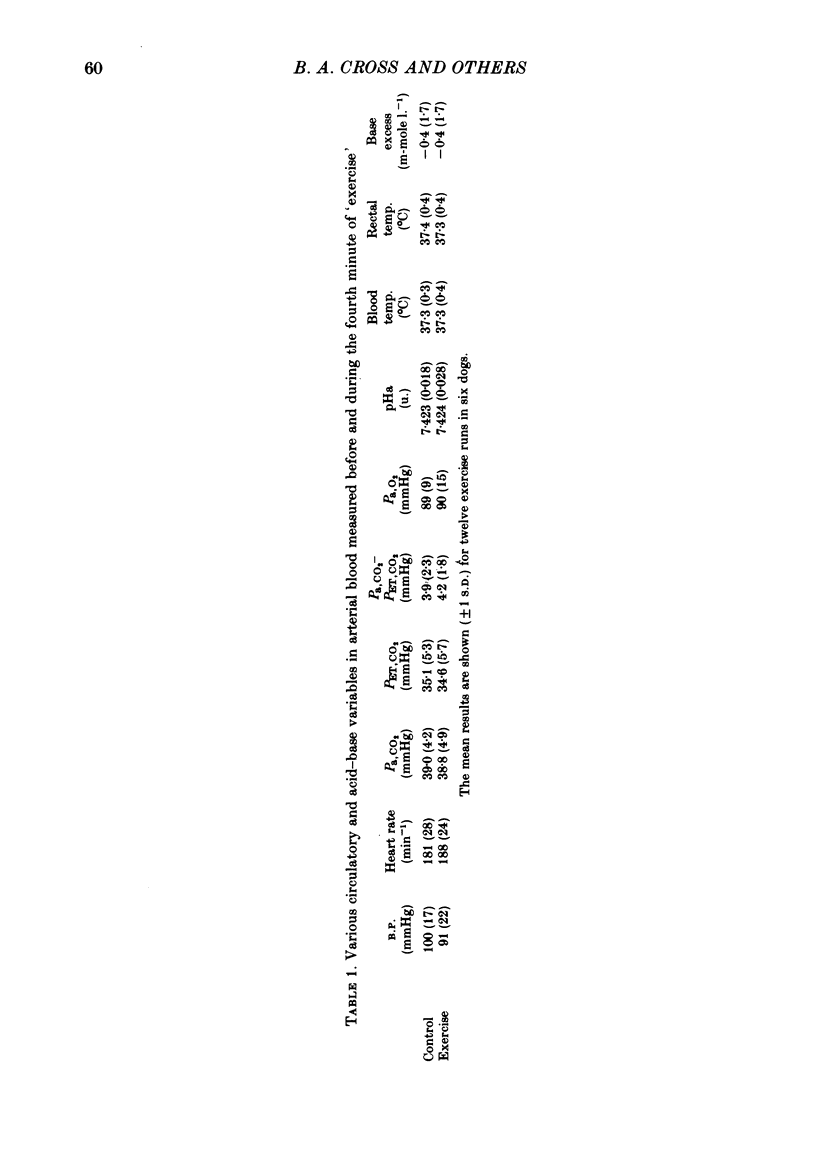
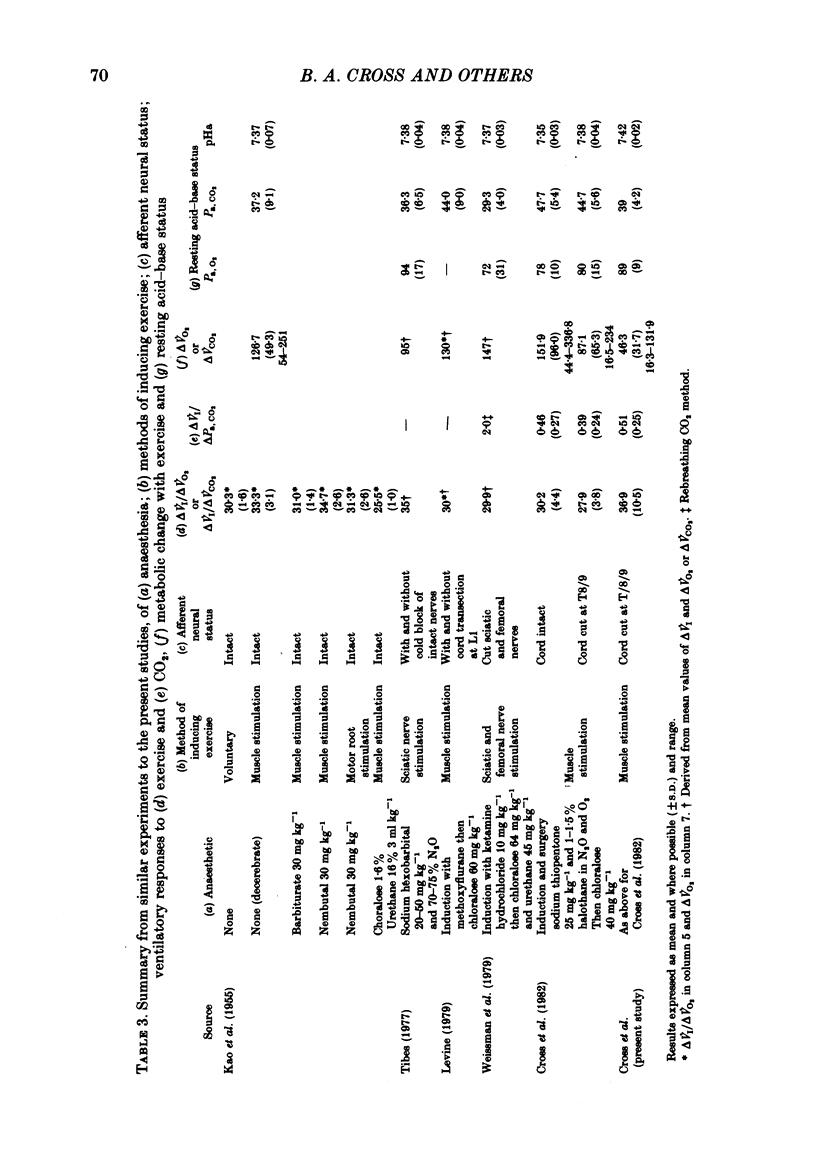
3. Consistent with the findings of the previous paper (Cross et al. 1982), the ventilatory response to exercise could not be explained on the basis of sensitivity to CO2; the Δ˙VI/ΔPa, CO2 was significantly greater for `exercise' than for CO2 inhalation.
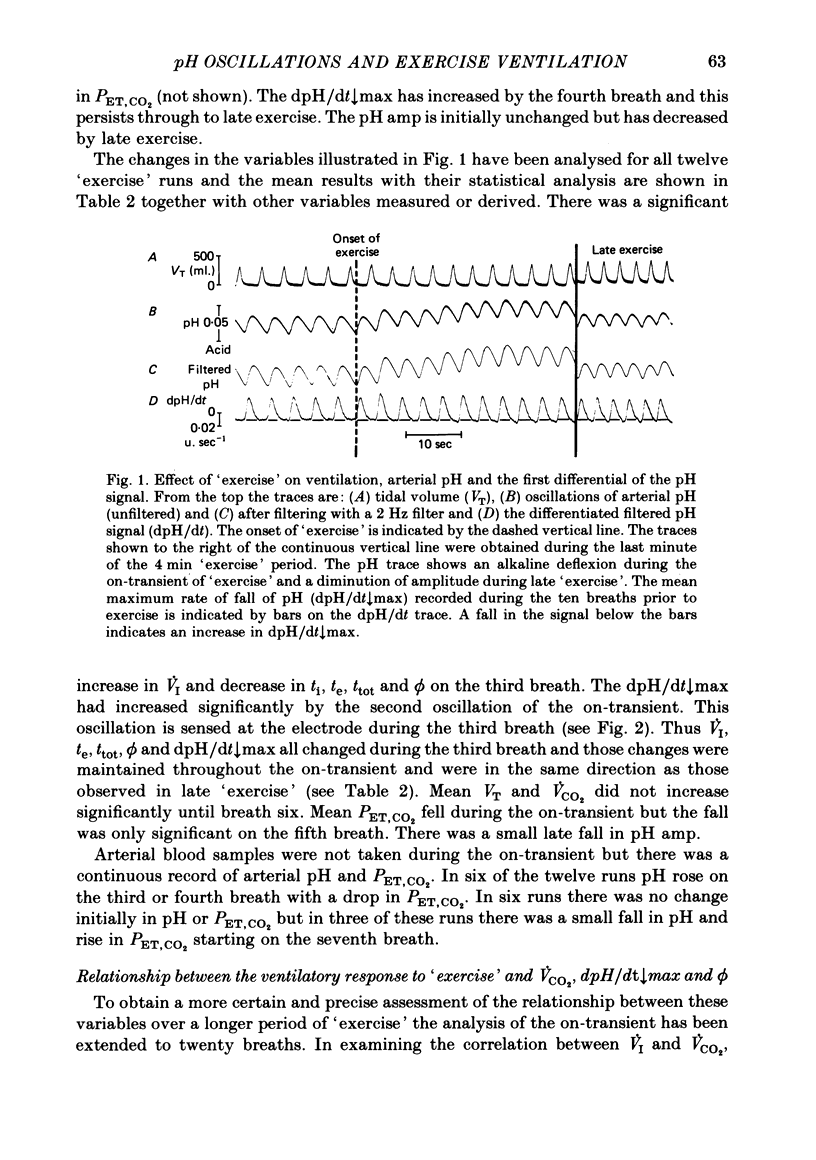
4. On average, the amplitude of the pH oscillations decreased during `exercise'. The change in the phase relationship (ϕ) between respiratory and pH cycles, although significant from the second breath onwards, was not thought to be responsible for the increased ventilation ˙VI; the direction of the change was opposite to that previously found to increase ˙VI.
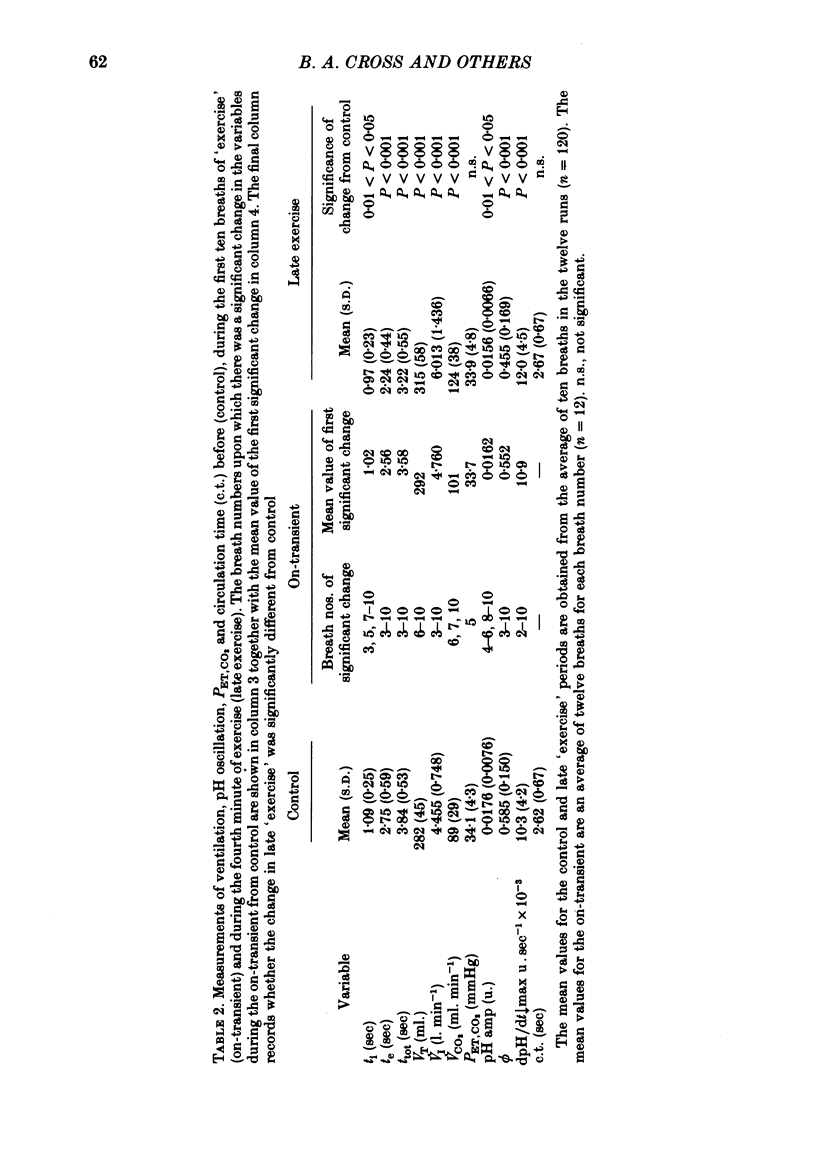
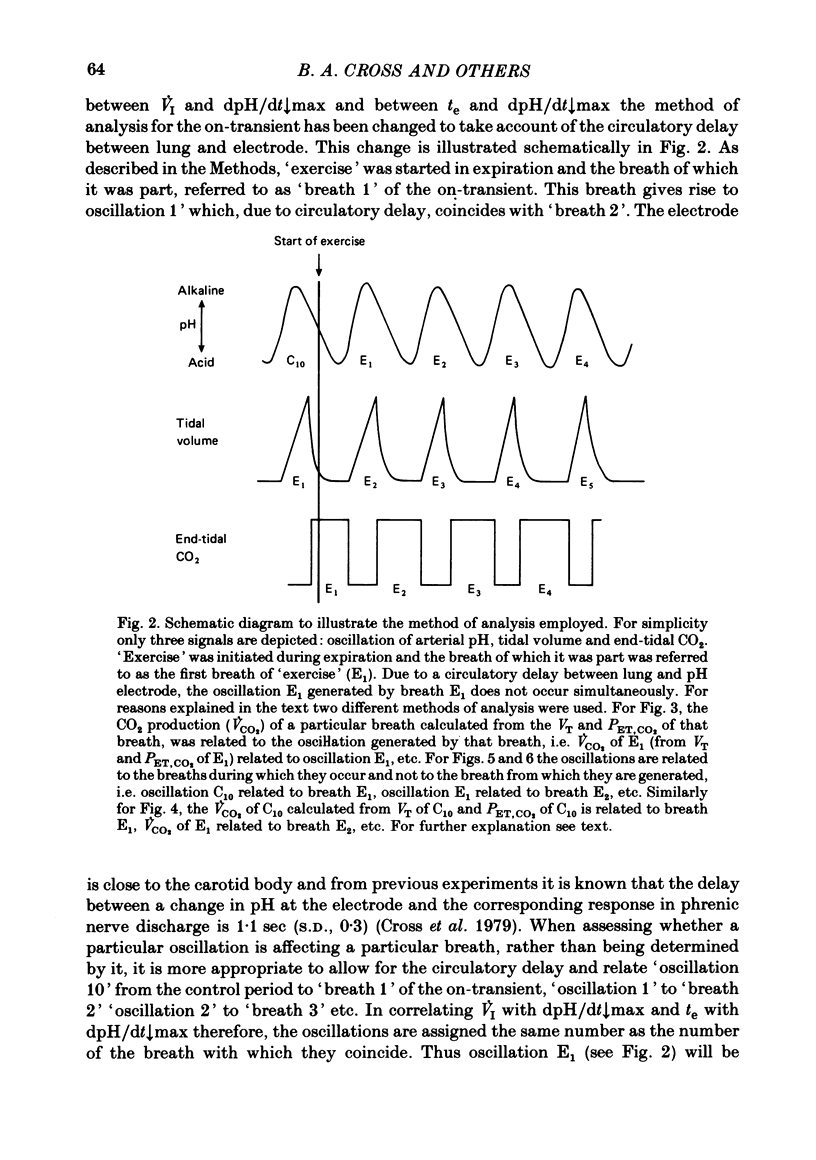
