Abstract
Unstable expression of transferred genes is a major obstacle to successful gene therapy of hematopoietic diseases. We have investigated in a canine large-animal model whether expression of transduced genes can be recovered in vivo. Mixed-breed dogs had undergone autologous bone marrow transplantation (BMT) with stem cell factor and granulocyte–colony-stimulating factor-mobilized retrovirally marked hematopoietic cells. The bicistronic retroviral vector construct allowed for coexpression of MDR1 and human IL-2 receptor common γ-chain cDNAs. The latter gene is deficient in X-linked severe combined immunodeficiency. After initial high-level expression, P-glycoprotein and the γ-chain were undetectable in blood and bone marrow 17 months post-BMT. Six months later, one dog was treated i.v. with 125 mg/m2 paclitaxel. Three administrations restored expression of the two linked genes to high levels in blood and bone marrow. Two dogs treated with higher paclitaxel doses died from myelosuppression after the first administration. As determined by flow cytometry, both genes were expressed in granulocytes, monocytes, and lymphocytes of the surviving animal. PCR analysis of DNA from peripheral blood confirmed that the retroviral cDNA was increased after paclitaxel treatment, suggesting enrichment of transduced cells. P-glycoprotein was detectable for more than 1 year after cessation of paclitaxel. Repeated analyses of blood and bone marrow aspirates gave no indication of hematopoietic disturbance after BMT with transduced cells and paclitaxel treatment. In summary, we have shown that with the use of a drug-selectable marker gene, chemotherapy can select for cells that express an otherwise nonselected therapeutic gene in blood and bone marrow.
Although gene replacement therapy is thought to have the potential to cure hematopoietic disorders, this treatment approach is hampered by loss of expression of genes transferred into bone marrow cells either by silencing or dilution of expressing cells by nontransduced cells. In most, but not all, clinical trials of retrovirus-mediated transduction, expression of proteins encoded by transgenes tends to decrease after several weeks or months. Recent advances in vector development have allowed for high initial transduction rates. Efficient transduction of hematopoietic progenitor cells does not, however, ensure long-term expression of transgenes in bone marrow. Thus, it would be advantageous if gene expression could be restored in bone marrow of patients if the levels decrease.
It has been suggested that drug resistance genes such as the multidrug transporter (MDR1), also known as P-glycoprotein (P-gp), may be useful for augmentation of gene expression in vivo (1). P-gp is an energy-dependent efflux pump that extrudes cytotoxic compounds, including clinically used anticancer drugs, from cells. Among the various substrates of P-gp are daunorubicin, vincristine, etoposide, colchicine, paclitaxel (Taxol, Bristol-Myers Squibb), and other natural toxins and commonly used pharmaceuticals (2). Similarly, transfer of other drug resistance genes, including members of the multidrug resistance-associated protein gene family, the mitoxantrone resistance protein (MXR, BCRP, or ABCP) gene, or mutated dihydrofolate reductase genes, renders chemosensitive cells chemoresistant (3, 4).
Cells that express a drug resistance gene gain a selective advantage over nontransfected or nontransduced cells upon addition of anticancer or other appropriate drugs. In tissue culture, selection with anticancer drugs has conclusively been demonstrated (reviewed in ref. 1). Mice transplanted with MDR1-transduced hematopoietic cells express increased levels of the MDR1 transgene after treatment with cytotoxic substrates of the multidrug transporter, e.g., paclitaxel (5, 6). Moreover, after several rounds of transplantation with drug-treated bone marrow, recipient mice tolerate dose levels that were lethal to normal animals of the respective background strain (7). We have recently shown that hematopoietic progenitor cells treated ex vivo with high concentrations of colchicine or daunorubicin retained their ability to engraft lethally irradiated mice (8). Furthermore, recent clinical trials have suggested that enrichment of MDR1-transduced hematopoietic cells may be feasible in bone marrow of patients by selection with etoposide or paclitaxel (9, 10). Similarly, a retroviral vector that contained a mutated dihydrofolate reductase (DHFR) cDNA allowed for selective enrichment of transduced hematopoietic cells in murine bone marrow (11).
It has been suggested that this selective advantage conferred by overexpression of MDR1 can be exploited to ensure the expression of an accompanying but otherwise nonselectable transgene in target cells (1). To this end, bicistronic retroviral vectors were constructed in which the MDR1 gene is combined with wild-type genes defective in disorders of the hematopoietic system, e.g., Gaucher disease, X-linked severe combined immunodeficiency (XSCID), and chronic granulomatous disease (12–14). In these vectors, both MDR1 and the respective nonselectable gene are transcribed from a common promoter with the use of an internal ribosomal entry site (IRES) (15). As previously reported, a bicistronic vector facilitated high-level expression of the human common γ-chain (γc) of IL-2 receptor in target canine cells (16).
Human γc, the disease gene for XSCID, is part of several cytokine receptor complexes on the plasma membrane of leukocytes including IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 (17, 18). Allogeneic bone marrow transplantation (BMT) is life saving for infants with XSCID, who would otherwise die of infections (19). However, limitations of BMT combined with the selective advantage of lymphocytes expressing the normal γc over those with the mutated protein makes gene transfer therapy attractive for XSCID. Moreover, because of their immunologic incompetence, patients are unlikely to reject hematopoietic cells that express γc from a virally transduced transgene. Gene transfer of the normal γc gene to two XSCID infants has already demonstrated short-term clinical benefit (20). The long-term efficacy of this treatment remains to be evaluated.
