Abstract
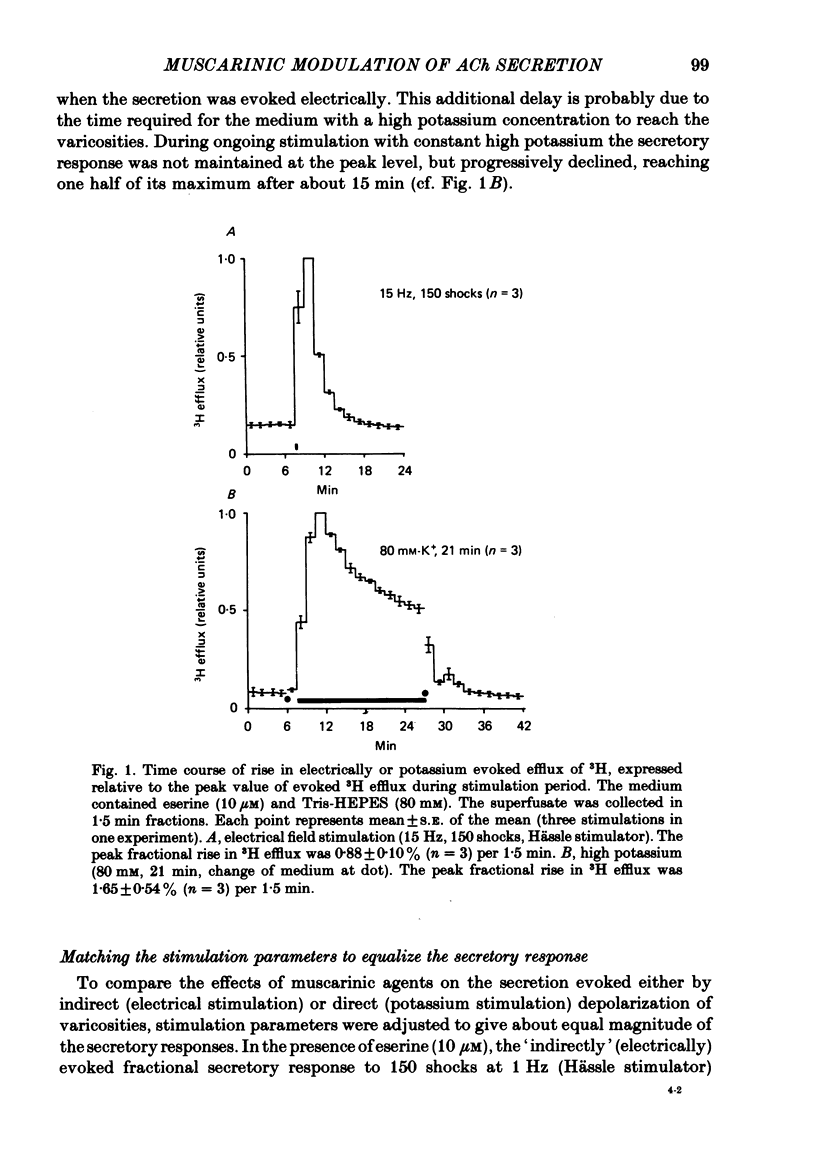
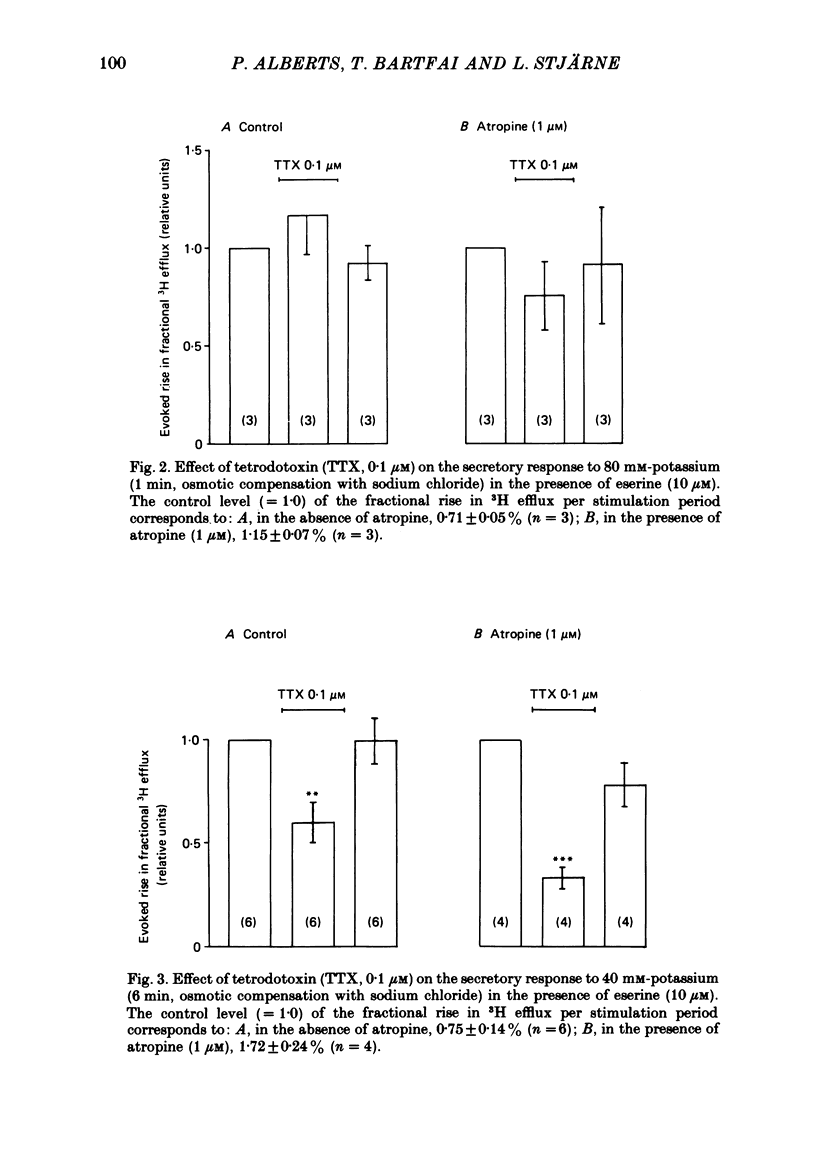
1. The myenteric plexus-longitudinal muscle preparation of the guinea-pig ileum was incubated with [3H]choline (1·125-1·5 μM), and then superfused with Tyrode solution containing hemicholinium-3 (10 μM). Secretion of [3H]acetylcholine ([3H]ACh) was evoked either (a) by electrical field stimulation (0·5-15 Hz, 150 shocks per