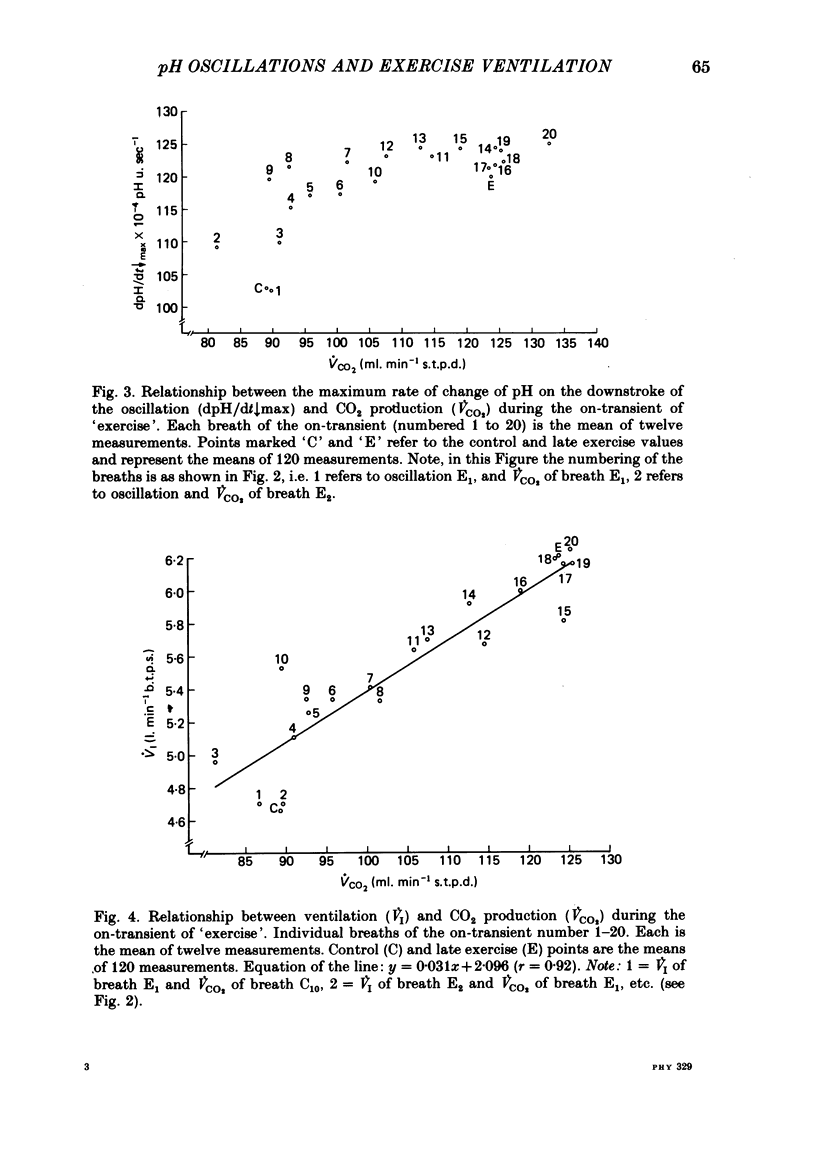
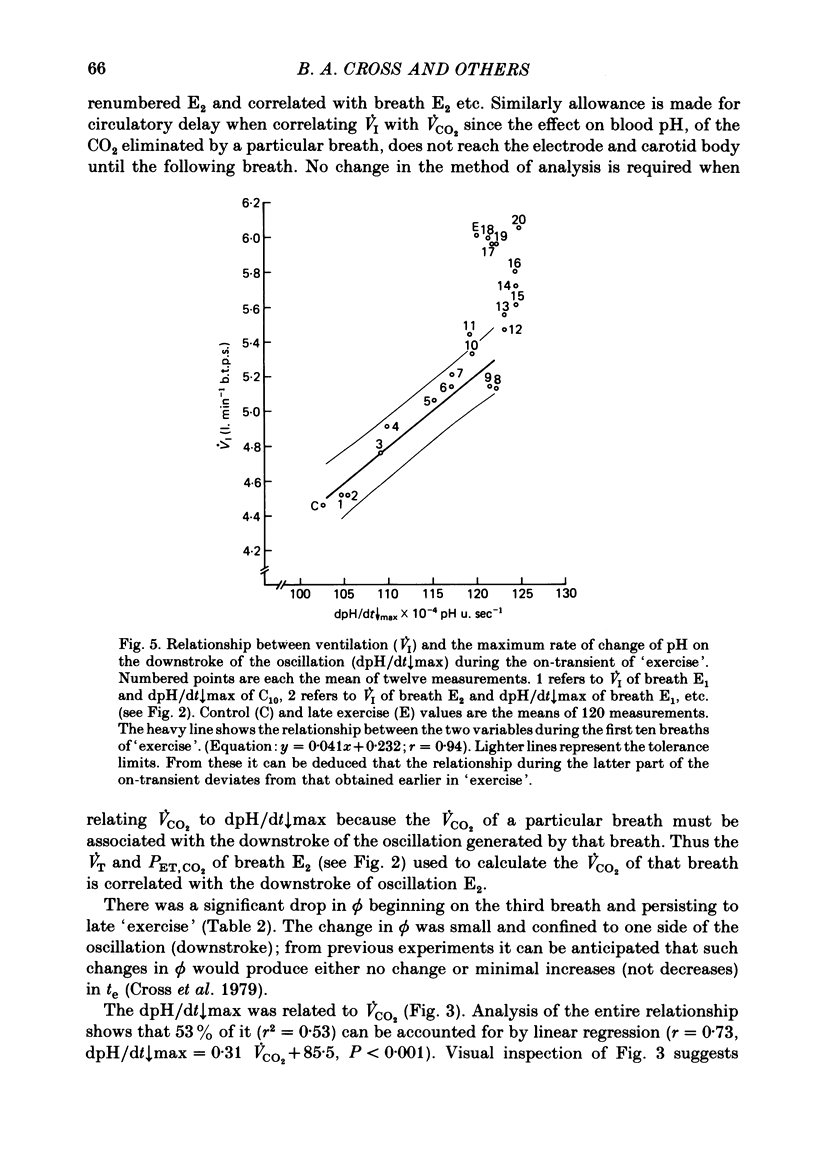
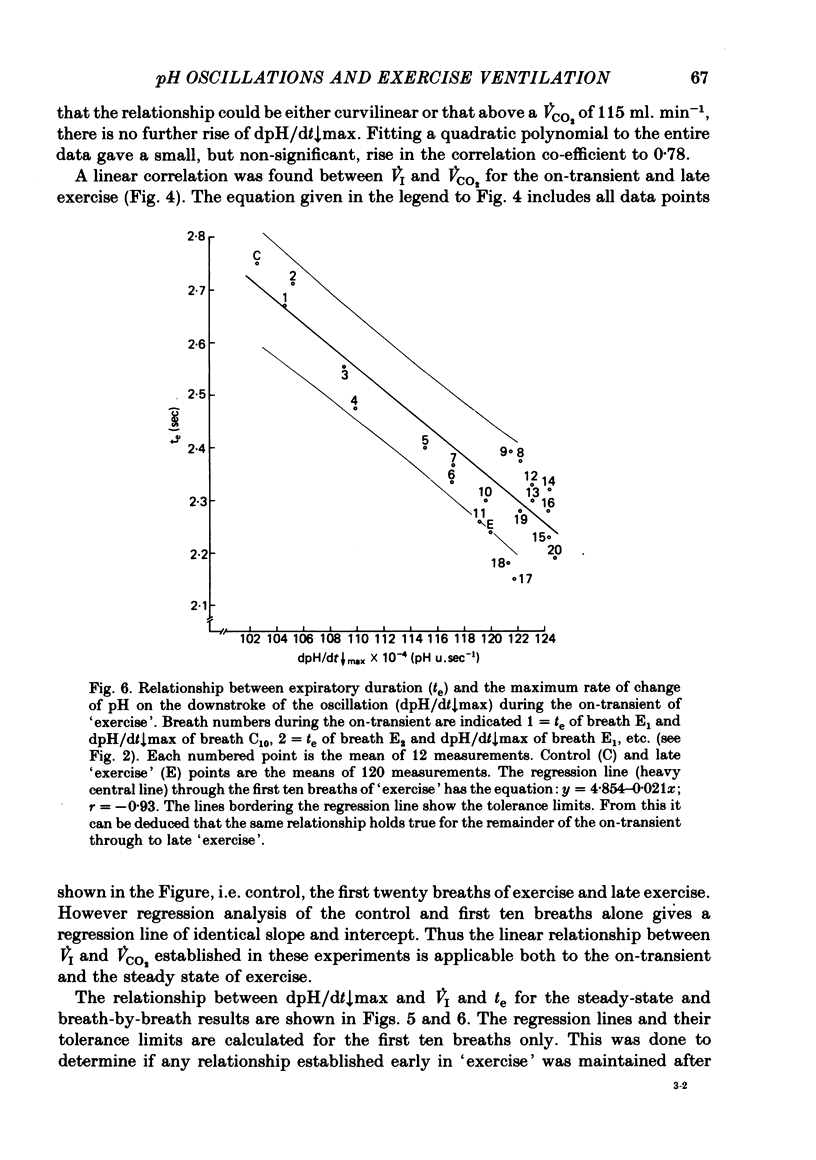
5. Inspiratory duration (ti), expiratory duration (te), ˙VI and the dpH/dt↓max changed significantly by the third breath of `exercise'. A significantly linear relationship was obtained between te and dpH/dt↓max during the on-transient (first ten breaths) of `exercise'. This relationship was maintained throughout `exercise'. ˙VI and dpH/dt↓max were also linearly related during the on-transient, although the same relationship did not hold true throughout `exercise'.
6. The dpH/dt↓max was related to CO2 production (˙VCO2) lending support to the prediction that the slope of the downstroke of the pH oscillation is a function of ˙VCO2.
