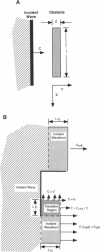
Abstract
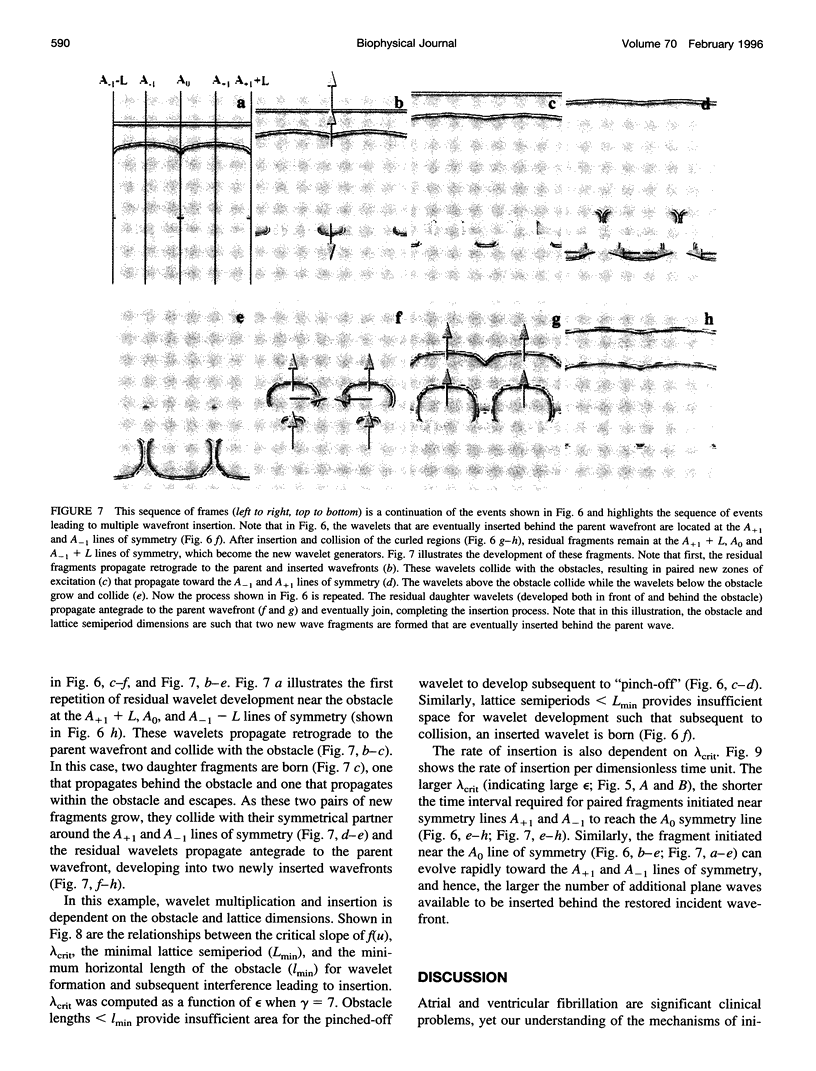
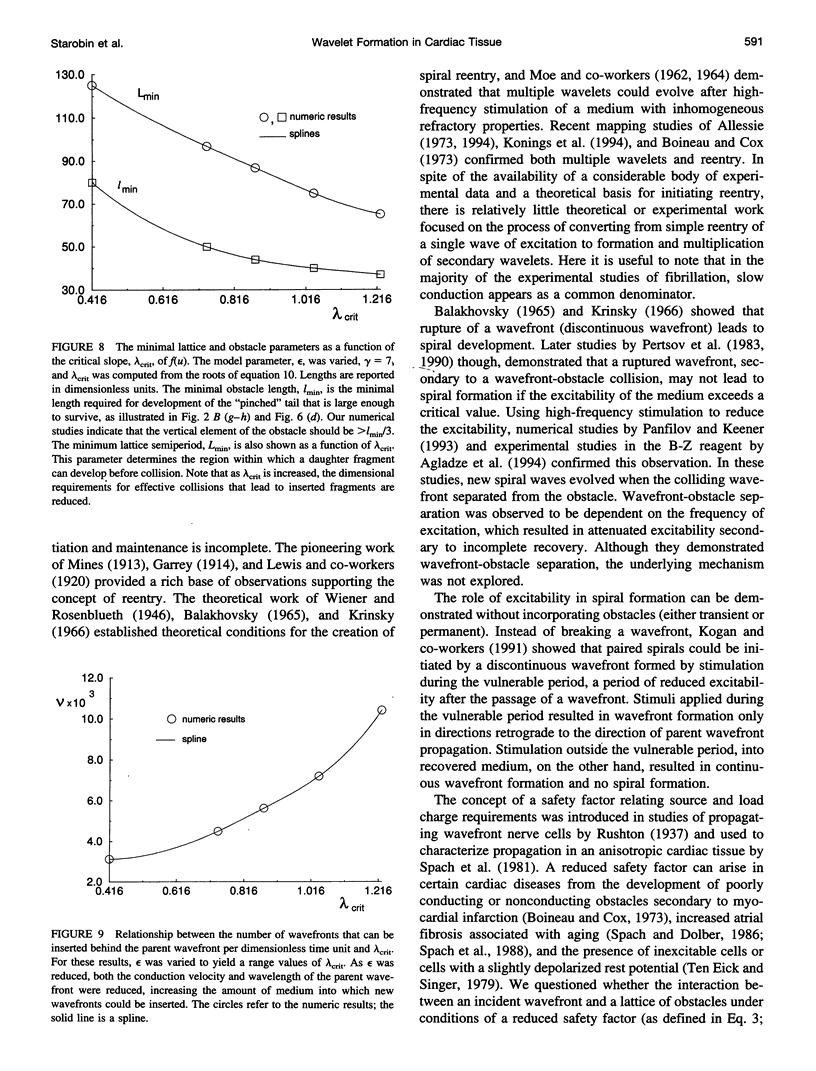
High-frequency arrhythmias leading to fibrillation are often associated with the presence of inhomogeneities (obstacles) in cardiac tissue and reduced excitability of cardiac cells. Studies of antiarrhythmic drugs in patients surviving myocardial infarction revealed an increased rate of sudden cardiac death compared with untreated patients. These drugs block the cardiac sodium channel, thereby reducing excitability, which may alter wavefront-obstacle interactions. In diseased atrial tissue, excitability is reduced by diminished sodium channel availability secondary to depolarized rest potentials and cellular decoupling secondary to intercellular fibrosis. Excitability can also be reduced by incomplete recovery between successive excitations. In all of these cases, wavefront-obstacle interactions in a poorly excitable medium may reflect an arrhythmogenic process that permits formation of reentrant wavelets leading to flutter, fibrillation, and sudden cardiac death. To probe the relationship between excitability and arrhythmogenesis, we explored conditions for new wavelet formation after collision of a plane wave with an obstacle in an otherwise homogeneous excitable medium. Formulating our approach in terms of the balance between charge available in the wavefront and the excitation charge requirements of adjacent medium, we found analytically the critical medium parameters that defined conditions for wavefront-obstacle separation. Under these conditions, when a parent wavefront collided with a primitive obstacle, the resultant fragments separated from the obstacle boundaries, subsequently curled, and spawned new "daughter" wavelets. We identified spatial arrangements of obstacles such that wavefront-obstacle collisions leading to spawning of new wavelets could produce high-frequency wavelet trains similar to fibrillation-like arrhythmias.