To achieve stable γc expression in lymphocytes, gene transfer should ideally be targeted to hematopoietic stem cells. As opposed to more mature, lymphoid progenitor cells, hematopoietic stem cells have the capacity of unlimited self-renewal, maximal proliferative capacity, and differentiation into all hematopoietic lineages. In the case of gene therapy for XSCID, expression of the transgene in hematopoietic stem cells should not interfere with their function because in the murine system γc is expressed in hematopoietic cells of all lineages (21, 22).
Transfer of γc to hematopoietic stem cells has been investigated in a canine animal model (16). In this study, efficient expression was achieved in stem cell factor and granulocyte–colony-stimulating factor-mobilized hematopoietic cells from bone marrow without drug selection, but it disappeared after 19–34 weeks. The observation in this study that expression of transgenes in hematopoietic cells disappeared after a few months closely resembled previous data from several marking studies of immunocompetent animals and humans. Several mechanisms may contribute to inactivation of virally transferred genes, including methylation of promoters (23) or stem cell-specific silencer elements (24), or loss of positive cells may reflect the limited life span of transduced progenitor cell clones (25) or their elimination by host immune attack.
We have now investigated whether expression of γc can be recovered in vivo after being undetectable for a long period. Because the γc transgene was cotransduced in a bicistronic vector with the MDR1 gene, recipient dogs were treated with paclitaxel, a cytotoxic substrate of P-gp. Although paclitaxel was unexpectedly toxic to some dogs, we found that both MDR1 and linked γc expression could be augmented to very high levels by low-dose paclitaxel treatment. Although no further chemotherapy was administered, both genes were stably expressed in hematopoietic cells 1 year after paclitaxel treatment.
Materials and Methods
Transplantation of Animals.
A female mixed-breed dog was raised in the animal colony of the School of Veterinary Medicine, University of Pennsylvania, under National Institutes of Health and U.S. Department of Agriculture guidelines for the care and use of animals in research. The dog, designated M862, was 8 weeks old at the beginning of the previously described study (16) and 25 months old at the beginning of the current phase of the experiment. Harvest of bone marrow, transduction of bone marrow cells, and transplantation of transduced bone marrow have been described (16). Briefly, recombinant canine stem cell factor at 25 mg/kg per day and recombinant canine granulocyte colony-stimulating factor at 10 mg/kg per day (both from Amgen Biologicals) were administered s.c. for 4 consecutive days.
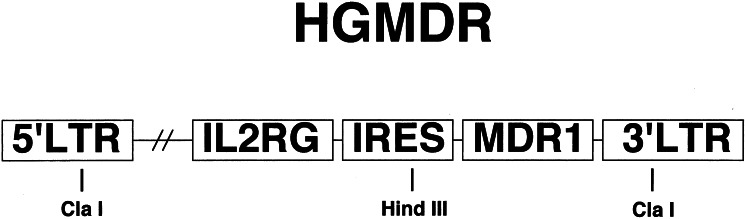
Ten days after the final treatment, bone marrow was aspirated from six sites in the pelvis and long bones, washed in PBS, and depleted of red cells. Bone marrow cells were transduced by means of a retroviral vector. Construction of HGMDR, the Harvey-sarcoma virus-based retroviral vector used for gene transfer, and production of amphotropic retroviruses by the packaging line GP+AM12 has been reported (14). This vector contained a full-length cDNA for the human IL-2 receptor common γc gene, followed by an IRES, and a full-length cDNA of the human MDR1 gene (Fig. 1). One day before graft infusion, the dog received a single sublethal dose of 200 cGy total body irradiation from parallel opposed portals in a 6-million electron-volt (MeV) linear accelerator. Two additional dogs were raised and transplanted under similar conditions as described (16).
Figure 1.
Retroviral vector in this study. HGMDR, Harvey murine sarcoma virus backbone; human IL2RG cDNA, IRES, human MDR1 cDNA. Selected restriction sites used for construction and analysis of HGMDR are indicated. Drawing is not to scale. LTR, long terminal repeat.
Treatment with Cytotoxic Drug.
The dog received 125 mg/m2 paclitaxel (Taxol; Bristol-Myers Squibb) i.v. at 25, 26, and 28 months of age. Beginning 5 days before each paclitaxel administration, the dog received 0.4–0.6 mg/kg dexamethasone s.c. twice a day and 2.9 mg/kg L-glutamine orally twice a day. On the day of the paclitaxel administration, the dog received i.v. injections of dexamethasone (2.2 mg/kg) and diphenhydramine (1.0–2.5 mg/kg) to control hypersensitivity reactions and was given acepromazine (0.5–1.0 mg/kg) and diazepam (0.25–0.5 mg/kg) i.v., as needed for sedation, during the infusion. Paclitaxel was diluted in 100 ml of 0.9% NaCl to a final concentration of 0.62–0.65 mg/ml and was delivered through the Primary IV Pump Set-OL (Abbott) with a 0.22-μm filter over 3–4 h.
