Abstract
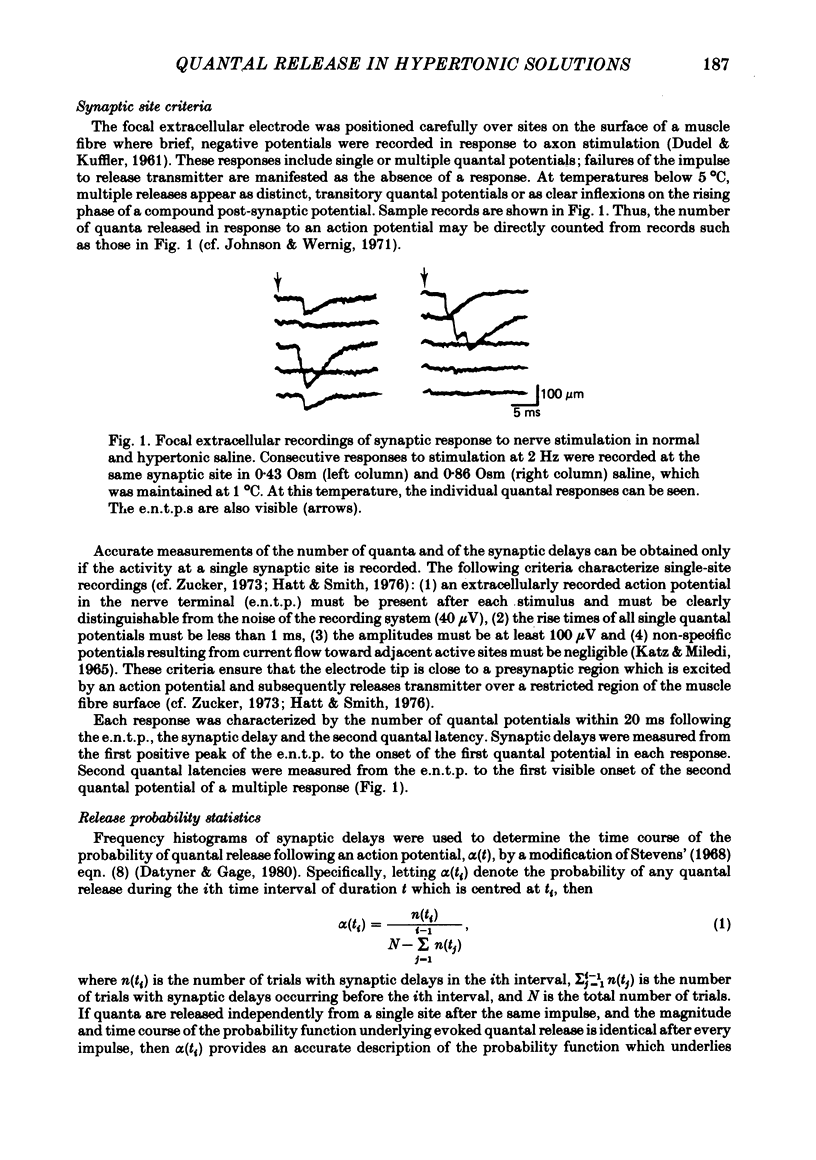
1. The effects of a hypertonic bathing medium, containing either NaCl or melezitose, on the average number (m) and the time course of quantal release following an action potential were studied using focal extracellular recording methods at