Abstract
1. The electrical activity of up to eight concurrently active motor units has been recorded from the human deltoid and first dorsal interosseous muscles. The resulting composite myoelectric signals have been decomposed into their constituent motor-unit action potential trains using a recently developed technique.
2. A computer cross-correlation analysis has been performed on motor-unit firing rate and muscle-force output records obtained from both constant-force and triangular force-varying isometric contractions performed by normal subjects, and three groups of highly trained performers (long-distance swimmers, powerlifters and pianists).
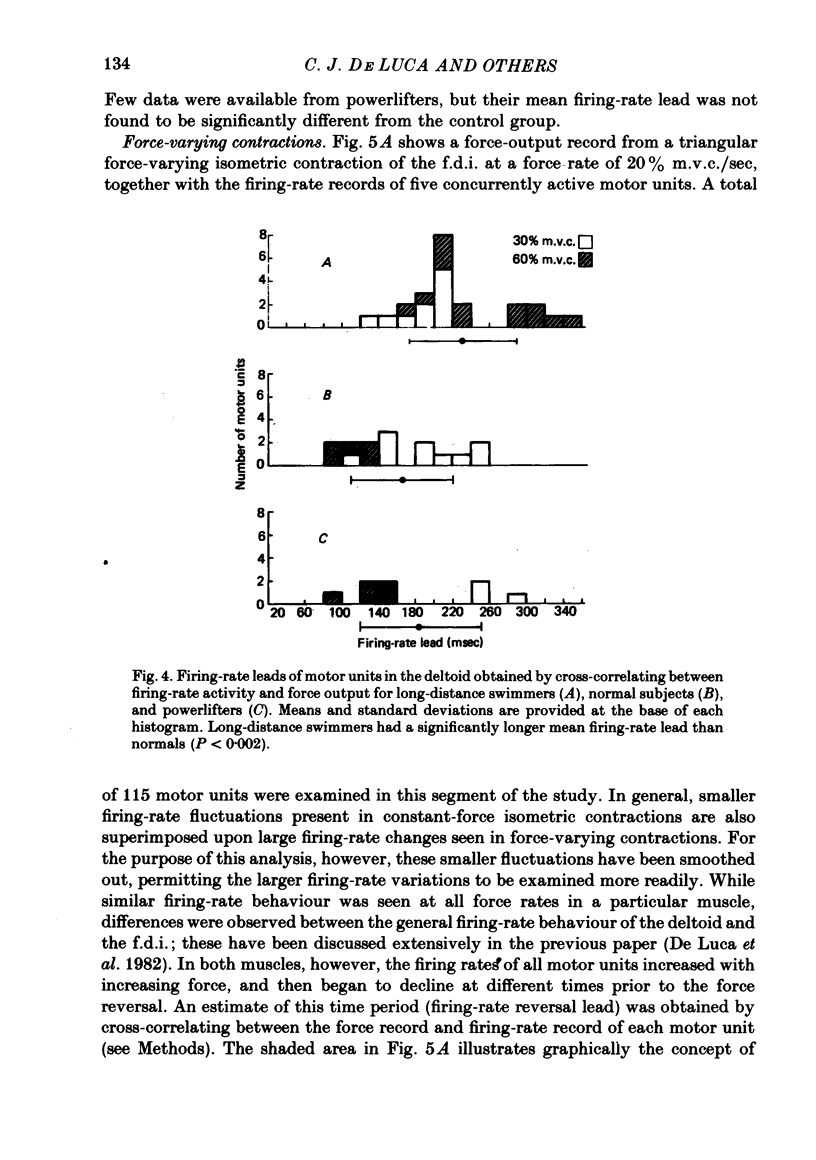
3. The temporal relationships between firing rate activity and force output have provided evidence that the deltoid of long-distance swimmers has a significantly higher percentage of slowly fatiguing fibres than that of normal subjects.