The dog received prophylactic amoxicillin/clavulanic acid (12.5 mg/kg) and was maintained on L-glutamine for 7–10 days after paclitaxel administration. The dog was housed in a class-100 hepafiltered room from the time of paclitaxel administration until the white blood cell count normalized. Signs of myelosuppression and hepatotoxicity were monitored because two previous dogs from the same transplantation experiment (16) died after receiving 175 mg/m2 and 140 mg/m2 paclitaxel i.v., respectively (unpublished observation).
Blood from the dog was periodically submitted for PCR, FACS for expression of γc and MDR1, complete blood count, and chemistry analysis. Bone marrow aspirates were submitted for cytological analysis at 31 and 53 months of age (29 and 51 months after the initial autologous transplantation).
Detection of P-gp and γc in Canine Blood and Bone Marrow.
Blood and marrow samples were washed twice with PBS containing 5 mM EDTA and 0.5% BSA (PBS-E/B). Mononuclear bone marrow cells were isolated by density gradient centrifugation with Ficoll/Paque (Amersham Pharmacia), followed by additional washing with PBS-E/B.
For detection of human P-gp, 5 × 105 peripheral blood cells or 1 × 106 bone marrow cells were stained at 4°C with 5 μg of mAb MRK16, specific for the product of the human MDR1 gene. As a control, cells were incubated under identical conditions with an isotype-identical, nonbinding antibody (Becton Dickinson). After 20 min, cells were washed, followed by a 15-min incubation with a FITC-labeled rat mAb to murine IgG2a. Cells were then washed twice with PBS-E/B. FITC fluorescence was analyzed with a FACSCalibur cytometer by using CELLQUEST software (Becton Dickinson).
Expression of the human γc (CD132) was assessed with a phycoerythrin-conjugated mAb (PharMingen). After washing and density gradient centrifugation, cells were stained for 20 min with this mAb or a phycoerythrin-labeled isotype control antibody. Cells were then washed extensively with PBS-E/B to overcome nonspecific binding with the anti-CD132 antibody. Phycoerythrin fluorescence was analyzed by flow cytometry.
PCR Analysis of Canine Blood.
DNA was extracted from canine blood with the Puregene DNA isolation kit (Gentra Systems, Minneapolis) or the Qiagen (Chatsworth, CA) blood kit. PCR was performed with the AmpliTaq Gold DNA polymerase kit (Perkin–Elmer). A 145-bp fragment of the transferred bicistronic cDNA was amplified by PCR with primers 5′-CCCCATGTTACACCCTAAAGCCTGAA-3′ (located in the γc sequence) and 5′-AGGTTTCCGGGCCCTCACATTG-3′ (located in the IRES sequence). As a control for DNA concentration, a 134-bp sequence from the corticotrophin-releasing hormone was amplified with primers 5′-GGACGAGGCGCCGCAACTTTTT-3′ and 5′-CTCTCCTCTCCGGGGTCTCTT-3′. A trace amount of 32P-labeled dCTP (Amersham Pharmacia) was added to the reaction mix; 200 ng of DNA were amplified in a total volume of 50 μl. After an initial denaturation step at 95°C for 10 min, 35 cycles were run at 94°C for 1 min/57°C for 1 min/72°C for 2 min. PCR products were separated on a 6% acrylamide/Tris-borate-EDTA (TBE) gel by using an XCell II electrophoresis system (NOVEX, San Diego). Radiation of [32P]dCTP incorporated into the PCR products was visualized on BioMax film (Kodak).
Results and Discussion
