Abstract
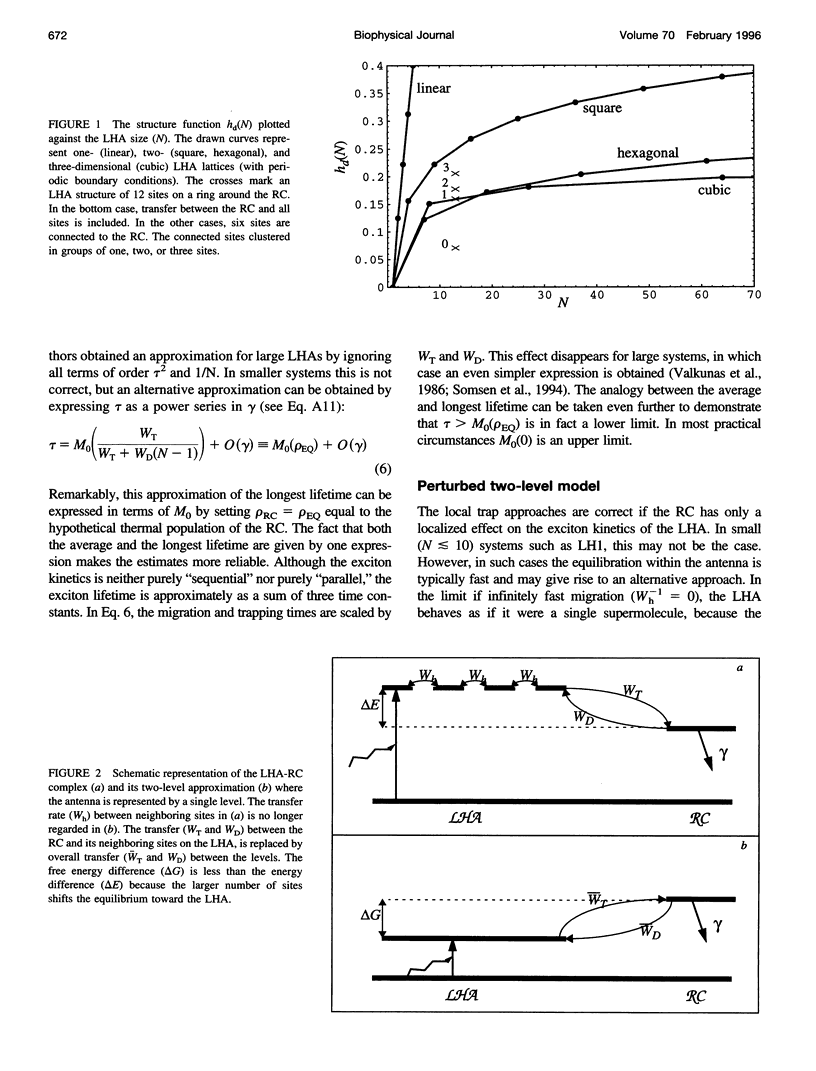
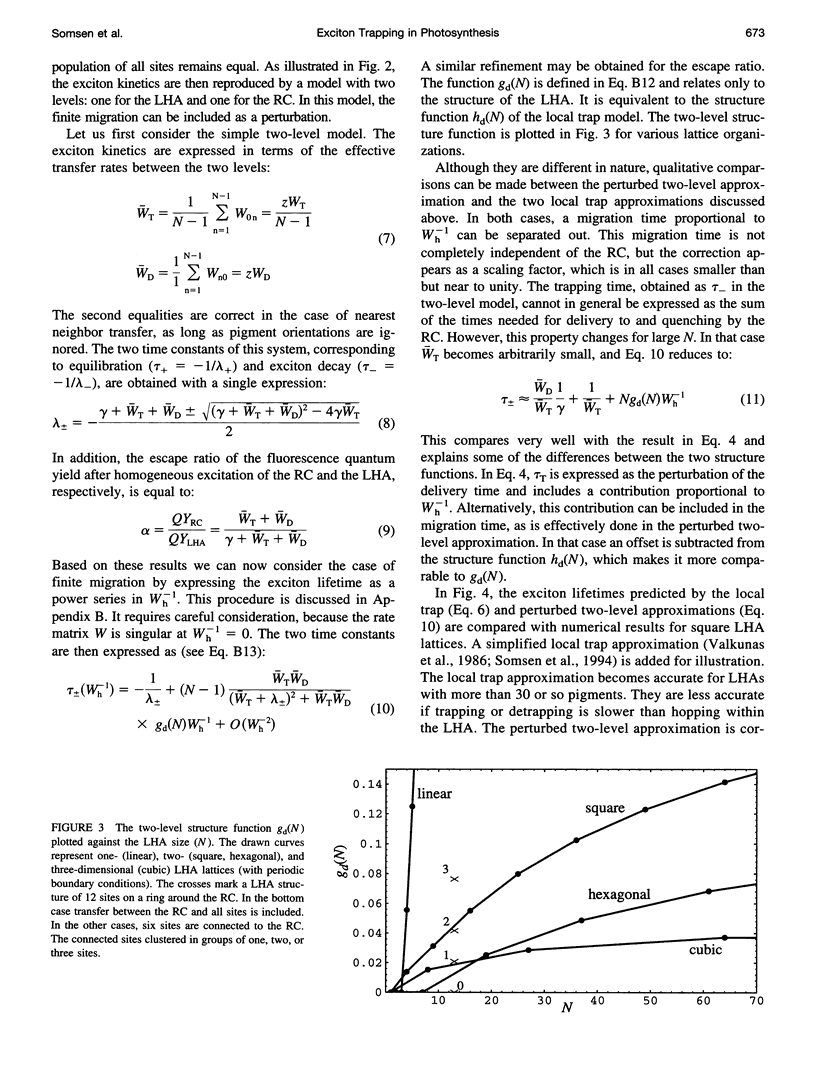
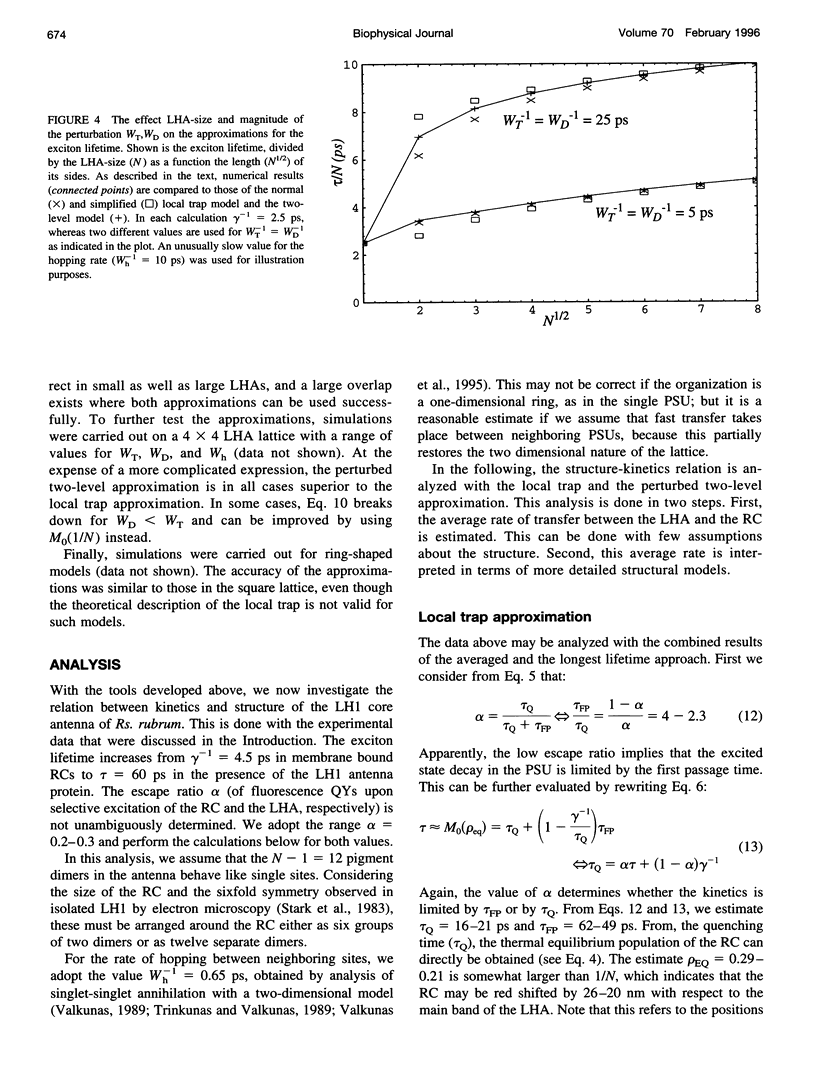
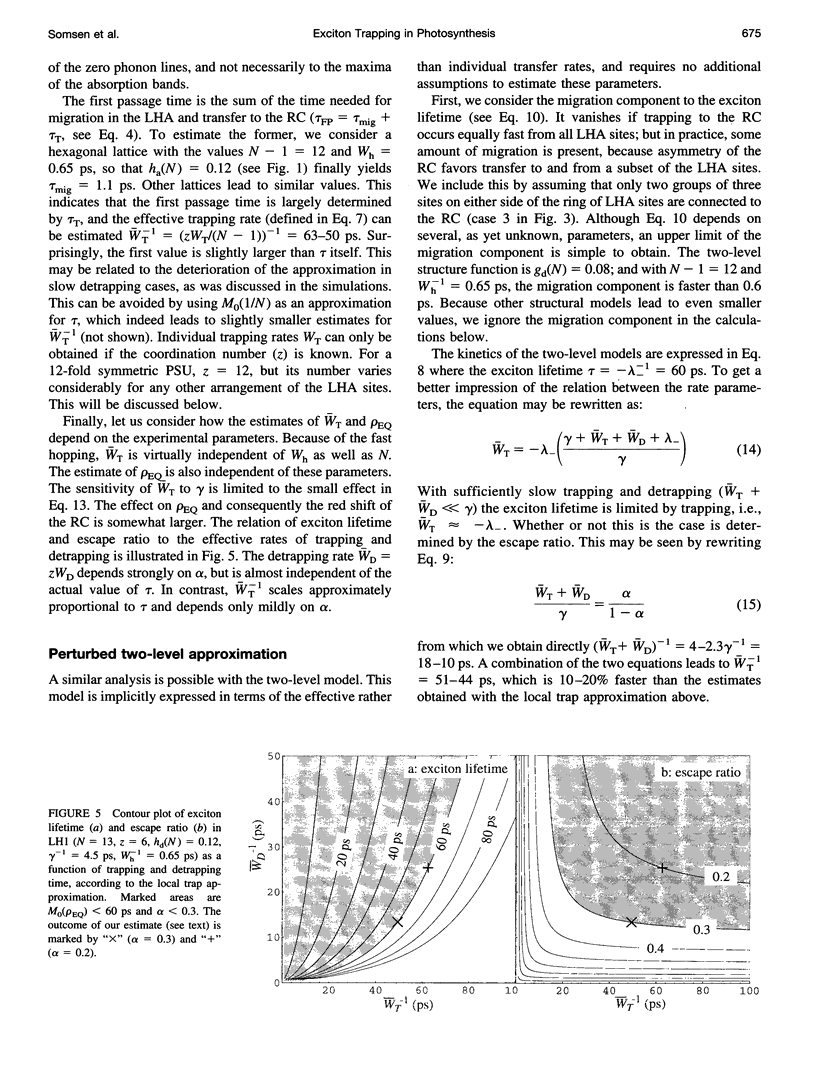
The study of exciton trapping in photosynthetic systems provides significant information about migration kinetics within the light harvesting antenna (LHA) and the reaction center (RC). We discuss two random walk models for systems with weakly coupled pigments, with a focus on the application to small systems (10-40 pigments/RC). Details of the exciton transfer to and from the RC are taken into consideration, as well as migration within the LHA and quenching in the RC. The first model is obtained by adapting earlier local trap models for application to small systems. The exciton lifetime is approximated by the sum of three contributions related to migration in the LHA, trapping by the RC, and quenching within the RC. The second model is more suitable for small systems and regards the finite rate of migration within the LHA as a perturbation of the simplified model, where the LHA and the RC are each represented by a single pigment level. In this approximation, the