Full text
PDF

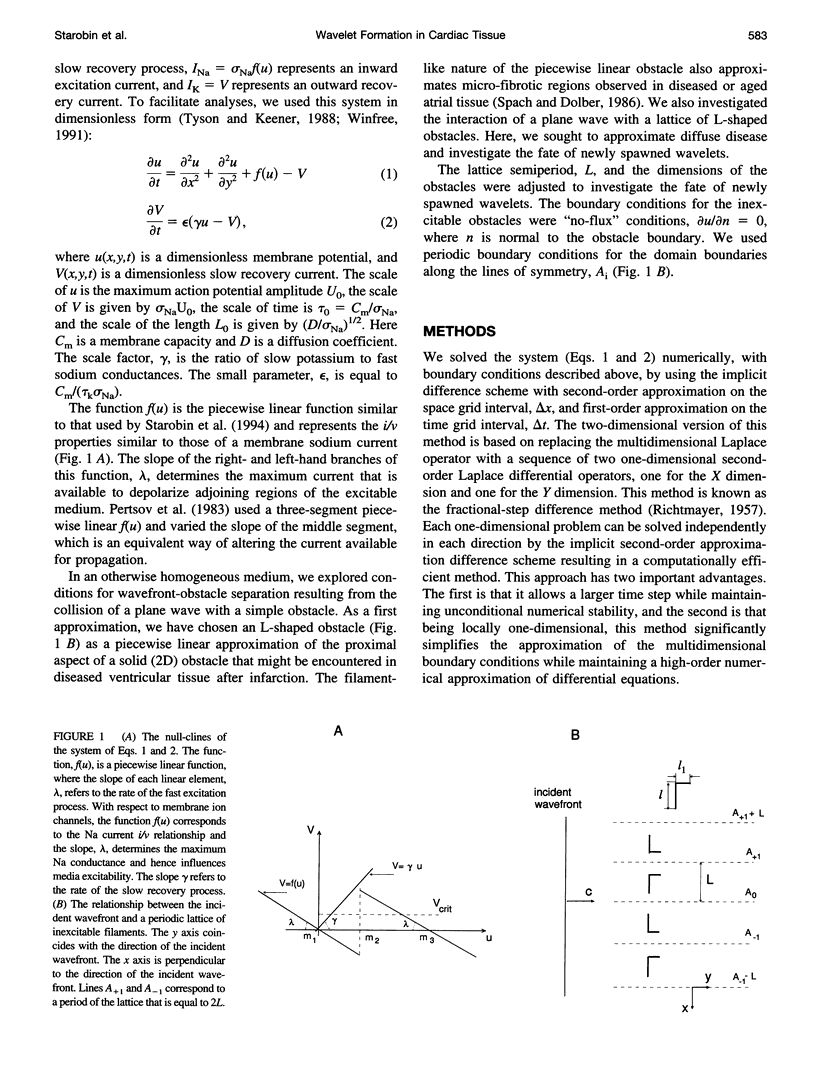

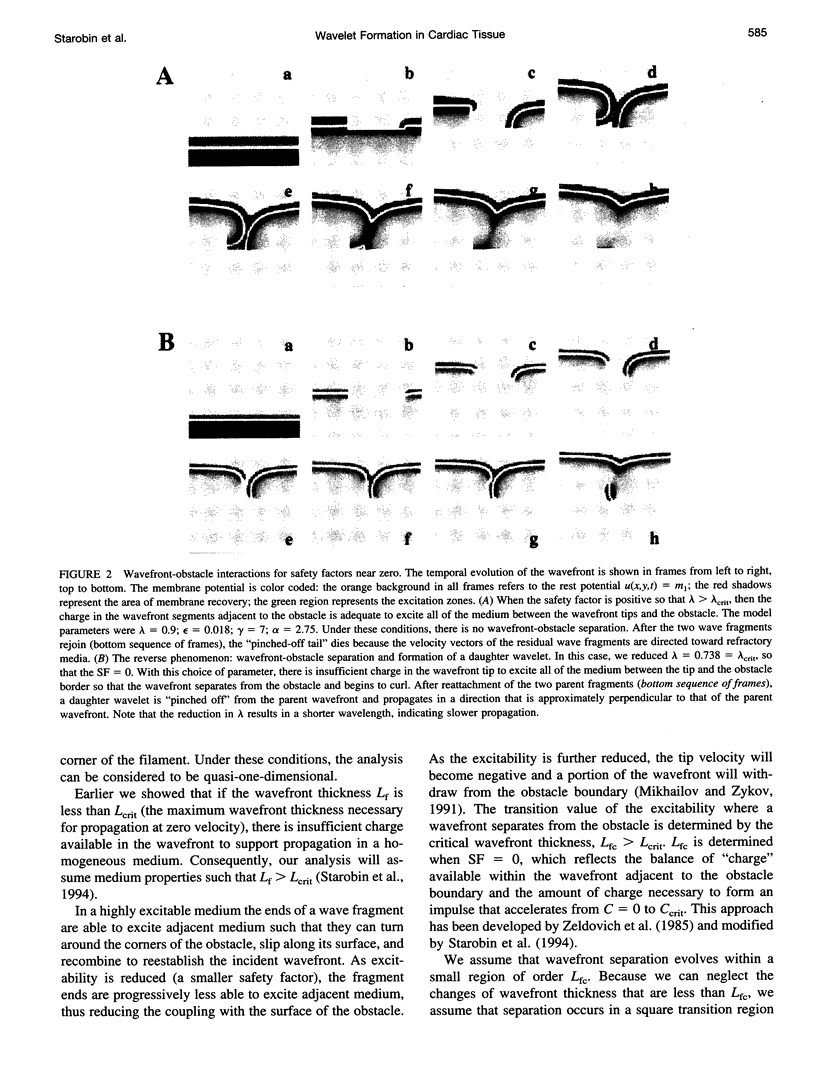



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agladze K., Keener J. P., Müller S. C., Panfilov A. Rotating spiral waves created by geometry. Science. 1994 Jun 17;264(5166):1746–1748. doi: 10.1126/science.264.5166.1746. [DOI] [PubMed] [Google Scholar]
- Allessie M. A., Bonke F. I., Schopman F. J. Circus movement in rabbit atrial muscle as a mechanism of tachycardia. III. The "leading circle" concept: a new model of circus movement in cardiac tissue without the involvement of an anatomical obstacle. Circ Res. 1977 Jul;41(1):9–18. doi: 10.1161/01.res.41.1.9. [DOI] [PubMed] [Google Scholar]
- Balakhovskii I. S. Nekotorye rezhimy dvizheniia vozbuzhdeniia v ideal'noi vozbudimoi tkani. Biofizika. 1965;10(6):1063–1067. [PubMed] [Google Scholar]
- Boineau J. P., Cox J. L. Slow ventricular activation in acute myocardial infarction. A source of re-entrant premature ventricular contractions. Circulation. 1973 Oct;48(4):702–713. doi: 10.1161/01.cir.48.4.702. [DOI] [PubMed] [Google Scholar]
- Buxton A. E., Waxman H. L., Marchlinski F. E., Josephson M. E. Atrial conduction: effects of extrastimuli with and without atrial dysrhythmias. Am J Cardiol. 1984 Oct 1;54(7):755–761. doi: 10.1016/s0002-9149(84)80203-9. [DOI] [PubMed] [Google Scholar]
- Fitzhugh R. Impulses and Physiological States in Theoretical Models of Nerve Membrane. Biophys J. 1961 Jul;1(6):445–466. doi: 10.1016/s0006-3495(61)86902-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordof A. J., Edie R., Malm J. R., Hoffman B. F., Rosen M. R. Electrophysiologic properties and response to pharmacologic agents of fibers from diseased human atria. Circulation. 1976 Nov;54(5):774–779. doi: 10.1161/01.cir.54.5.774. [DOI] [PubMed] [Google Scholar]
- Imanishi S., Arita M. Factors related to the low resting membrane potentials of diseased human atrial muscles. Jpn J Physiol. 1987;37(3):393–410. doi: 10.2170/jjphysiol.37.393. [DOI] [PubMed] [Google Scholar]
- Kirchhof C., Chorro F., Scheffer G. J., Brugada J., Konings K., Zetelaki Z., Allessie M. Regional entrainment of atrial fibrillation studied by high-resolution mapping in open-chest dogs. Circulation. 1993 Aug;88(2):736–749. doi: 10.1161/01.cir.88.2.736. [DOI] [PubMed] [Google Scholar]