synaptic sites on the opener muscle of the crayfish leg.
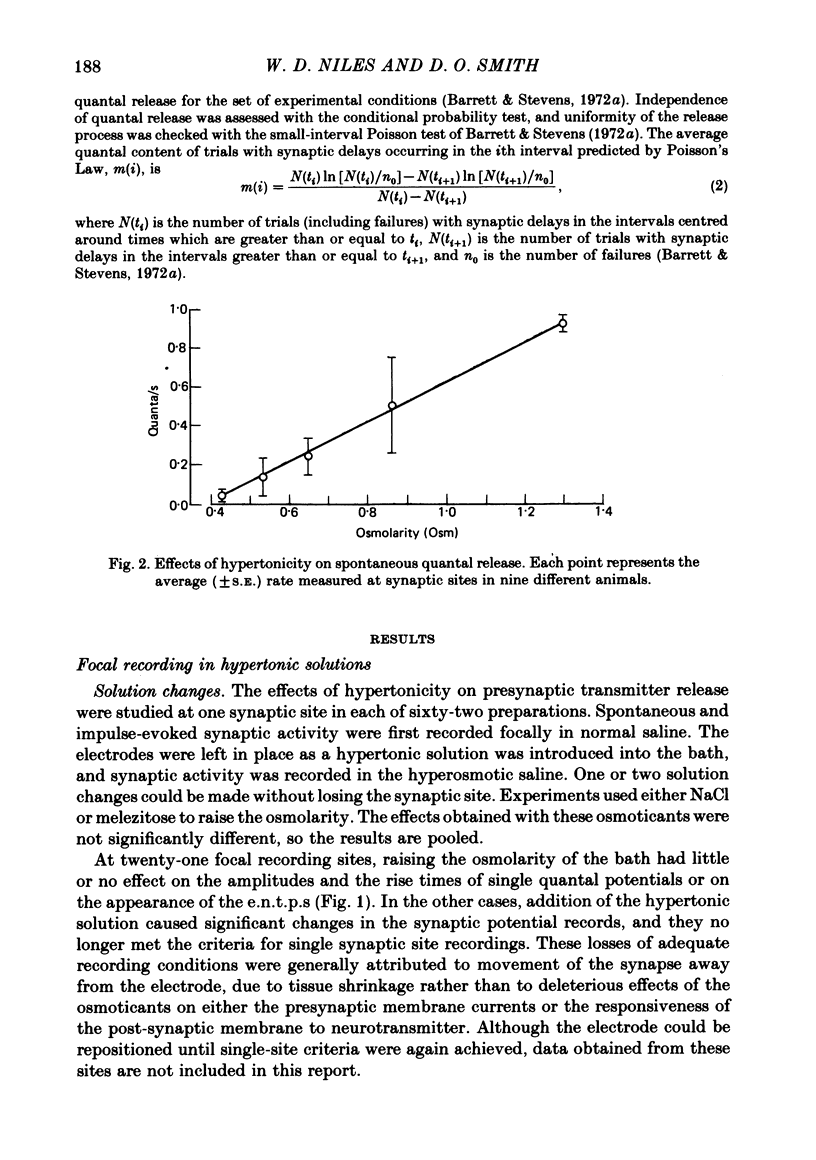
2. After the application of hypertonic saline, the rate of spontaneous quantal release increased but m decreased to a new level within 1 min; the extent of depression depended on the magnitude of the