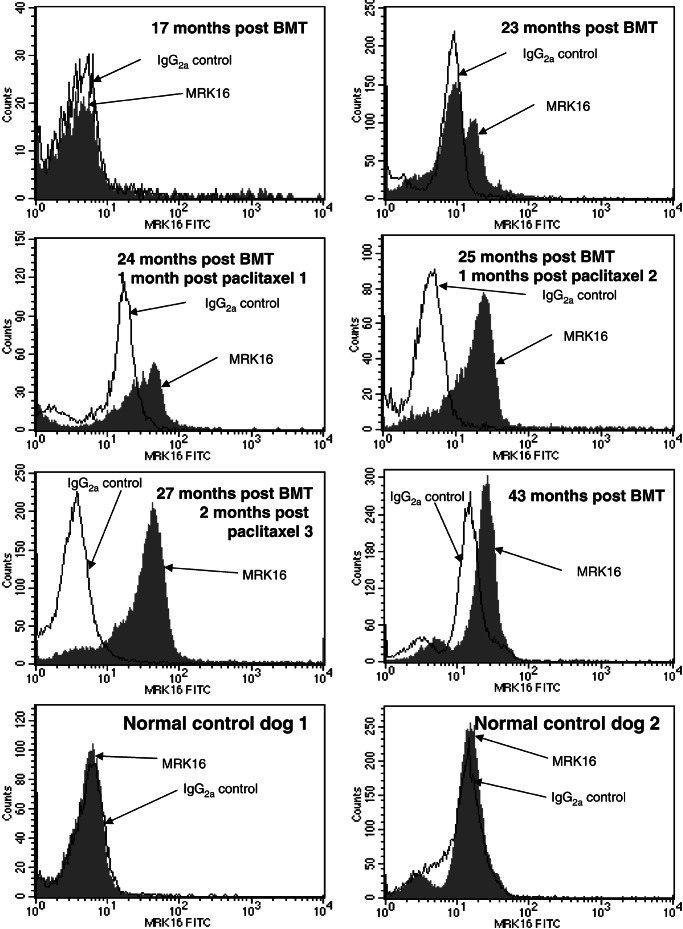
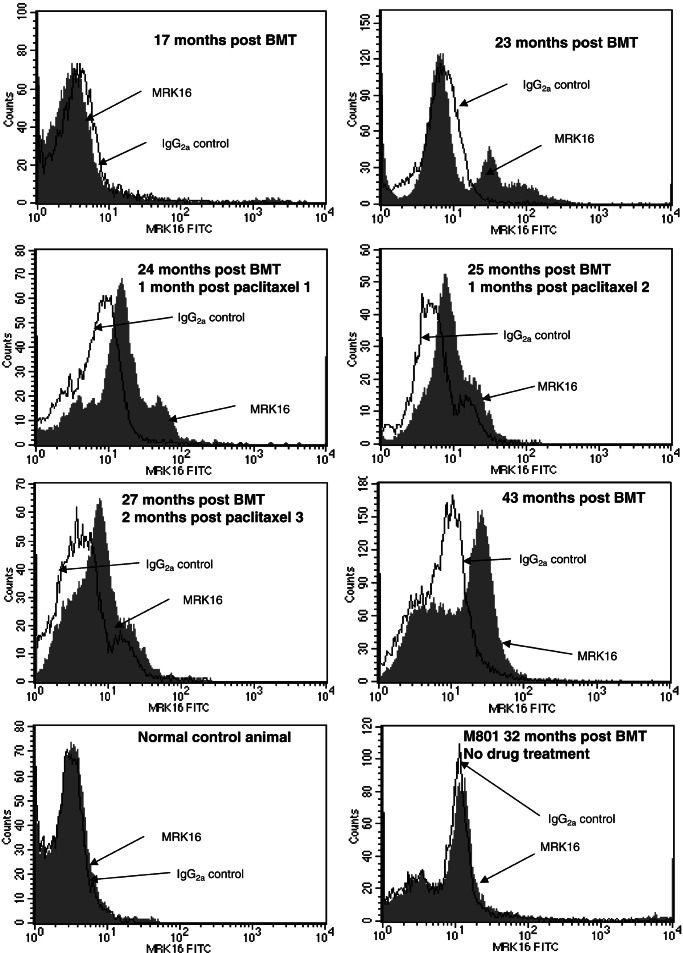
Recovery of transgene expression in hematopoietic cells after a long period of low or undetectable expression was analyzed in this canine large-animal model. As previously reported, transfer of a bicistronic vector containing both the human γc and the human MDR1 genes to bone marrow cells of normal dogs and transplantation with transduced marrow led to high-level expression of the γc protein in hematopoietic cells (16). When expression was lost after 19–34 weeks, immunosuppressive treatment transiently restored gene expression, but this expression subsequently disappeared. Seventeen months after BMT, P-gp was undetectable in peripheral blood (Fig. 2 Top Left) and bone marrow (Fig. 3 Top Left) of the dog in this study and two other recipient animals (not shown). Similarly, human γc (CD132) was undetectable in hematopoietic cells of recipient animals (not shown). The lack of expression of the transgenes could be a result of vector silencing or loss of transduced cells.
Figure 2.
Expression of P-gp in canine peripheral blood. For detection of P-gp, peripheral blood cells were stained with mAb MRK16 or a nonbinding isotype control, followed by staining with an FITC-labeled anti-mouse IgG antibody. Histograms display expression of P-gp in peripheral blood cells of animal M862 at indicated time points. As a negative control, blood cells from two normal, untreated dogs were analyzed (Bottom).
Figure 3.
Expression of P-gp in canine bone marrow. P-gp was analyzed by staining of mononuclear bone marrow cells with mAb MRK16 or a control antibody. Cells were then labeled with an FITC-labeled antibody to murine IgG, followed by flow cytometry. Shown is expression of P-gp in bone marrow at indicated time points. (Bottom Left) A normal control animal. (Bottom Right) Cells from a dog (M801) transplanted with the same retroviral vector are analyzed before chemotherapy with paclitaxel. This animal died after the first chemotherapy because of paclitaxel toxicity.
For drug selection, the dogs were then treated with paclitaxel. Two dogs received either 175 mg/m2 or 140 mg/m2 paclitaxel, dose levels well below the equivalent maximal tolerated dose in human patients. Unexpectedly, both animals died shortly after treatment. Pathologic analysis revealed myelosuppression. Whether or not normal dogs tolerate only lower doses of paclitaxel than humans requires further study. The hematopoiesis of the animals used in this study may have been unusually vulnerable because of prior BMT.
The third animal was treated successfully with 125 mg/m2 paclitaxel on three separate occasions. Varying degrees of hypersensitivity were noted during each paclitaxel infusion. Signs included vocalization, erythema, panting, and tachycardia; all were alleviated with either diphenhydramine or dexamethasone. Clinical signs on the days after paclitaxel infusion included mild loss of appetite and depression. Loose stools were noted occasionally, possibly because of mild mucositis. We also noted moderate hepatotoxicity with 3-fold elevations of serum alanine aminotransferase and 2-fold elevations of serum alkaline phosphatase. Mild to moderate hepatotoxicity has been described after administration of paclitaxel to patients (26).