- Konings K. T., Kirchhof C. J., Smeets J. R., Wellens H. J., Penn O. C., Allessie M. A. High-density mapping of electrically induced atrial fibrillation in humans. Circulation. 1994 Apr;89(4):1665–1680. doi: 10.1161/01.cir.89.4.1665. [DOI] [PubMed] [Google Scholar]
- Krinskii V. I. Rasprostranenie vozbuzhdeniia v neodnorodnoi srede (rezhimy, analogichnye fibrilliatsii serdtsa) Biofizika. 1966;11(4):676–683. [PubMed] [Google Scholar]
- Leier C. V., Meacham J. A., Schaal S. F. Prolonged atrial conduction. A major predisposing factor for the development of atrial flutter. Circulation. 1978 Feb;57(2):213–216. doi: 10.1161/01.cir.57.2.213. [DOI] [PubMed] [Google Scholar]
- MOE G. K., ABILDSKOV J. A. Atrial fibrillation as a self-sustaining arrhythmia independent of focal discharge. Am Heart J. 1959 Jul;58(1):59–70. doi: 10.1016/0002-8703(59)90274-1. [DOI] [PubMed] [Google Scholar]
- MOE G. K., RHEINBOLDT W. C., ABILDSKOV J. A. A COMPUTER MODEL OF ATRIAL FIBRILLATION. Am Heart J. 1964 Feb;67:200–220. doi: 10.1016/0002-8703(64)90371-0. [DOI] [PubMed] [Google Scholar]
- Mines G. R. On dynamic equilibrium in the heart. J Physiol. 1913 Jul 18;46(4-5):349–383. doi: 10.1113/jphysiol.1913.sp001596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesterenko V. V., Lastra A. A., Rosenshtraukh L. V., Starmer C. F. A proarrhythmic response to sodium channel blockade: modulation of the vulnerable period in guinea pig ventricular myocardium. J Cardiovasc Pharmacol. 1992 May;19(5):810–820. [PubMed] [Google Scholar]
- Ostrovskii L. A., Iakhno V. G. Formirovanie impul'sov v vozbudimoi srede. Biofizika. 1975 May-Jun;20(3):489–493. [PubMed] [Google Scholar]
- Panfilov A. V., Keener J. P. Effects of high frequency stimulation on cardiac tissue with an inexcitable obstacle. J Theor Biol. 1993 Aug 21;163(4):439–448. doi: 10.1006/jtbi.1993.1129. [DOI] [PubMed] [Google Scholar]
- Pertsov A. M., Davidenko J. M., Salomonsz R., Baxter W. T., Jalife J. Spiral waves of excitation underlie reentrant activity in isolated cardiac muscle. Circ Res. 1993 Mar;72(3):631–650. doi: 10.1161/01.res.72.3.631. [DOI] [PubMed] [Google Scholar]