increase in tonicity until the osmolarity was 50% greater than the normal value of 0·43 osmol/l (Osm), but greater increases in tonicity exerted little further effect.
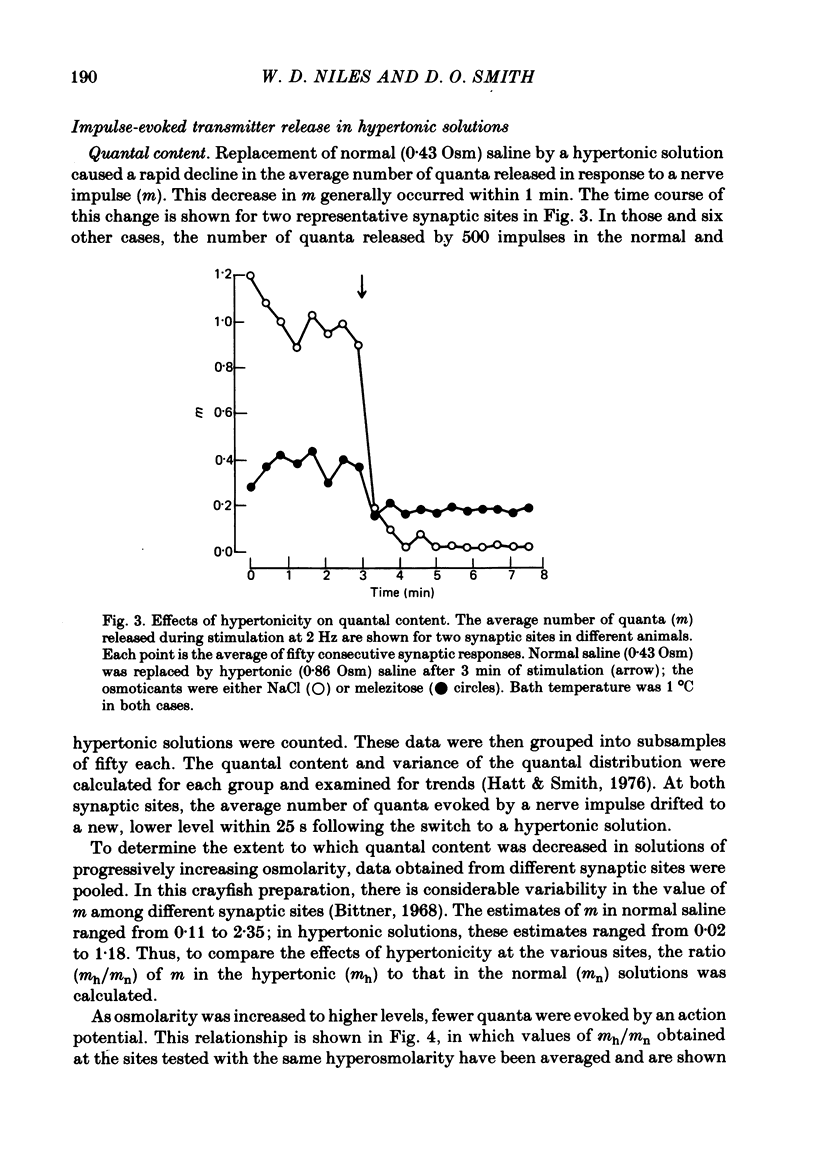
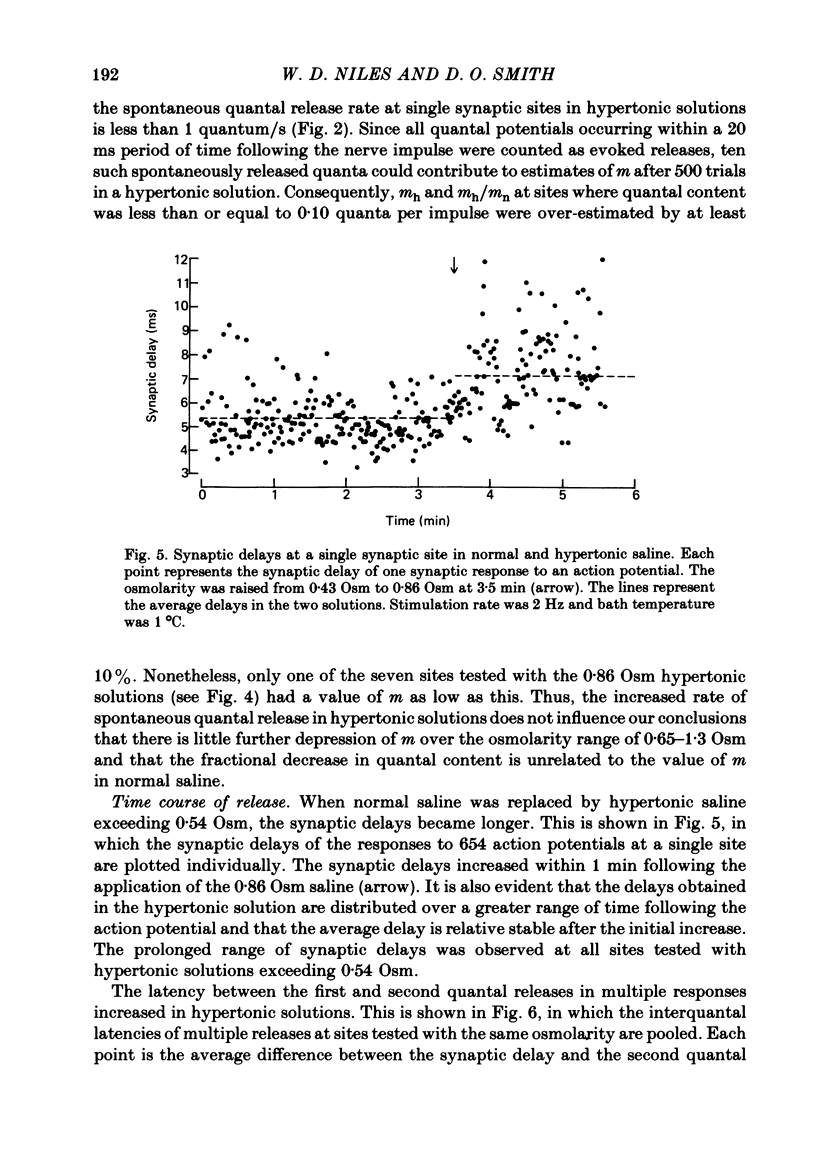
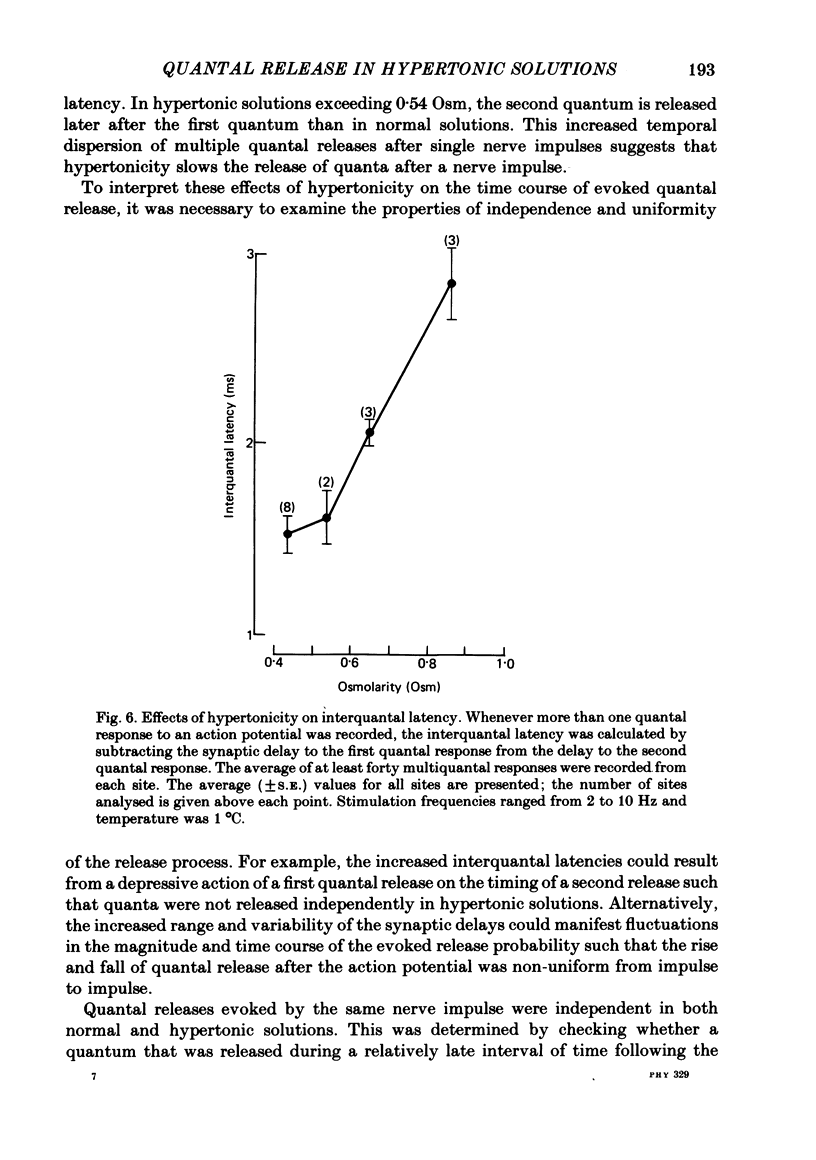