7. It was concluded that the dpH/dt↓max (dpCO2/dt↑max) is a potential humoral signal in `exercise' and could account totally for the shortening of te. Since there was a late rise in ˙VI (due to an increase in tidal volume VT) in the absence of a change in dpH/dt↓max, it was considered unlikely that the dpH/dt↓max was the only humoral signal present during `exercise'.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Band D. M., McClelland M., Phillips D. L., Saunders K. B., Wolff C. B. Sensitivity of the carotid body to within-breath changes in arterial PCO2. J Appl Physiol Respir Environ Exerc Physiol. 1978 Nov;45(5):768–777. doi: 10.1152/jappl.1978.45.5.768. [DOI] [PubMed] [Google Scholar]
- Band D. M., Semple S. J. Continuous measurement of blood pH with an indwelling arterial glass electrode. J Appl Physiol. 1967 Apr;22(4):854–857. doi: 10.1152/jappl.1967.22.4.854. [DOI] [PubMed] [Google Scholar]
- Band D. M., Wolff C. B., Ward J., Cochrane G. M., Prior J. Respiratory oscillations in arterial carbon dioxide tension as a control signal in exercise. Nature. 1980 Jan 3;283(5742):84–85. doi: 10.1038/283084a0. [DOI] [PubMed] [Google Scholar]
- Black A. M., McCloskey D. I., Torrance R. W. The responses of carotid body chemoreceptors in the cat to sudden changes of hypercapnic and hypoxic stimuli. Respir Physiol. 1971 Oct;13(1):36–49. doi: 10.1016/0034-5687(71)90063-6. [DOI] [PubMed] [Google Scholar]
- Brewer A., Cross B. A., Davey A., Guz A., Jones P., Katona P., MacLean M., Murphy K., Semple S. J., Solomon M. Effect of electrically induced exercise in anaesthetized dogs on ventilation and arterial pH [proceedings]. J Physiol. 1980 Jan;298:49P–50P. [PubMed] [Google Scholar]