exciton lifetime is the sum of a migration component and a single nonlinear expression for the trapping and quenching of the excitons. Numerical simulations demonstrate that both models provide accurate estimates of the exciton lifetime in the intermediate range of 20-50 sites/RC. In combination, they cover the entire range of very small to very large photosynthetic systems. Although initially intended for regular LHA lattices, the models can also be applied to less regular systems. This becomes essential as more details of the structure of these systems become available. Analysis with these models indicates that the excited state decay in LH1 is limited by the average rate at which excitons transfer to the RC from neighboring sites in the LHA. By comparing this to the average rate of transfer within the LHA, various structural models that have been proposed for the LH1 core antenna are discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aagaard J., Sistrom W. R. Control of synthesis of reaction center bacteriochlorophyll in photosynthetic bacteria. Photochem Photobiol. 1972 Feb;15(2):209–225. doi: 10.1111/j.1751-1097.1972.tb06240.x. [DOI] [PubMed] [Google Scholar]
- Beekman L. M., van Mourik F., Jones M. R., Visser H. M., Hunter C. N., van Grondelle R. Trapping kinetics in mutants of the photosynthetic purple bacterium Rhodobacter sphaeroides: influence of the charge separation rate and consequences for the rate-limiting step in the light-harvesting process. Biochemistry. 1994 Mar 22;33(11):3143–3147. doi: 10.1021/bi00177a001. [DOI] [PubMed] [Google Scholar]
- Karrasch S., Bullough P. A., Ghosh R. The 8.5 A projection map of the light-harvesting complex I from Rhodospirillum rubrum reveals a ring composed of 16 subunits. EMBO J. 1995 Feb 15;14(4):631–638. doi: 10.1002/j.1460-2075.1995.tb07041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox R. S. On the theory of trapping of excitation in the photosynthetic unit. J Theor Biol. 1968 Nov;21(2):244–259. doi: 10.1016/0022-5193(68)90073-8. [DOI] [PubMed] [Google Scholar]
- Kudzmauskas S., Valkunas L., Borisov A. Y. A theory of excitation transfer in photosynthetic units. J Theor Biol. 1983 Nov 7;105(1):13–23. doi: 10.1016/0022-5193(83)90421-6. [DOI] [PubMed] [Google Scholar]