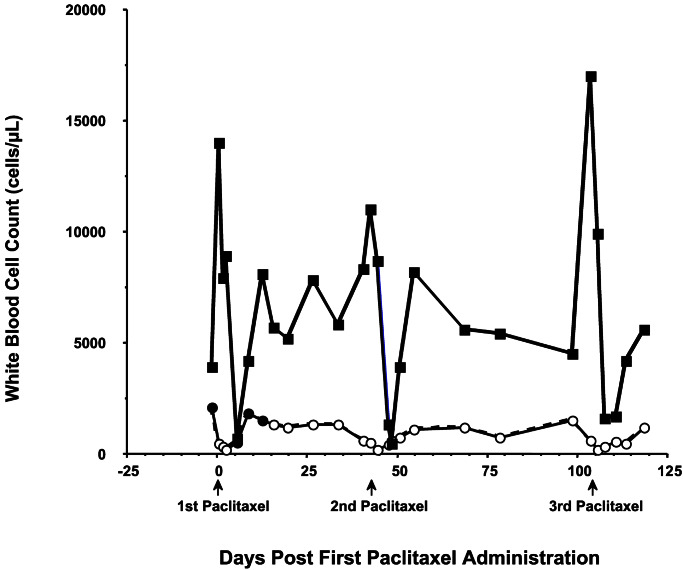
The white blood cell count of the surviving paclitaxel-treated dog fell below normal (6,700–18,000 cells per μl) between days 5 and 10 after each paclitaxel administration (Fig. 4). Myelosuppression was moderate, with the nadir average for the three paclitaxel administrations being 2,370 cells per μl. Accordingly, moderate neutropenia with an average neutrophil nadir of 930 cells per μl was observed (normal range 3,600 to 12,500 cells per μl). A brief increase of leukocyte and neutrophil counts before each paclitaxel administration was caused by pretreatment with dexamethasone. Conversely, the corticosteroid decreased lymphocyte counts below the normal range (1,000 to 4,800 cells per μl) on each of the days that paclitaxel was administered. Lymphocyte counts remained low until 5–10 days postpaclitaxel, with an average nadir of 190 cells per μl 2 days postpaclitaxel.
Figure 4.
Peripheral white blood counts during paclitaxel treatment. Neutrophil and lymphocyte counts were taken before and after three paclitaxel treatments (125 mg/m2). Normal canine neutrophil count is 3,600–12,500 cells per μl, and lymphocyte count is 1,000–4,800 cells per μl.
P-gp expression was increased in bone marrow after treatment with paclitaxel. After 2–3 treatment cycles, MRK16 fluorescence of the majority of the bone marrow cells shifted toward higher intensities (Fig. 3). The expression levels, however, seemed to be somewhat lower than in peripheral blood. P-gp was almost undetectable in erythrocytes as well as in erythroid progenitor cells in bone marrow, as revealed by immunocytochemical staining (data not shown). Furthermore, technical differences may also have contributed to the lower expression levels, e.g., depletion by density centrifugation of polynucleated granulocytes, which contained the highest amounts of P-gp (Fig. 5C), and different numbers of cells used for staining with mAbs.
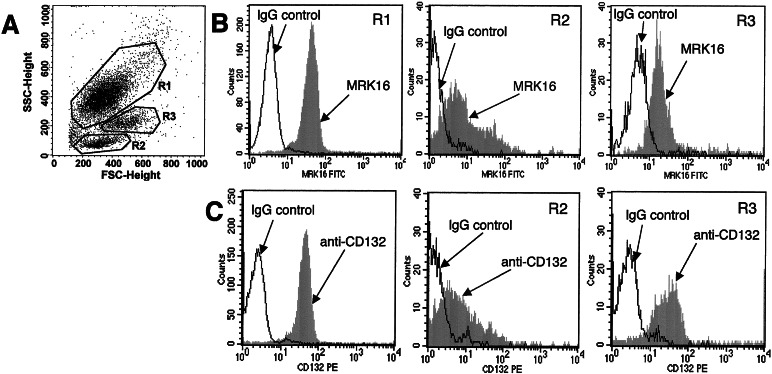
Figure 5.
Multilineage expression of P-gp. (A) Gating for granulocytes (R1), lymphocytes (R2), and monocytes (R3) in forward and side scatter plot. (B) Cells were stained for P-gp expression 28 months post-BMT. (C) Cells were stained for γc with a phycoerythrin-labeled anti-CD132 antibody 27 months post-BMT.