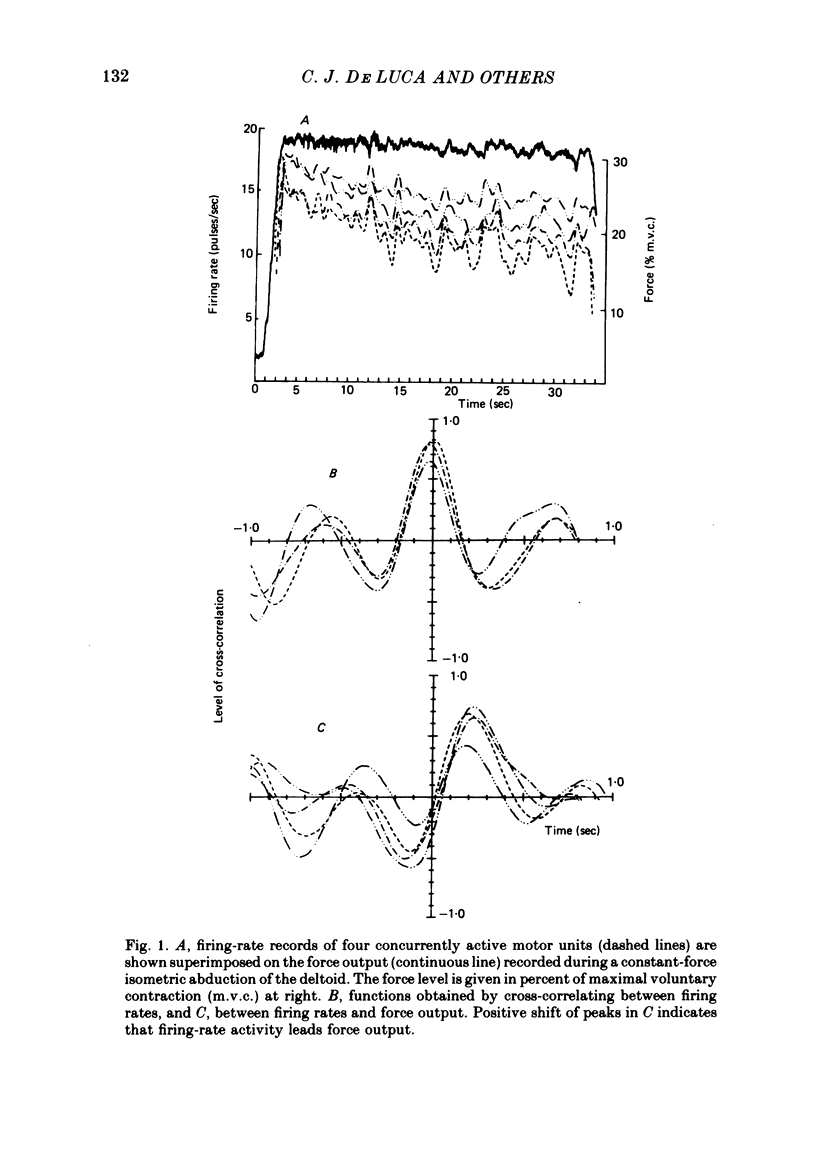
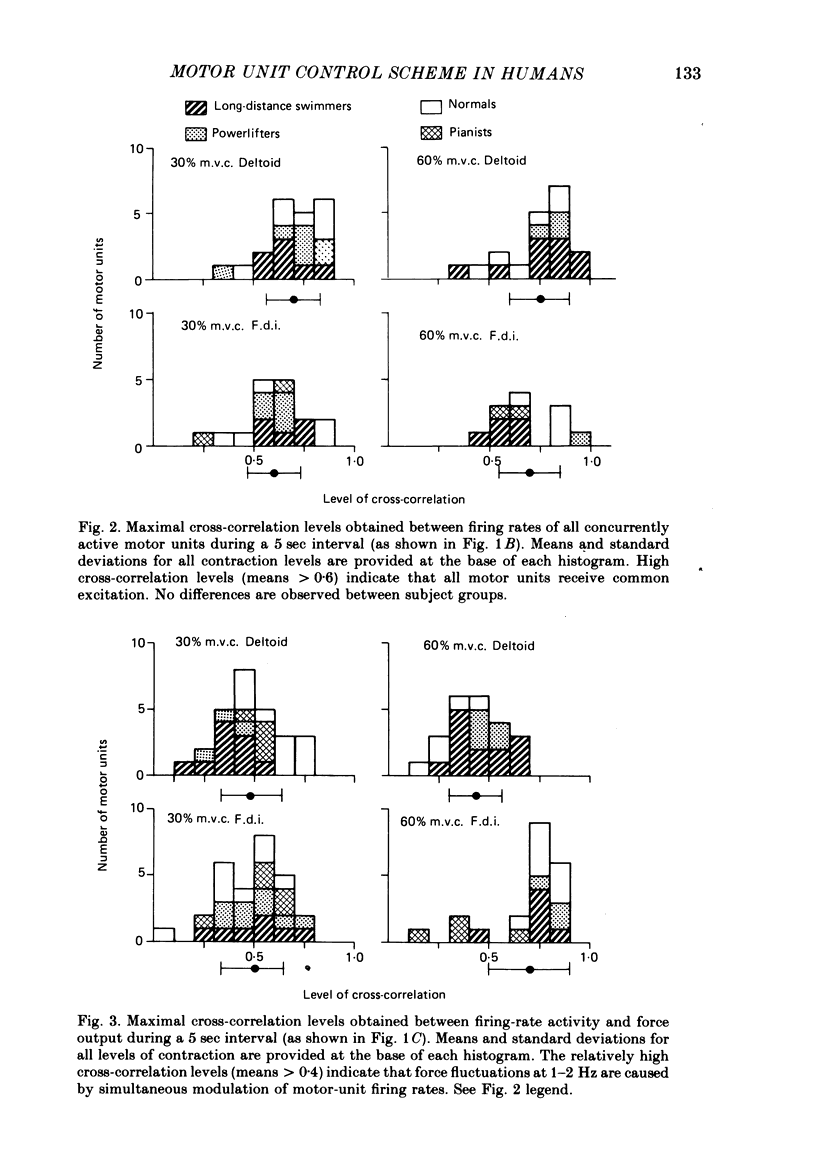
4. Results showed that both muscles are incapable of producing a purely isotonic contraction under isometric conditions. Small, possibly compensatory force variations at 1-2 Hz result from a common drive to all active motoneurones in a single muscle pool.
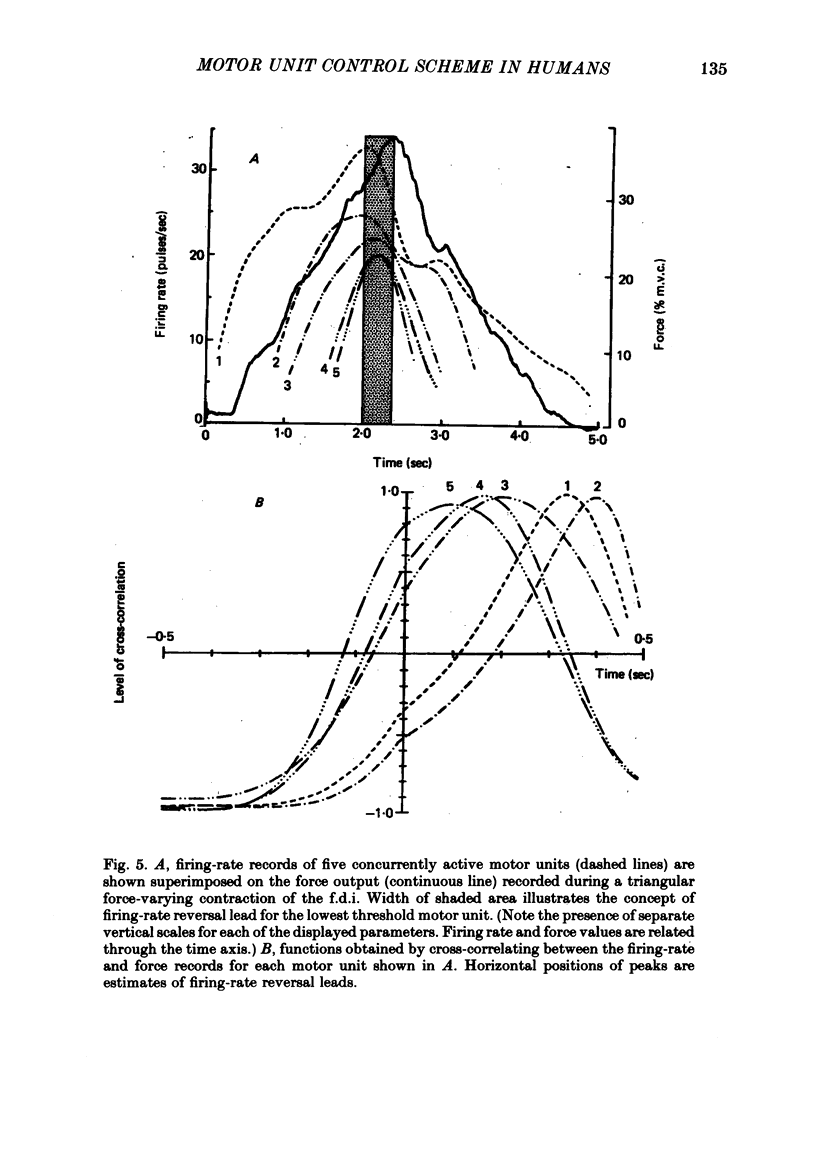
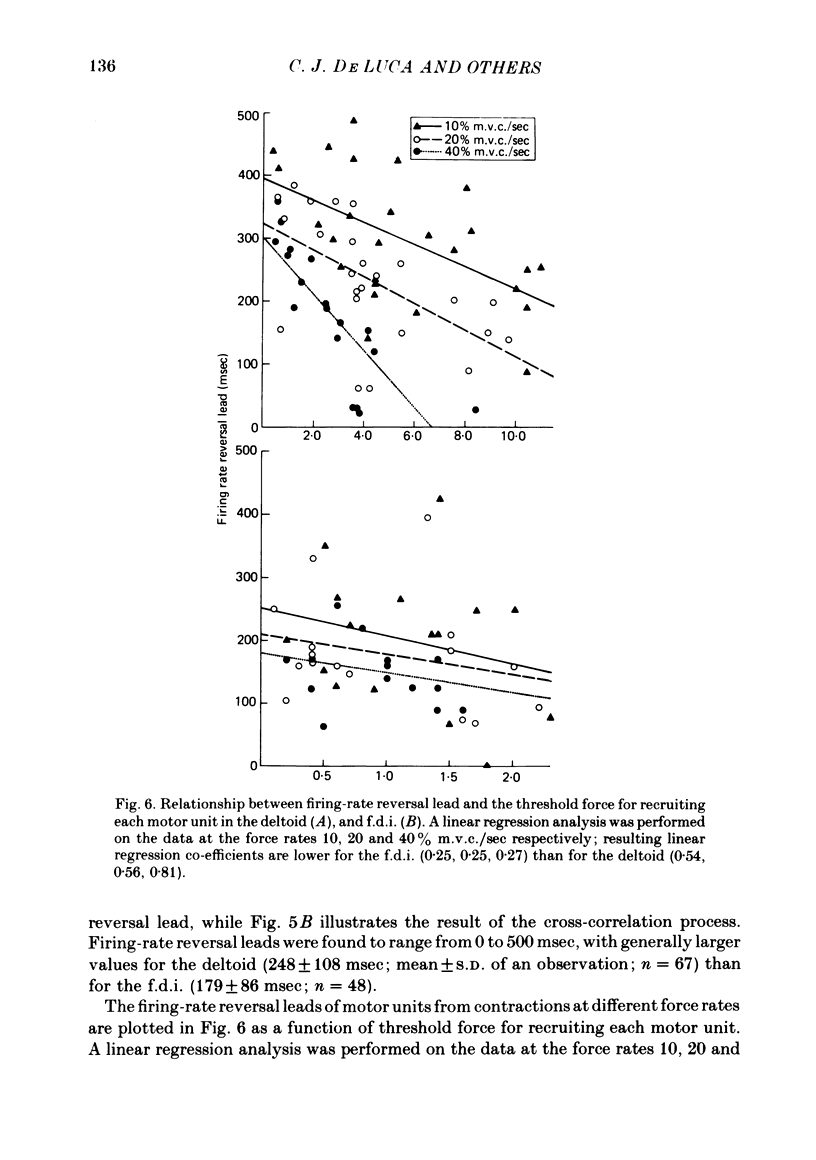
5. Rapid force reversals during triangular, force-varying isometric contractions appear to be accomplished through a size-related motor-unit control scheme. All firing rates decline prior to the force peak, but small motor units with slow-twitch responses tend to decrease their firing rates before large, fast-twitch motor units. This mechanism is not visually controlled, and does not depend on force rate in non-ballistic contractions.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bigland-Ritchie B. EMG and fatigue of human voluntary and stimulated contractions. Ciba Found Symp. 1981;82:130–156. doi: 10.1002/9780470715420.ch9. [DOI] [PubMed] [Google Scholar]
- Büdingen H. J., Freund H. J. The relationship between the rate of rise of isometric tension and motor unit recruitment in a human forearm muscle. Pflugers Arch. 1976 Mar 11;362(1):61–67. doi: 10.1007/BF00588682. [DOI] [PubMed] [Google Scholar]