period, 0·5 msec), used to `indirectly' depolarize the varicosities of nerve terminals, or (b) by high potassium (40 mM with 1 μM-tetrodotoxin, for 6 min, or 80 mM without tetrodotoxin, for 1 min), to `directly' depolarize varicosities.
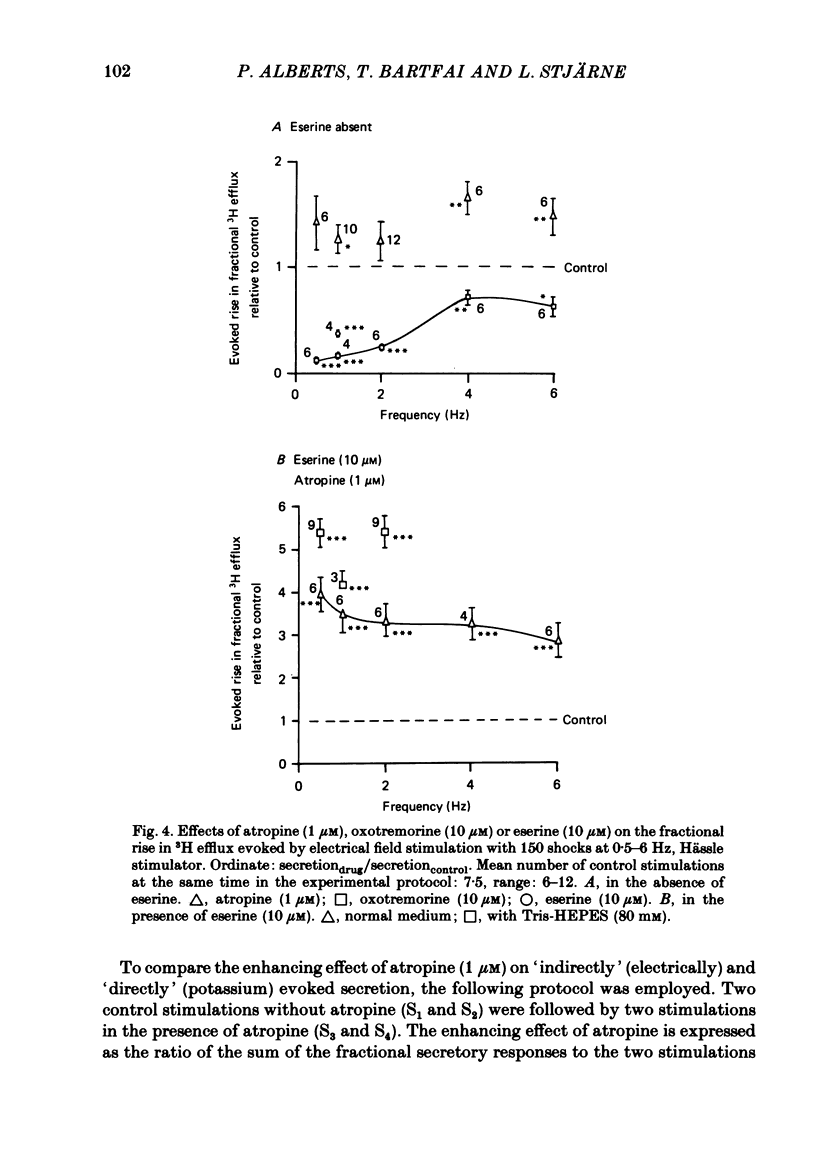
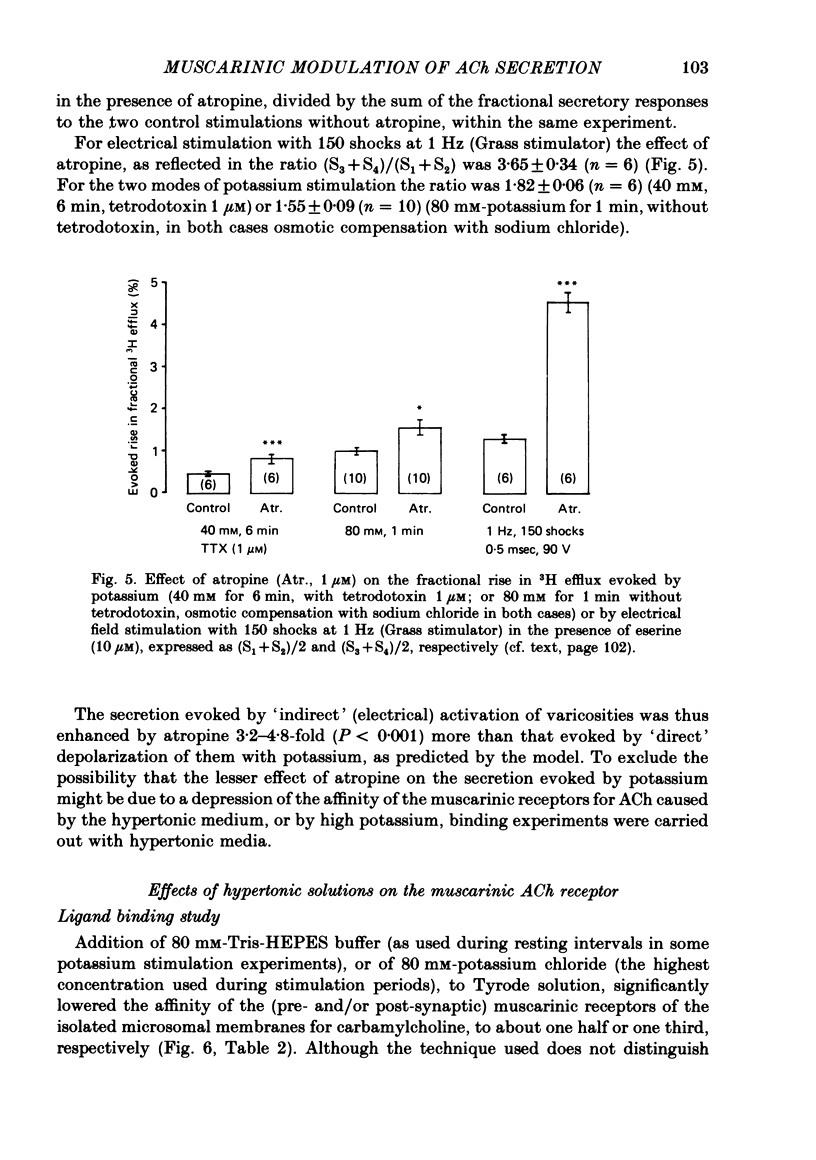
2. With these stimulation parameters, which yielded about the same fractional secretion of [3H]ACh, and with eserine (10 μM) present in the medium, atropine (1 μM) enhanced the `indirectly', electrically evoked secretion 3·65±0·34 (n = 6) fold, and that caused by 40 mM or 80 mM-potassium 1·82±0·06 (n = 6) or 1·55±0·09 (n = 10) fold, respectively. Atropine thus enhanced `indirectly', electrically evoked secretion 4-fold more than that caused by `direct' depolarization of varicosities with high potassium (P < 0·001).