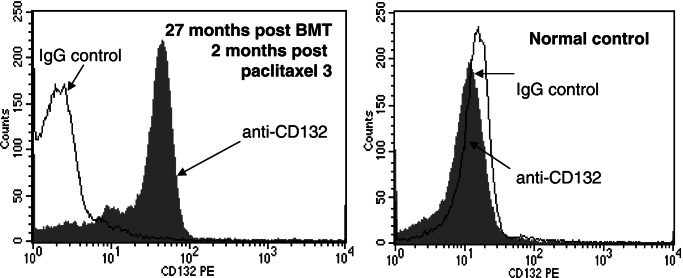
At the time of the first treatment (23 months post-BMT), a minority of cells in blood (Fig. 2) and bone marrow (Fig. 3) expressed P-gp. This was most likely related to clonal fluctuation. Shifts between individual cell clones in bone marrow after BMT are known from studies in mice (27, 28). Furthermore, calculations on stochastic differentiation in feline bone marrow have proven that clonal dominance may occur by chance after autologous transplantation (29). A number of P-gp-expressing cells in our dog were substantially increased by treatment with paclitaxel. Whereas the first treatment course resulted only in moderate increase in the percentage of cells expressing the human P-gp gene, almost the entire population of cells in peripheral blood expressed P-gp after the second and third treatment cycles (Fig. 2). Gating for leukocyte subpopulations demonstrated that human P-gp was expressed in granulocytes, lymphocytes, and monocytes of the dog over 2 years post-BMT (Fig. 5B). As shown in Fig. 6, expression of the human γc protein (CD132) was specific in the treated dog. No staining was seen in a control dog. Furthermore, human γc was also expressed in leukocytes of all hematopoietic lineages (Fig. 5C).
Figure 6.
Expression of γc in blood. (Left) Peripheral blood cells from dog M862 were analyzed after three courses of paclitaxel treatment. (Right) Cells from an untreated, normal dog were stained with a phycoerythrin-labeled mAb to CD132 or a phycoerythrin-labeled control antibody.
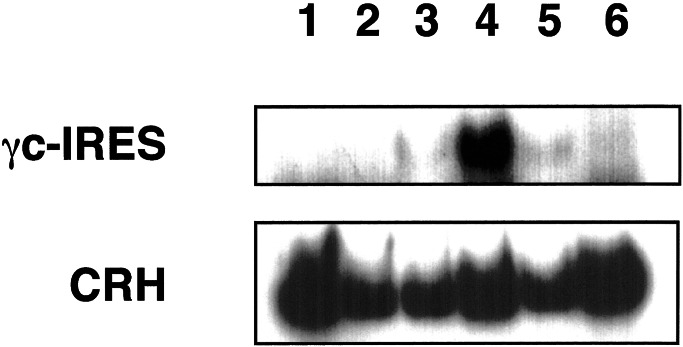
To confirm that the γc-IRES-MDR1 provirus was present in the dog after selection, a PCR analysis was performed. The primers used were highly specific for the transduced bicistronic γc-IRES-MDR1 sequence, with the sense primer starting in the γc portion and the antisense primer starting in the IRES portion of the cDNA. Although specific, the yield of amplification with this primer pair was, for reasons unknown to us, poor. We observed a strong signal after the third cycle of drug treatment, whereas the cDNA was not unequivocally detectable at earlier time points (Fig. 7). A smaller signal was detected more than 1 year after the end of drug treatment, confirming the presence of the transferred cDNA. The apparent increase in the levels of transgene cDNA suggests that higher expression after drug treatment was caused by clonal selection rather than transcriptional activation of the transgene.
Figure 7.
PCR detection of the transgene in peripheral blood of the dog. A fragment of the bicistronic γc-IRES-MDR1 cDNA was amplified from 200 ng of DNA with primers specific for the γc (sense primer) and IRES (antisense primer). As a control, a fragment from the canine corticotrophin-releasing hormone gene was amplified under identical conditions. Shown are analyses from blood at the following time points: 17 months post-BMT (lane 1), 25 months (lane 2), 27 months (lane 3), 28 months (lane 4), and 43 months post-BMT (lane 5). As a negative control, blood from a normal, untreated animal is shown in lane 6.
Expression of P-gp was stable for extended periods even in the absence of continuing drug selection. As displayed in Figs. 2 and 3, significant proportions of blood and bone marrow cells expressed P-gp at a late time point, 16 months after the end of paclitaxel treatment. Thus, drug selection exerted a lasting impact on transgene expression in hematopoietic cells. Although this study does not formally prove that hematopoietic stem cells were selected for, we conclude that drug selection occurred at the level of long-living progenitor cells of multiple lineages. Although complete T cell reconstitution was achieved in the recent XSCID clinical trial (20), fewer than 1% of B cells had provirus. A selectable marker such as MDR1 could potentially augment the natural selective advantage of γc in future XSCID trials. Moreover, for other diseases that lack a natural selective advantage of corrected cells (e.g., Gaucher, chronic granulomatous disease), the selectable bicistronic vector approach may offer high enough rates of expressing cells to achieve therapeutic benefit.
No major aberration of hematopoiesis has been observed in the recipient dog. A bone marrow aspirate obtained 29 months after transplantation with the retrovirally transduced bone marrow cells, and 6 months after the first dose of paclitaxel, revealed no significant abnormalities. A second bone marrow aspirate obtained 51 months after transplantation (28 months after the first dose of paclitaxel) revealed mild erythroid hypoplasia and plasmacytosis, which was of no clinical significance. Furthermore, no indication for the development of a myeloproliferative syndrome was observed in differential blood counts of peripheral blood (data not shown). MDR1-transduced and ex vivo expanded bone marrow cells caused a myeloproliferative syndrome in mice (30). This syndrome has not been observed, however, in mice treated with transduced bone marrow that was not expanded ex vivo (5, 6, 31), in either non-human primates (32) or in patients transplanted with MDR1-transduced hematopoietic cells (9, 10, 33–35).