- Clamann H. P., Gillies J. D., Henneman E. Effects of inhibitory inputs on critical firing level and rank order of motoneurons. J Neurophysiol. 1974 Nov;37(6):1350–1360. doi: 10.1152/jn.1974.37.6.1350. [DOI] [PubMed] [Google Scholar]
- De Luca C. J., Forrest W. J. Some properties of motor unit action potential trains recorded during constant force isometric contractions in man. Kybernetik. 1973 Mar;12(3):160–168. doi: 10.1007/BF00289169. [DOI] [PubMed] [Google Scholar]
- De Luca C. J., LeFever R. S., McCue M. P., Xenakis A. P. Behaviour of human motor units in different muscles during linearly varying contractions. J Physiol. 1982 Aug;329:113–128. doi: 10.1113/jphysiol.1982.sp014293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca C. J. Physiology and mathematics of myoelectric signals. IEEE Trans Biomed Eng. 1979 Jun;26(6):313–325. doi: 10.1109/tbme.1979.326534. [DOI] [PubMed] [Google Scholar]
- Edwards R. H., Hill D. K., Jones D. A. Metabolic changes associated with the slowing of relaxation in fatigued mouse muscle. J Physiol. 1975 Oct;251(2):287–301. doi: 10.1113/jphysiol.1975.sp011093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEINSTEIN B., LINDEGARD B., NYMAN E., WOHLFART G. Morphologic studies of motor units in normal human muscles. Acta Anat (Basel) 1955;23(2):127–142. doi: 10.1159/000140989. [DOI] [PubMed] [Google Scholar]