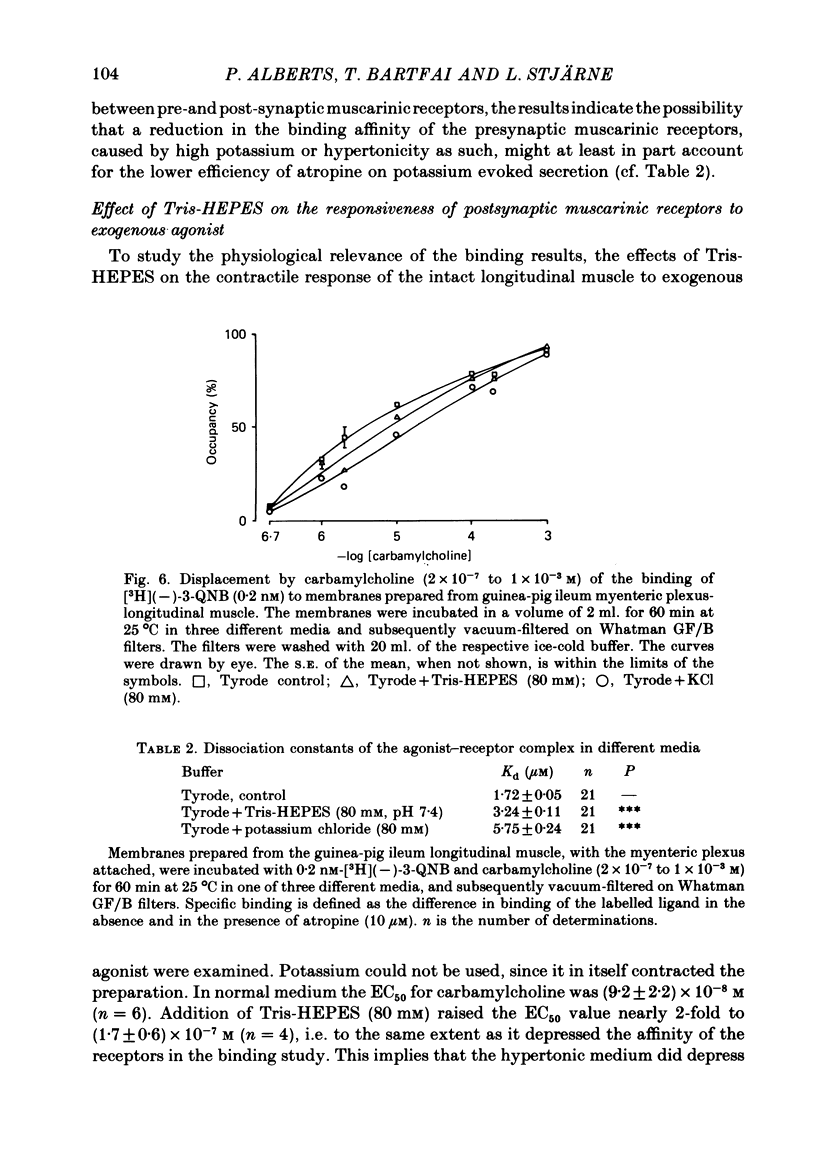
3. This difference is not likely to be caused by depression of the sensitivity of the presynaptic muscarinic receptors to ACh released by nerve stimulation, caused by the hypertonicity of the medium in the potassium stimulation experiments. The medium made hypertonic by addition of Tris-HEPES (80 mM) did lower the binding affinity of membrane preparations of (pre- and post-synaptic) muscarinic receptors, to carbamylcholine, and also the contractile responsiveness of the longitudinal muscle to this agent, in both cases to about one half. But it did not appear to alter the responsiveness of either pre- or post-synaptic muscarinic receptors to endogenous ACh, released by nerve stimulation.