3. The synaptic delays were increased and distributed over a longer range of time in hypertonic solutions; also, the latency between the first and second quantal releases in a multiple response to a single nerve impulse was also increased.
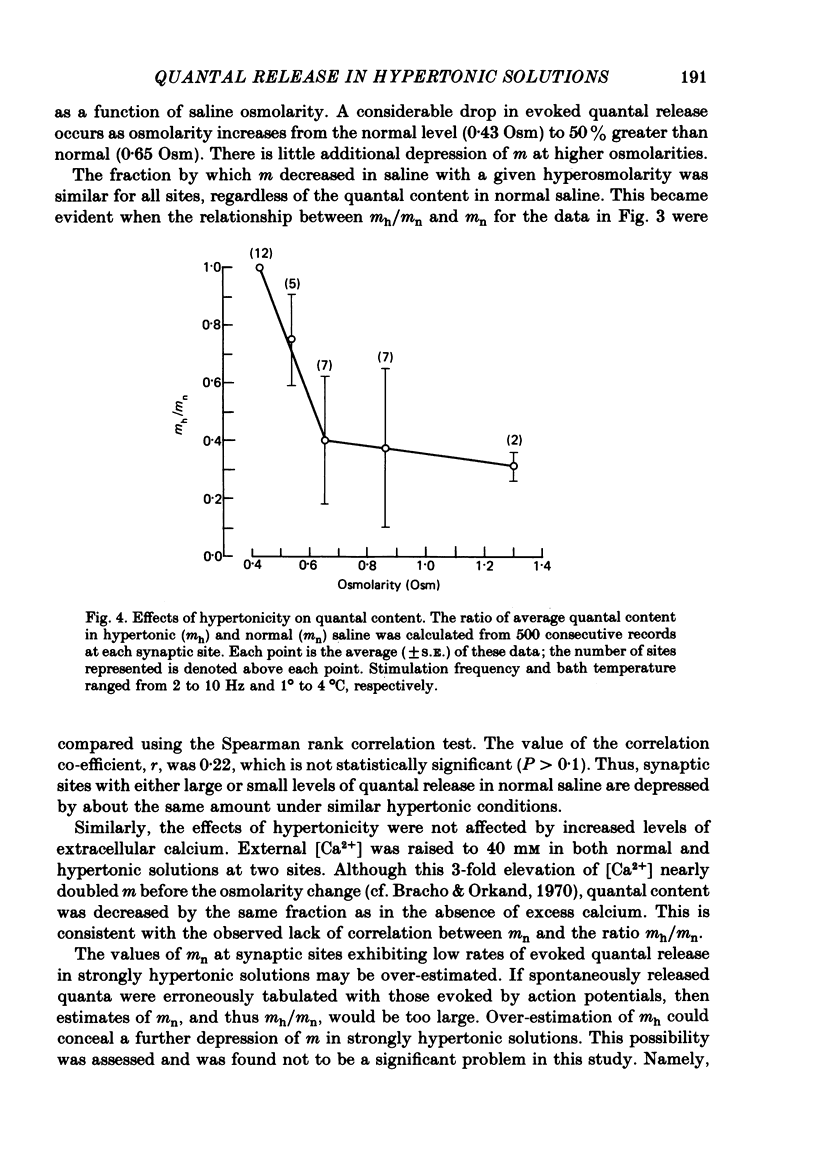