- Gollnick P. D., Armstrong R. B., Saltin B., Saubert C. W., 4th, Sembrowich W. L., Shepherd R. E. Effect of training on enzyme activity and fiber composition of human skeletal muscle. J Appl Physiol. 1973 Jan;34(1):107–111. doi: 10.1152/jappl.1973.34.1.107. [DOI] [PubMed] [Google Scholar]
- HENNEMAN E., SOMJEN G., CARPENTER D. O. FUNCTIONAL SIGNIFICANCE OF CELL SIZE IN SPINAL MOTONEURONS. J Neurophysiol. 1965 May;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Henneman E., Clamann H. P., Gillies J. D., Skinner R. D. Rank order of motoneurons within a pool: law of combination. J Neurophysiol. 1974 Nov;37(6):1338–1349. doi: 10.1152/jn.1974.37.6.1338. [DOI] [PubMed] [Google Scholar]
- Johnson M. A., Polgar J., Weightman D., Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973 Jan;18(1):111–129. doi: 10.1016/0022-510x(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Kosarov D., Gydikov A. Dependence of the discharge frequency of motor units in different human muscles upon the level of the isometric muscle tension. Electromyogr Clin Neurophysiol. 1976 Aug-Sep;16(4):293–306. [PubMed] [Google Scholar]
- LeFever R. S., De Luca C. J. A procedure for decomposing the myoelectric signal into its constituent action potentials--Part I: Technique, theory, and implementation. IEEE Trans Biomed Eng. 1982 Mar;29(3):149–157. doi: 10.1109/tbme.1982.324881. [DOI] [PubMed] [Google Scholar]