- CROPP G. J., COMROE J. H., Jr Role of mixed venous blood PCO2 in respiratory control. J Appl Physiol. 1961 Nov;16:1029–1033. doi: 10.1152/jappl.1961.16.6.1029. [DOI] [PubMed] [Google Scholar]
- Cowell T. K., Band D. M., Semple S. J. A fast-response pH meter. J Appl Physiol. 1967 Apr;22(4):858–860. doi: 10.1152/jappl.1967.22.4.858. [DOI] [PubMed] [Google Scholar]
- Cross B. A., Davey A., Guz A., Katona P. G., MacLean M., Murphy K., Semple S. J., Stidwill R. The role of spinal cord transmission in the ventilatory response to electrically induced exercise in the anaesthetized dog. J Physiol. 1982 Aug;329:37–55. doi: 10.1113/jphysiol.1982.sp014289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross B. A., Grant B. J., Guz A., Jones P. W., Semple S. J., Stidwill R. P. Dependence of phrenic motoneurone output on the oscillatory component of arterial blood gas composition. J Physiol. 1979 May;290(2):163–184. doi: 10.1113/jphysiol.1979.sp012766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOIS A. B., BRITT A. G., FENN W. O. Alveolar CO2 during the respiratory cycle. J Appl Physiol. 1952 Jan;4(7):535–548. doi: 10.1152/jappl.1952.4.7.535. [DOI] [PubMed] [Google Scholar]
- Duval A., Léoty C. Comparison between the delayed outward current in slow and fast twitch skeletal muscle in the rat. J Physiol. 1980 Oct;307:43–57. doi: 10.1113/jphysiol.1980.sp013422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt J. R., Winn R. K., Hildebrandt J. Cardiorespiratory responses to sudden release of circulatory occlusion during exercise. Respir Physiol. 1979 Sep;38(1):83–92. doi: 10.1016/0034-5687(79)90008-2. [DOI] [PubMed] [Google Scholar]