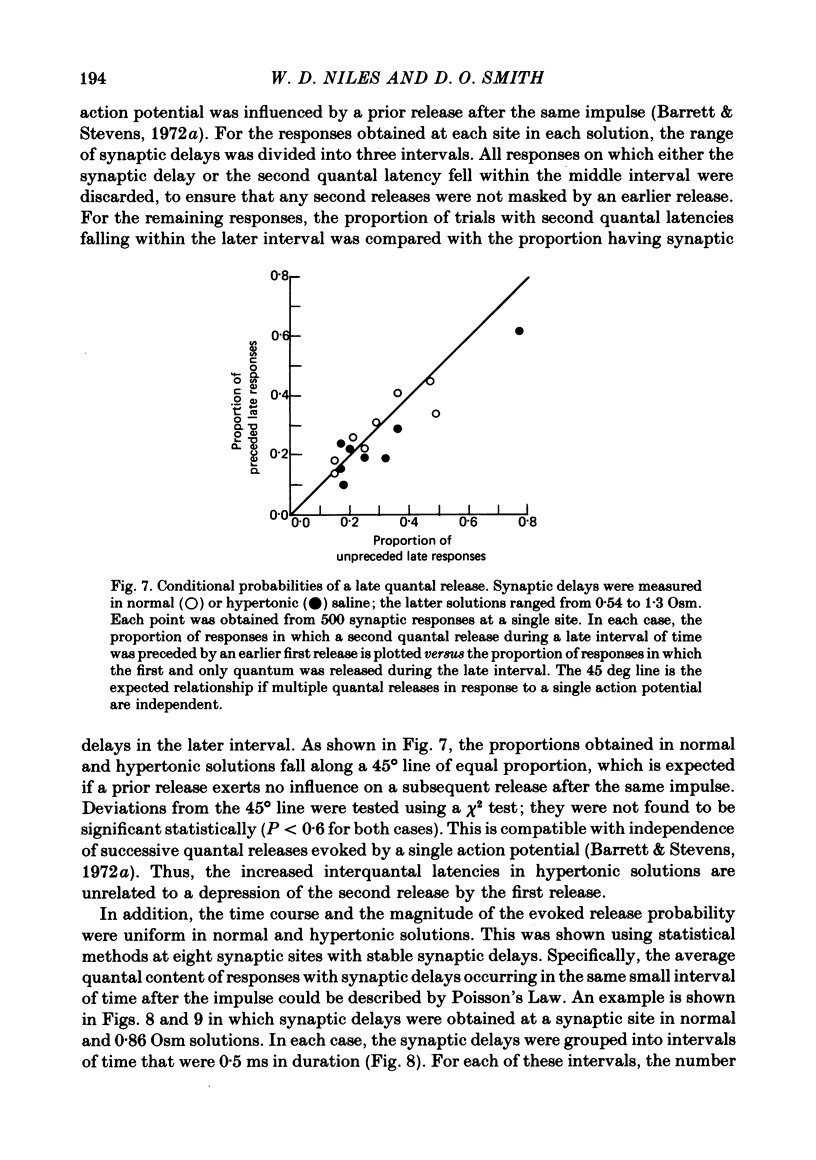
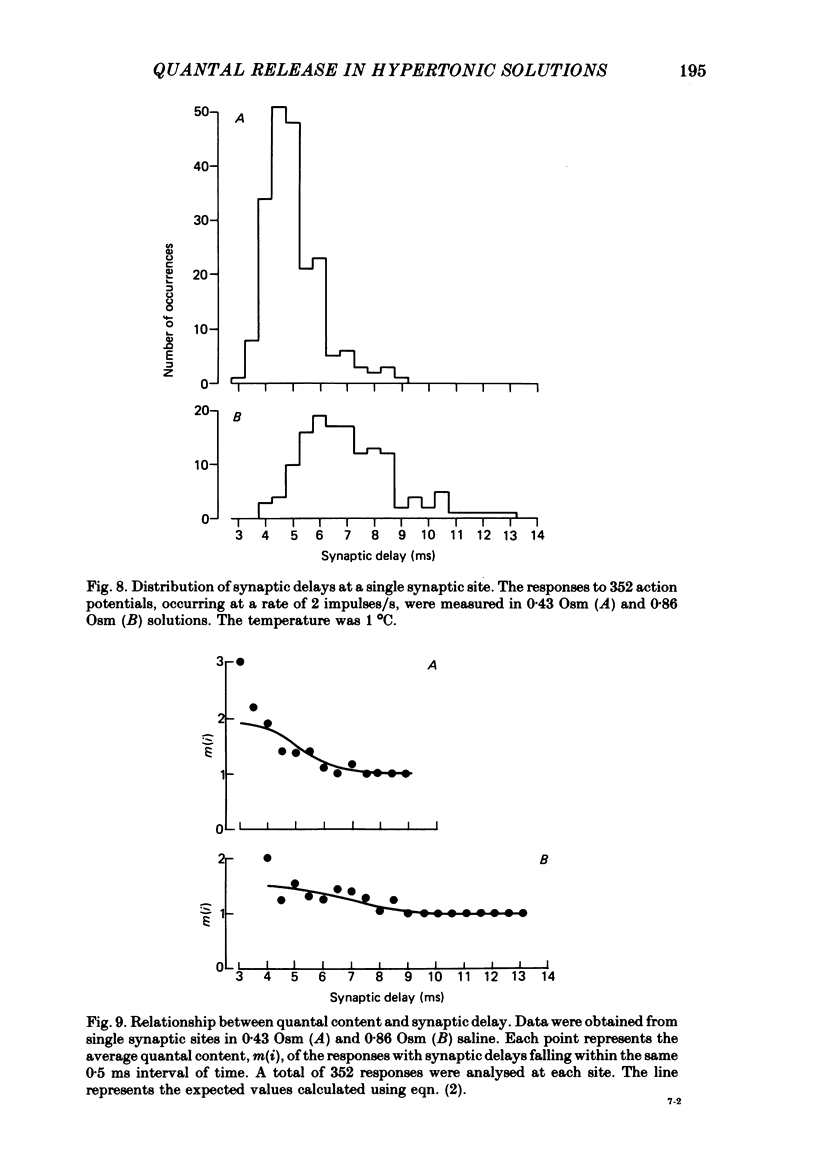
4. Hypertonicity had no significant effect on the conduction velocity of the action potential, the independence of successive quantal releases in the same response, or the uniformity of the rise and fall of the probability of quanta