- LeFever R. S., Xenakis A. P., De Luca C. J. A procedure for decomposing the myoelectric signal into its constituent action potentials--Part II: Execution and test for accuracy. IEEE Trans Biomed Eng. 1982 Mar;29(3):158–164. doi: 10.1109/TBME.1982.324882. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Henneman E. How the size of motoneurones determines their susceptibility to discharge. Nature. 1979 Dec 20;282(5741):859–861. doi: 10.1038/282859a0. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: location, density, and distribution within a pool of 300 homonymous motoneurons. J Neurophysiol. 1971 Jan;34(1):171–187. doi: 10.1152/jn.1971.34.1.171. [DOI] [PubMed] [Google Scholar]
- Milner-Brown H. S., Stein R. B., Lee R. G. Synchronization of human motor units: possible roles of exercise and supraspinal reflexes. Electroencephalogr Clin Neurophysiol. 1975 Mar;38(3):245–254. doi: 10.1016/0013-4694(75)90245-x. [DOI] [PubMed] [Google Scholar]
- Milner-Brown H. S., Stein R. B., Yemm R. Changes in firing rate of human motor units during linearly changing voluntary contractions. J Physiol. 1973 Apr;230(2):371–390. doi: 10.1113/jphysiol.1973.sp010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner-Brown H. S., Stein R. B., Yemm R. The contractile properties of human motor units during voluntary isometric contractions. J Physiol. 