- Pertsov A. M., Panfilov A. V., Medvedeva F. U. Neustoichivosti avtovoln v vozbudimykh sredakh, sviazannye s iavleniem kriticheskoi krivizny. Biofizika. 1983 Jan-Feb;28(1):100–102. [PubMed] [Google Scholar]
- Schalij M. J., Lammers W. J., Rensma P. L., Allessie M. A. Anisotropic conduction and reentry in perfused epicardium of rabbit left ventricle. Am J Physiol. 1992 Nov;263(5 Pt 2):H1466–H1478. doi: 10.1152/ajpheart.1992.263.5.H1466. [DOI] [PubMed] [Google Scholar]
- Schuessler R. B., Grayson T. M., Bromberg B. I., Cox J. L., Boineau J. P. Cholinergically mediated tachyarrhythmias induced by a single extrastimulus in the isolated canine right atrium. Circ Res. 1992 Nov;71(5):1254–1267. doi: 10.1161/01.res.71.5.1254. [DOI] [PubMed] [Google Scholar]
- Schuessler R. B., Kawamoto T., Hand D. E., Mitsuno M., Bromberg B. I., Cox J. L., Boineau J. P. Simultaneous epicardial and endocardial activation sequence mapping in the isolated canine right atrium. Circulation. 1993 Jul;88(1):250–263. doi: 10.1161/01.cir.88.1.250. [DOI] [PubMed] [Google Scholar]
- Spach M. S., Dolber P. C., Heidlage J. F. Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium conductance in human atrial muscle. A model of reentry based on anisotropic discontinuous propagation. Circ Res. 1988 Apr;62(4):811–832. doi: 10.1161/01.res.62.4.811. [DOI] [PubMed] [Google Scholar]
- Spach M. S., Dolber P. C. Relating extracellular potentials and their derivatives to anisotropic propagation at a microscopic level in human cardiac muscle. Evidence for electrical uncoupling of side-to-side fiber connections with increasing age. Circ Res. 1986 Mar;58(3):356–371. doi: 10.1161/01.res.58.3.356. [DOI] [PubMed] [Google Scholar]
- Spach M. S., Heidlage J. F., Darken E. R., Hofer E., Raines K. H., Starmer C. F. Cellular Vmax reflects both membrane properties and the load presented by adjoining cells. Am J Physiol. 1992 Dec;263(6 Pt 2):H1855–H1863. doi: 10.1152/ajpheart.1992.263.6.H1855. [DOI] [PubMed] [Google Scholar]
- Spach M. S., Miller W. T., 3rd, Dolber P. C., Kootsey J. M., Sommer J. R., Mosher C. E., Jr The functional role of structural complexities in the propagation of depolarization in the atrium of the dog. Cardiac conduction disturbances due to discontinuities of effective axial resistivity. Circ Res. 1982 Feb;50(2):175–191. doi: 10.1161/01.res.50.2.175. [DOI] [PubMed] [Google Scholar]