release following an action potential, α(t).
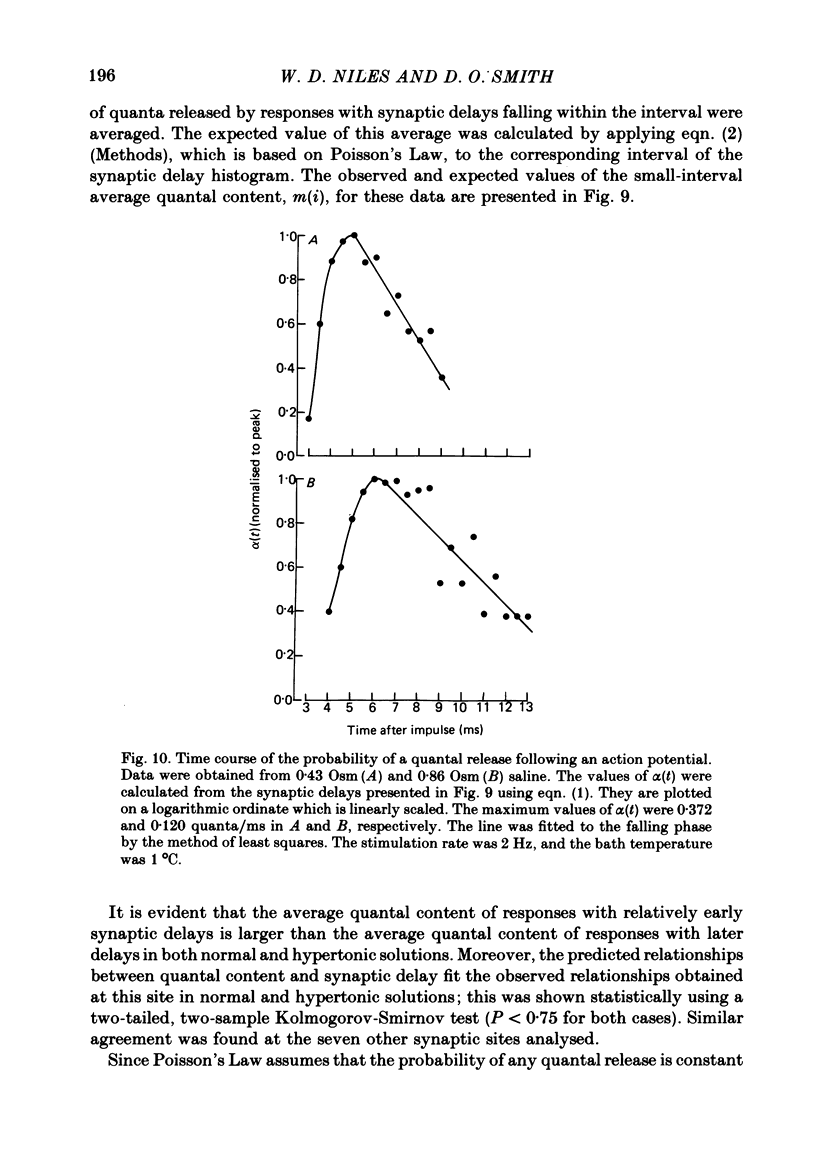
5. The time course of α(t) is prolonged in hypertonic solutions; this was manifested as an increase in the time constant of the exponential decline in α(t) from its peak value following the nerve impulse.