This article demonstrates that the expression of a retrovirally introduced transgene for correction of a hematopoietic disorder can be recovered in a live animal with the use of a drug-selectable marker gene. Studies in tissue culture systems have shown that complete restoration of the function of an otherwise nonselectable gene was achievable with the use of bicistronic vectors (13, 36). In such vectors, the two transgenes could be arranged with the selectable marker gene being either in the 3′ or in the 5′ position with respect to the IRES (15). A high number of drug-resistant cell clones were obtained if MDR1 was located in the 5′ position. Conversely, if MDR1 was located in the 3′ position, the number of drug-resistant clones was lower because the gene at the 3′ position is expressed at generally lower rates. The advantage of the latter strategy is that all drug-resistant cells expressed a high level of the otherwise nonselectable, therapeutic gene cloned in the 5′ position.
The construction of vector HGMDR, in which MDR1 is located downstream of the IRES and γc is upstream, may therefore explain both the striking elevation of γc gene expression after selection and the low protection from the toxicity of paclitaxel at high doses. Hence, in this configuration major selective effects on the therapeutic or linked gene can be observed at relatively low doses of anticancer drugs. Side effects from the toxicity to other organ systems therefore may be comparably mild. Although our results demonstrate that rare cells expressing transgenes can be selected to high levels among hematopoietic cells in vivo, further studies are required to determine the optimal modalities of drug selection for improvement of gene replacement therapy.
Acknowledgments
We thank Margaret Weil and Patricia O'Donnell for technical support and care of the animals and Pat Miller-Wilson for animal irradiation. This work was supported by National Institutes of Health Grants AI33177 (to P.H.) and R01DK-32475, P40RR-02512, and T32RR-07063 (to M.H.).
Abbreviations
- P-gp
P-glycoprotein
- γc
common γ-chain
- IRES
internal ribosomal entry site
- BMT
bone marrow transplantation
- XSCID
X-linked severe combined immunodeficiency
Footnotes
Present address: Institute for the Study of Fertility, Serling Maternity Hospital, 16 Ein Dor Street, Tel Aviv 67441 Israel.
References
- 1.Gottesman M M, Hrycyna C A, Schoenlein P V, Germann U A, Pastan I. Annu Rev Genet. 1995;29:607–649. doi: 10.1146/annurev.ge.29.120195.003135. [DOI] [PubMed] [Google Scholar]
- 2.Ambudkar S V, Dey S, Hrycyna C A, Ramachandra M, Pastan I, Gottesman M M. Annu Rev Pharmacol Toxicol. 1999;39:361–398. doi: 10.1146/annurev.pharmtox.39.1.361. [DOI] [PubMed] [Google Scholar]
- 3.Banerjee D, Schweitzer B I, Volkenandt M, Li M X, Waltham M, Mineishi S, Zhao S C, Bertino J R. Gene. 1994;139:269–274. doi: 10.1016/0378-1119(94)90768-4. [DOI] [PubMed] [Google Scholar]
- 4.Machiels J P, Govaerts A S, Guillaume T, Bayat B, Feyens A M, Lenoir E, Goeminne J C, Cole S, Deeley R, Caruso M, et al. Hum Gene Ther. 1999;10:801–811. doi: 10.1089/10430349950018553. [DOI] [PubMed] [Google Scholar]
- 5.Sorrentino B P, Brandt S J, Bodine D, Gottesman M M, Pastan I, Cline A, Nienhuis A W. Science. 1992;257:99–103. doi: 10.1126/science.1352414. [DOI] [PubMed] [Google Scholar]
- 6.Podda S, Ward M, Himelstein A, Richardson C, de la Flor-Weiss E, Smith L, Gottesman M M, Pastan I, Bank A. Proc Natl Acad Sci USA. 1992;89:9676–9680. doi: 10.1073/pnas.89.20.9676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanania E G, Deisseroth A B. Cancer Gene Ther. 1994;1:21–25. [PubMed] [Google Scholar]
- 8.Licht T, Goldenberg S K, Vieira W D, Gottesman M M, Pastan I. Gene Ther. 2000;7:348–358. doi: 10.1038/sj.gt.3301087. [DOI] [PubMed] [Google Scholar]
- 9.Moscow J A, Huang H, Carter C, Hines K, Zujewski J, Cusack G, Chow C, Venzon D, Sorrentino B, Chiang Y, et al. Blood. 1999;94:52–61. [PubMed] [Google Scholar]
- 10.Abonour R, Williams D A, Einhorn L, Hall K M, Chen J, Coffman J, Traycoff C M, Bank A, Kato I, Ward M, et al. Nat Med. 2000;6:652–658. doi: 10.1038/76225. [DOI] [PubMed] [Google Scholar]