- Spach M. S., Miller W. T., 3rd, Geselowitz D. B., Barr R. C., Kootsey J. M., Johnson E. A. The discontinuous nature of propagation in normal canine cardiac muscle. Evidence for recurrent discontinuities of intracellular resistance that affect the membrane currents. Circ Res. 1981 Jan;48(1):39–54. doi: 10.1161/01.res.48.1.39. [DOI] [PubMed] [Google Scholar]
- Starmer C. F., Lancaster A. R., Lastra A. A., Grant A. O. Cardiac instability amplified by use-dependent Na channel blockade. Am J Physiol. 1992 Apr;262(4 Pt 2):H1305–H1310. doi: 10.1152/ajpheart.1992.262.4.H1305. [DOI] [PubMed] [Google Scholar]
- Starmer C. F., Lastra A. A., Nesterenko V. V., Grant A. O. Proarrhythmic response to sodium channel blockade. Theoretical model and numerical experiments. Circulation. 1991 Sep;84(3):1364–1377. doi: 10.1161/01.cir.84.3.1364. [DOI] [PubMed] [Google Scholar]
- Ten Eick R. E., Singer D. H. Electrophysiological properties of diseased human atrium. I. Low diastolic potential and altered cellular response to potassium. Circ Res. 1979 Apr;44(4):545–557. doi: 10.1161/01.res.44.4.545. [DOI] [PubMed] [Google Scholar]
- Winfree A. T. Electrical instability in cardiac muscle: phase singularities and rotors. J Theor Biol. 1989 Jun 8;138(3):353–405. doi: 10.1016/s0022-5193(89)80200-0. [DOI] [PubMed] [Google Scholar]
- Winfree Arthur T. Varieties of spiral wave behavior: An experimentalist's approach to the theory of excitable media. Chaos. 1991 Oct;1(3):303–334. doi: 10.1063/1.165844. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Oikawa N., Inoue H., Murakawa Y., Nakajima T., Usui M., Ajiki K., Ohkawa S., Sugimoto T. Slow abnormal conduction in the low right atrium: its anatomic basis and relevance to atrial reentry. Am Heart J. 1994 Feb;127(2):353–359. doi: 10.1016/0002-8703(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Zilberter YuI, Starmer C. F., Starobin J., Grant A. O. Late Na channels in cardiac cells: the physiological role of background Na channels. Biophys J. 1994 Jul;67(1):153–160. doi: 10.1016/S0006-3495(94)80464-3. [DOI] [PMC free article] [PubMed] [Google Scholar]