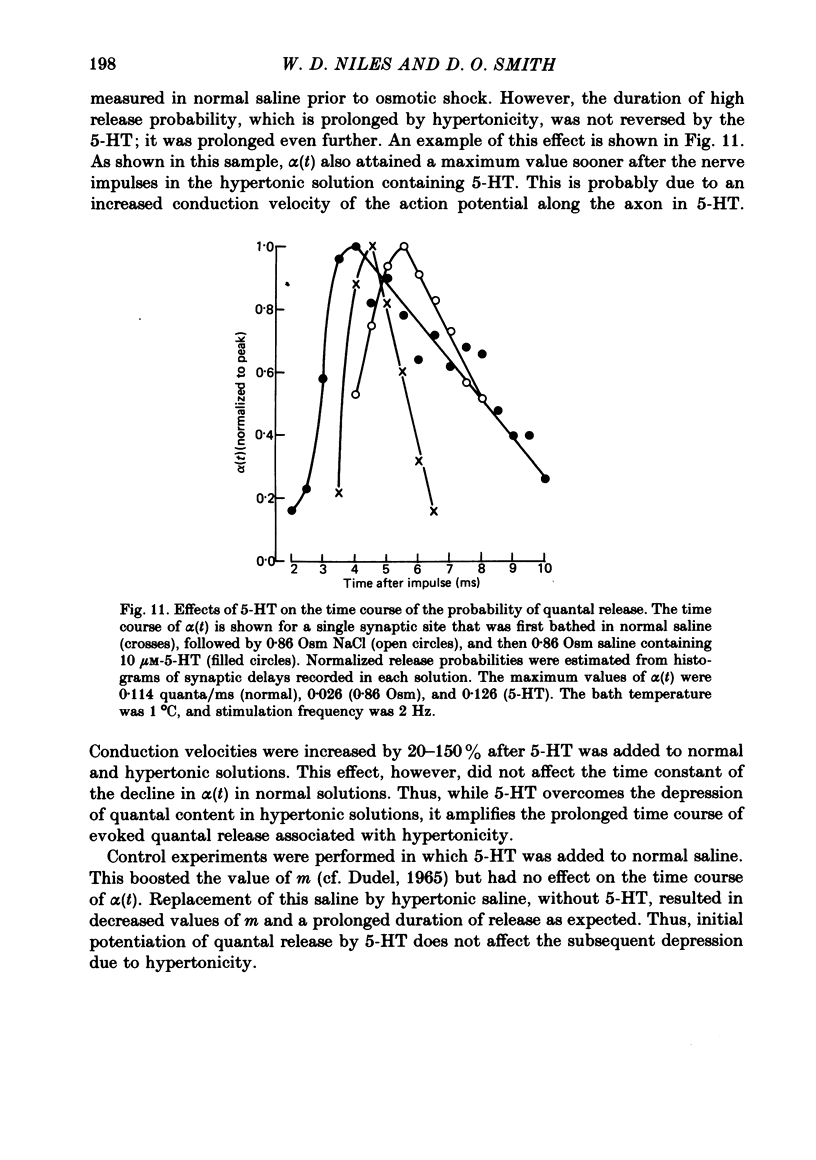
5. When the potentiating agent 5-hydroxytryptamine (5-HT) was added to the hypertonic saline, m was increased, and the time course of α(t) was prolonged further than in the hypertonic solution alone; 5-HT produced no change in the time course of α(t) when it was added to normal saline, although m was increased.