- 11.Allay J A, Persons D A, Galipeau J, Riberdy J M, Ashmun R A, Blakley R L, Sorrentino B P. Nat Med. 1998;4:1136–1143. doi: 10.1038/2632. [DOI] [PubMed] [Google Scholar]
- 12.Aran J M, Gottesman M M, Pastan I. Proc Natl Acad Sci USA. 1994;91:3176–3180. doi: 10.1073/pnas.91.8.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sokolic R A, Sekhsaria S, Sugimoto Y, Whiting-Theobald N, Linton G F, Li F, Gottesman M M, Malech H L. Blood. 1996;87:42–50. [PubMed] [Google Scholar]
- 14.Kleiman S E, Pastan I, Puck J M, Gottesman M M. Gene Ther. 1998;5:671–676. doi: 10.1038/sj.gt.3300651. [DOI] [PubMed] [Google Scholar]
- 15.Sugimoto Y, Aksentijevich I, Gottesman M M, Pastan I. Biotechnology. 1994;12:694–698. doi: 10.1038/nbt0794-694. [DOI] [PubMed] [Google Scholar]
- 16.Whitwam T, Haskins M E, Henthorn P S, Kraszewski J N, Kleiman S E, Seidel N E, Bodine D M, Puck J M. Blood. 1998;92:1565–1575. [PubMed] [Google Scholar]
- 17.Leonard W J. Annu Rev Med. 1996;47:229–239. doi: 10.1146/annurev.med.47.1.229. [DOI] [PubMed] [Google Scholar]
- 18.Asao H, Okuyama C, Kumaki S, Ishii N, Tsuchiya S, Foster D, Sugamura K. J Immunol. 2001;167:1–5. doi: 10.4049/jimmunol.167.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Buckley R H, Schiff R I, Schiff S E, Markert M L, Williams L W, Harville T O, Roberts J L, Puck J M. J Pediatr. 1997;130:378–387. doi: 10.1016/s0022-3476(97)70199-9. [DOI] [PubMed] [Google Scholar]
- 20.Cavazzanna-Calvo, M., Hacein-Bey, S., de Saint Basile, G., Gross, F., Yvon, E., Nusbaum, P., Selz, F., Hue, C., Certain, S., Casanova, J.-L., et al. (2000) 288, 669–672. [DOI] [PubMed]
- 21.Ishii N, Takeshita T, Kimura Y, Tada K, Kondo M, Nakamura M, Sugamura K. Int Immunol. 1994;6:1273–1277. doi: 10.1093/intimm/6.8.1273. [DOI] [PubMed] [Google Scholar]
- 22.Orlic D, Girard L J, Lee D, Anderson S M, Puck J M, Bodine D M. Exp Hematol. 1997;25:217–222. [PubMed] [Google Scholar]
- 23.Hoeben R C, Migchielsen A A, van der Jagt R C, van Ormondt H, van der Eb A J. J Virol. 1991;65:904–912. doi: 10.1128/jvi.65.2.904-912.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen R, Kempler G, Barklis E. Mol Cell Biol. 1991;11:1214–1221. doi: 10.1128/mcb.11.3.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capel B, Hawley R G, Mintz B. Blood. 1990;75:2267–2270. [PubMed] [Google Scholar]
- 26.Oettle H, Arnold D, Esser M, Huhn D, Riess H. Anticancer Drugs. 2000;11:635–638. doi: 10.1097/00001813-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Snodgrass R, Keller G. EMBO J. 1987;6:3955–3960. doi: 10.1002/j.1460-2075.1987.tb02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capel B, Hawley R, Covarrubias L, Hawley T, Mintz B. Proc Natl Acad Sci USA. 1989;86:4564–4568. doi: 10.1073/pnas.86.12.4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abkowitz J L, Catlin S N, Guttorp P. Nat Med. 1996;2:190–197. doi: 10.1038/nm0296-190. [DOI] [PubMed] [Google Scholar]
- 30.Bunting K D, Galipeau J, Topham D, Benaim E, Sorrentino B P. Blood. 1998;92:2269–2279. [PubMed] [Google Scholar]
- 31.Bodine D M, Seidel N E, Gale M S, Nienhuis A W, Orlic D. Blood. 1997;84:1482–1491. [PubMed] [Google Scholar]
- 32.Sellers S E, Tisdale J F, Agricola B A, Metzger M E, Donahue R E, Dunbar C E, Sorrentino B P. Blood. 2001;97:1888–1891. doi: 10.1182/blood.v97.6.1888. [DOI] [PubMed] [Google Scholar]
- 33.Hanania E G, Giles R E, Kavanagh J, Fu S Q, Ellerson D, Zu Z, Wang T, Su Y, Kudelka A, Rahman Z, et al. Proc Natl Acad Sci USA. 1996;93:15346–15351. doi: 10.1073/pnas.93.26.15346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hesdorffer C, Ayello J, Ward M, Kaubisch A, Vahdat L, Balmaceda C, Garrett T, Fetell M, Reiss R, Bank A, Antman K. J Clin Oncol. 1998;16:165–172. doi: 10.1200/JCO.1998.16.1.165. [DOI] [PubMed] [Google Scholar]
- 35.Cowan K H, Moscow J A, Huang H, Zujewski J, O'Shaughnessy J, Sorrentino B, Hines K, Carter C, Schneider E, Cusack G, et al. Clin Cancer Res. 1999;5:1619–1628. [PubMed] [Google Scholar]
- 36.Aran J M, Licht T, Gottesman M M, Pastan I. Hum Gene Ther. 1996;7:2165–2175. doi: 10.1089/hum.1996.7.17-2165. [DOI] [PubMed] [Google Scholar]