4. The results support a dual-mode model for the muscarinic negative feed-back control of ACh secretion from the nerve terminals of this preparation, mainly operating by restriction of the invasion of terminals, and only secondarily by depression of the efficiency of depolarization—secretion coupling in invaded varicosities.
5. Since this model has earlier been proposed to apply for the control of secretion of [3H]noradrenaline from the micro-anatomically similar nerve terminals of noradrenergic nerves, the present findings suggest that the model may have a wider biological significance, and possibly apply to the control of the secretory activity of boutons-en-passant nerve terminals in general.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts P., Bartfai T., Stjärne L. Site(s) and ionic basis of alpha-autoinhibition and facilitation of "3H'noradrenaline secretion in guinea-pig vas deferens. J Physiol. 1981 Mar;312:297–334. doi: 10.1113/jphysiol.1981.sp013630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsall N. J., Burgen A. S., Hulme E. C., Wells J. W. The effects of ions on the binding of agonists and antagonists to muscarinic receptors. Br J Pharmacol. 1979 Nov;67(3):371–377. doi: 10.1111/j.1476-5381.1979.tb08690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzieniszewski P., Kilbinger H. Muscarinic modulation of acetylcholine release evoked by dimethylphenylpiperazinium and high potassium from guinea-pig myenteric plexus. Eur J Pharmacol. 1978 Aug 15;50(4):385–391. doi: 10.1016/0014-2999(78)90144-9. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Isolation of choline esters from aqueous solutions by extraction with sodium tetraphenylboron in organic solvents. Biochem J. 1969 Jun;113(2):291–298. doi: 10.1042/bj1130291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabella G. Fine structure of the myenteric plexus in the guinea-pig ileum. J Anat. 1972 Jan;111(Pt 1):69–97. [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. M., McCaman R. E. The determination of picomole amounts of acetylcholine in mammalian brain. J Neurochem. 1973 Jan;20(1):1–8. doi: 10.1111/j.1471-4159.1973.tb12097.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson L., Hedqvist P., Lundgren G. Pre- and postjunctional effects of prostaglandin E2, prostaglandin synthetase inhibitors and atropine on cholinergic neurotransmission in guinea pig ileum and bovine iris. Acta Physiol Scand. 1980 Dec;110(4):401–411. doi: 10.1111/j.1748-1716.1980.tb06687.x. [DOI] [PubMed] [Google Scholar]
- Göthert M., Nawroth P., Neumeyer H. Inhibitory effects of verapamil, prenylamine and D 600 on Ca2+-dependent noradrenaline release from the sympathetic nerves of isolated rabbit hearts. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(1):11–19. doi: 10.1007/BF00499869. [DOI] [PubMed] [Google Scholar]
- Hadházy P., Szerb J. C. The effect of cholinergic drugs on [3H]acetylcholine release from slices of rat hippocampus, striatum and cortex. Brain Res. 1977 Mar 11;123(2):311–322. doi: 10.1016/0006-8993(77)90482-6. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Haubrich D. R. Partial purification and properties of choline kinase (EC 2. 7. 1. 32) from rabbit brain: measurement of acetylcholine. J Neurochem. 1973 Aug;21(2):315–328. doi: 10.1111/j.1471-4159.1973.tb04252.x. [DOI] [PubMed] [Google Scholar]
- Järv J., Hedlund B., Bartfai T. Isomerization of the muscarinic receptor . antagonist complex. J Biol Chem. 1979 Jul 10;254(13):5595–5598. [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbinger H. Modulation by oxotremorine and atropine of acetylcholine release evoked by electrical stimulation of the myenteric plexus of the guinea-pig ileum. Naunyn Schmiedebergs Arch Pharmacol. 1977 Nov;300(2):145–151. doi: 10.1007/BF00505045. [DOI] [PubMed] [Google Scholar]