1973 Jan;228(2):285–306. doi: 10.1113/jphysiol.1973.sp010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner-Brown H. S., Stein R. B., Yemm R. The orderly recruitment of human motor units during voluntary isometric contractions. J Physiol. 1973 Apr;230(2):359–370. doi: 10.1113/jphysiol.1973.sp010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monster A. W. Firing rate behavior of human motor units during isometric voluntary contraction: relation to unit size. Brain Res. 1979 Aug 3;171(2):349–354. doi: 10.1016/0006-8993(79)90341-x. [DOI] [PubMed] [Google Scholar]
- Olson C. B., Carpenter D. O., Henneman E. Orderly recruitment of muscle action potentials. Arch Neurol. 1968 Dec;19(6):591–597. doi: 10.1001/archneur.1968.00480060061008. [DOI] [PubMed] [Google Scholar]
- Person R. S., Kudina L. P. Discharge frequency and discharge pattern of human motor units during voluntary contraction of muscle. Electroencephalogr Clin Neurophysiol. 1972 May;32(5):471–483. doi: 10.1016/0013-4694(72)90058-2. [DOI] [PubMed] [Google Scholar]
- Stein R. B., French A. S., Mannard A., Yemm R. New methods for analysing motor function in man and animals. Brain Res. 1972 May 12;40(1):187–192. doi: 10.1016/0006-8993(72)90126-6. [DOI] [PubMed] [Google Scholar]