- KAO F. F., SCHLIG B. B., BROOKS C. M. Regulation of respiration during induced muscular work in decerebrate dogs. J Appl Physiol. 1955 Jan;7(4):379–386. doi: 10.1152/jappl.1955.7.4.379. [DOI] [PubMed] [Google Scholar]
- Levine S. Ventilatory response to muscular exercise: observations regarding a humoral pathway. J Appl Physiol Respir Environ Exerc Physiol. 1979 Jul;47(1):126–137. doi: 10.1152/jappl.1979.47.1.126. [DOI] [PubMed] [Google Scholar]
- Phillipson E. A., Hickey R. F., Bainton C. R., Nadel J. A. Effect of vagal blockade on regulation of breathing in conscious dogs. J Appl Physiol. 1970 Oct;29(4):475–479. doi: 10.1152/jappl.1970.29.4.475. [DOI] [PubMed] [Google Scholar]
- STOREY W. F., BUTLER J. Evidence that the PCO2 of mixed venous blood is not a regulator of ventilation during exercise. J Appl Physiol. 1963 Mar;18:345–348. doi: 10.1152/jappl.1963.18.2.345. [DOI] [PubMed] [Google Scholar]
- Saunders K. B. Oscillations of arterial CO2 tension in a respiratory model: some implications for the control of breathing in exercise. J Theor Biol. 1980 May 7;84(1):163–179. doi: 10.1016/s0022-5193(80)81042-3. [DOI] [PubMed] [Google Scholar]
- Weissman M. L., Wasserman K., Huntsman D. J., Whipp B. J. Ventilation and gas exchange during phasic hindlimb exercise in the dog. J Appl Physiol Respir Environ Exerc Physiol. 1979 May;46(5):878–884. doi: 10.1152/jappl.1979.46.5.878. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO W. S., EDWARDS M. W., Jr Homeostasis of carbon dioxide during intravenous infusion of carbon dioxide. J Appl Physiol. 1960 Sep;15:807–818. doi: 10.1152/jappl.1960.15.5.807. [DOI] [PubMed] [Google Scholar]