- Kilbinger H., Wagner P. Inhibition by oxotremorine of acetylcholine resting release from guinea pig-ileum longitudinal muscle strips. Naunyn Schmiedebergs Arch Pharmacol. 1975;287(1):47–60. doi: 10.1007/BF00632637. [DOI] [PubMed] [Google Scholar]
- Kilbinger H., Wessler I. Inhibition by acetylcholine of the stimulation-evoked release of [3H]acetylcholine from the guinea-pig myenteric plexus. Neuroscience. 1980;5(7):1331–1340. doi: 10.1016/0306-4522(80)90205-5. [DOI] [PubMed] [Google Scholar]
- Kilbinger H., Wessler I. Pre- and postsynaptic effects of muscarinic agonists in the guinea-pig ileum. Naunyn Schmiedebergs Arch Pharmacol. 1980 Nov;314(3):259–266. doi: 10.1007/BF00498547. [DOI] [PubMed] [Google Scholar]
- Kosterlitz H. W., Lydon R. J., Watt A. J. The effects of adrenaline, noradrenaline and isoprenaline on inhibitory alpha- and beta-adrenoceptors in the longitudinal muscle of the guinea-pig ileum. Br J Pharmacol. 1970 Jun;39(2):398–413. doi: 10.1111/j.1476-5381.1970.tb12903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzati M., Clavilier L., Pere-Aubert G., Slonimski P. P. Contrôle biochimique de la recombinaison chez Saccharomyces cerevisiae. I. Inhibition par la L-histidine de la recombinaison intragenique mitotique au locus ad 3. Mol Gen Genet. 1971;111(2):120–137. doi: 10.1007/BF00267787. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Bártfai T. Discrimination between mathematical models of biological systems exemplified by enzyme steady state kinetics. Acta Biol Med Ger. 1973;31(2):203–215. [PubMed] [Google Scholar]
- McGee R., Simpson P., Christian C., Mata M., Nelson P., Nirenberg M. Regulation of acetylcholine release from neuroblastoma x glioma hybrid cells. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1314–1318. doi: 10.1073/pnas.75.3.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., North R. A. Clonidine activates membrane potassium conductance in myenteric neurones. Br J Pharmacol. 1981 Oct;74(2):419–428. doi: 10.1111/j.1476-5381.1981.tb09987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K., North R. A. Opiates and enkephalin reduce the excitability of neuronal processes. Neuroscience. 1981;6(10):1943–1951. doi: 10.1016/0306-4522(81)90034-8. [DOI] [PubMed] [Google Scholar]
- Nemeth E. F., Cooper J. R. Effect of somatostatin on acetylcholine release from rat hippocampal synaptosomes. Brain Res. 1979 Apr 6;165(1):166–170. doi: 10.1016/0006-8993(79)90057-x. [DOI] [PubMed] [Google Scholar]
- Nordström O., Bartfai T. Muscarinic autoreceptor regulates acetylcholine release in rat hippocampus: in vitro evidence. Acta Physiol Scand. 1980 Apr;108(4):347–353. doi: 10.1111/j.1748-1716.1980.tb06543.x. [DOI] [PubMed] [Google Scholar]
- North R. A. Electrophysiology of the enteric nervous system. Neuroscience. 1982 Feb;7(2):315–325. doi: 10.1016/0306-4522(82)90269-x. [DOI] [PubMed] [Google Scholar]
- Paton W. D., Vizi E. S. The inhibitory action of noradrenaline and adrenaline on acetylcholine output by guinea-pig ileum longitudinal muscle strip. Br J Pharmacol. 1969 Jan;35(1):10–28. doi: 10.1111/j.1476-5381.1969.tb07964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton W. D., Zar M. A. The origin of acetylcholine released from guinea-pig intestine and longitudinal muscle strips. J Physiol. 1968 Jan;194(1):13–33. doi: 10.1113/jphysiol.1968.sp008392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANG H. P. STIMULANT ACTIONS OF VOLATILE ANAESTHETICS ON SMOOTH MUSCLE. Br J Pharmacol Chemother. 