6. It is concluded that in this preparation hypertonicity decreases the rate of release of each quantum from the nerve terminal following a nerve impulse.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett E. F., Stevens C. F. Quantal independence and uniformity of presynaptic release kinetics at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):665–689. doi: 10.1113/jphysiol.1972.sp010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett E. F., Stevens C. F. The kinetics of transmitter release at the frog neuromuscular junction. J Physiol. 1972 Dec;227(3):691–708. doi: 10.1113/jphysiol.1972.sp010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass L., Moore W. J. Electrokinetic mechanism of miniature postsynaptic potentials. Proc Natl Acad Sci U S A. 1966 May;55(5):1214–1217. doi: 10.1073/pnas.55.5.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner G. D. Differentiation of nerve terminals in the crayfish opener muscle and its functional significance. J Gen Physiol. 1968 Jun;51(6):731–758. doi: 10.1085/jgp.51.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blioch Z. L., Glagoleva I. M., Liberman E. A., Nenashev V. A. A study of the mechanism of quantal transmitter release at a chemical synapse. J Physiol. 1968 Nov;199(1):11–35. doi: 10.1113/jphysiol.1968.sp008637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracho H., Orkand R. K. Effect of calcium on excitatory neuromuscular transmission in the crayfish. J Physiol. 1970 Jan;206(1):61–71. doi: 10.1113/jphysiol.1970.sp008997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Localization of active spots within the neuromuscular junction of the frog. J Physiol. 1956 Jun 28;132(3):630–649. doi: 10.1113/jphysiol.1956.sp005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDEL J. FACILITATORY EFFECTS OF 5-HYDROXY-TRYPTAMINE ON THE CRAYFISH NEUROMUSCULAR JUNCTION. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1965 Jan 8;249:515–528. doi: 10.1007/BF00246558. [DOI] [PubMed] [Google Scholar]
- DUDEL J., KUFFLER S. W. The quantal nature of transmission and spontaneous miniature potentials at the crayfish neuromuscular junction. J Physiol. 1961 Mar;155:514–529. doi: 10.1113/jphysiol.1961.sp006644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datyner N. B., Gage P. W. Phasic secretion of acetylcholine at a mammalian neuromuscular junction. J Physiol. 1980 Jun;303:299–314. doi: 10.1113/jphysiol.1980.sp013286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J Physiol. 1952 May;117(1):109–128. [PMC free article] [PubMed] [Google Scholar]
- FURSHPAN E. J. The effects of osmotic pressure changes on the spontaneous activity at motor nerve endings. J Physiol. 1956 Dec 28;134(3):689–697. doi: 10.1113/jphysiol.1956.sp005675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatt H., Smith D. O. Synaptic depression related to presynaptic axon conduction block. J Physiol. 1976 Jul;259(2):367–393. doi: 10.1113/jphysiol.1976.sp011471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard J. I., Jones S. F., Landau E. M. An examination of the effects of osmotic pressure changes upon transmitter release from mammalian motor nerve terminals. J Physiol. 1968 Aug;197(3):639–657. doi: 10.1113/jphysiol.1968.sp008579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahromi S. S., Atwood H. L. Three-dimensional ultrastructure of the crayfish neuromuscular apparatus. J Cell Biol. 1974 Nov;63(2 Pt 1):599–613. doi: 10.1083/jcb.63.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. W., Wernig A. The binomial nature of transmitter release at the crayfish neuromuscular junction. J Physiol. 1971 Nov;218(3):757–767. doi: 10.1113/jphysiol.1971.sp009642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. PROPAGATION OF ELECTRIC ACTIVITY IN MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:453–482. doi: 10.1098/rspb.1965.0015. [DOI] [PubMed] [Google Scholar]
- Kita H., van der Kloot W. Time course and magnitude of effects of changes in tonicity on acetylcholine release at frog neuromuscular junction. J Neurophysiol. 1977 Mar;40(2):212–224. doi: 10.1152/jn.1977.40.2.212. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. An investigation of spontaneous activity at the neuromuscular junction of the rat. J Physiol. 1956 Jun 28;132(3):650–666. doi: 10.1113/jphysiol.1956.sp005555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni Y., Alnaes E., Rahamimoff R. Is hyperosmotic neurosecretion from motor nerve endings a calcium-dependent process? Nature. 1977 May 12;267(5607):170–172. doi: 10.1038/267170a0. [DOI] [PubMed] [Google Scholar]
- Smith D. O. Mechanisms of action potential propagation failure at sites of axon branching in the crayfish. J Physiol. 1980 Apr;301:243–259. doi: 10.1113/jphysiol.1980.sp013202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. O. Ultrastructural specificity of synaptic sites in nerve terminals mediating both presynaptic and postsynaptic inhibition. J Comp Neurol. 1978 Dec 15;182(4 Pt 2):839–849. doi: 10.1002/cne.901820507. [DOI] [PubMed] [Google Scholar]
- THESLEFF S. Motor end-plate 'desensitization' by repetitive nerve stimuli. J Physiol. 1959 Oct;148:659–664. doi: 10.1113/jphysiol.1959.sp006314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILLEGAS R., VILLEGAS G. M. Characterization of the membranes in the giant nerve fiber of the squid. J Gen Physiol. 1960 May;43:73–103. doi: 10.1085/jgp.43.5.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Kloot W., Kita H. The possible role of fixed membrane surface charges in acetylcholine release at the frog neuromuscular junction. J Membr Biol. 1973;14(4):365–382. doi: 10.1007/BF01868085. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J., Cohen F. S., Finkelstein A. Micromolar Ca2+ stimulates fusion of lipid vesicles with planar bilayers containing a calcium-binding protein. Science. 1980 Nov 21;210(4472):906–908. doi: 10.1126/science.7434004. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Changes in the statistics of transmitter release during facilitation. J Physiol. 1973 Mar;229(3):787–810. doi: 10.1113/jphysiol.1973.sp010167. [DOI] [PMC free article] [PubMed] [Google Scholar]