1964 Apr;22:356–365. doi: 10.1111/j.1476-5381.1964.tb02040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson I. W., Szerb J. C. The release of labelled acetylcholine and choline from cerebral cortical slices stimulated electrically. Br J Pharmacol. 1974 Dec;52(4):499–507. doi: 10.1111/j.1476-5381.1974.tb09717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawynok J., Jhamandas K. Muscarinic feedback inhibition of acetylcholine release from the myenteric plexus in the quinea pig ileum and its status after chronic exposure to morphine. Can J Physiol Pharmacol. 1977 Aug;55(4):909–916. doi: 10.1139/y77-121. [DOI] [PubMed] [Google Scholar]
- Stjärne L., Bartfai T., Alberts P. The influence of 8-Br 3', 5'-cyclic nucleotide analogs and of inhibitors of 3', 5'-cyclic nucleotide phosphodiesterase, on noradrenaline secretion and neuromuscular transmission in guinea-pig vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1979 Aug;308(2):99–105. doi: 10.1007/BF00499050. [DOI] [PubMed] [Google Scholar]
- Stjärne L. Facilitation and receptor-mediated regulation of noradrenaline secretion by control of recruitment of varicosities as well as by control of electro-secretory coupling. Neuroscience. 1978;3(12):1147–1155. doi: 10.1016/0306-4522(78)90135-5. [DOI] [PubMed] [Google Scholar]
- Swedin G. Postnatal development of the mechanical response of the isolated rat vas deferens to nerve stimulation. Acta Physiol Scand. 1972 Feb;84(2):217–223. doi: 10.1111/j.1748-1716.1972.tb05172.x. [DOI] [PubMed] [Google Scholar]
- Szerb J. C. Effect of low calcium and of oxotremorine on the kinetics of the evoked release of [3H]acetylcholine from the guinea-pig myenteric plexus; comparison with morphine. Naunyn Schmiedebergs Arch Pharmacol. 1980 Mar;311(2):119–127. doi: 10.1007/BF00510250. [DOI] [PubMed] [Google Scholar]
- Szerb J. C. Endogenous acetylcholine release and labelled acetylcholine formation from [3H]choline in the myenteric plexus of the guinea-pig ileum. Can J Physiol Pharmacol. 1975 Aug;53(4):566–574. doi: 10.1139/y75-080. [DOI] [PubMed] [Google Scholar]
- Szerb J. C., Somogyi G. T. Depression of acetylcholine release from cerebral cortical slices by cholinesterase inhibition and by oxotremorine. Nat New Biol. 1973 Jan 24;241(108):121–122. doi: 10.1038/newbio241121a0. [DOI] [PubMed] [Google Scholar]
- Szerb J. C. Storage and release of labelled acetylcholine in the myenteric plexus of the guinea-pig ileum. Can J Physiol Pharmacol. 1976 Feb;54(1):12–22. doi: 10.1139/y76-003. [DOI] [PubMed] [Google Scholar]
- Wikberg J. E., Axelsson K. L. A simple and efficient method for studying neurotransmitter release in vitro by a radiotracer technique. Acta Physiol Scand. 1980 Jun;109(2):123–129. doi: 10.1111/j.1748-1716.1980.tb06576.x. [DOI] [PubMed] [Google Scholar]
- Wikberg J. Localization of adrenergic receptors in guinea pig ileum and rabbit jejunum to cholinergic neurons and to smooth muscle cells. Acta Physiol Scand. 1977 Feb;99(2):190–207. doi: 10.1111/j.1748-1716.1977.tb10370.x. [DOI] [PubMed] [Google Scholar]
- Wikberg J. Release of 3H-acetylcholine from isolated guinea pig ileum. A radiochemical method for studying the release on the cholinergic neurotransmitter in the intestine. Acta Physiol Scand. 1977 Nov;101(3):302–317. doi: 10.1111/j.1748-1716.1977.tb06012.x. [DOI] [PubMed] [Google Scholar]
- Yamamura H. I., Snyder S. H. Muscarinic cholinergic binding in rat brain. Proc Natl Acad Sci U S A. 1974 May;71(5):1725–1729. doi: 10.1073/pnas.71.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]