- Martin J. L., Breton J., Hoff A. J., Migus A., Antonetti A. Femtosecond spectroscopy of electron transfer in the reaction center of the photosynthetic bacterium Rhodopseudomonas sphaeroides R-26: Direct electron transfer from the dimeric bacteriochlorophyll primary donor to the bacteriopheophytin acceptor with a time constant of 2.8 +/- 0.2 psec. Proc Natl Acad Sci U S A. 1986 Feb;83(4):957–961. doi: 10.1073/pnas.83.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckenstock R. U., Brunisholz R. A., Zuber H. The light-harvesting core-complex and the B820-subunit from Rhodopseudomonas marina. Part I. Purification and characterisation. FEBS Lett. 1992 Oct 19;311(2):128–134. doi: 10.1016/0014-5793(92)81383-w. [DOI] [PubMed] [Google Scholar]
- Monger T. G., Parson W. W. Singlet-triplet fusion in Rhodopseudomonas sphaeroides chromatophores. A probe of the organization of the photosynthetic apparatus. Biochim Biophys Acta. 1977 Jun 9;460(3):393–407. doi: 10.1016/0005-2728(77)90080-9. [DOI] [PubMed] [Google Scholar]
- Pullerits T., Freiberg A. Kinetic model of primary energy transfer and trapping in photosynthetic membranes. Biophys J. 1992 Oct;63(4):879–896. doi: 10.1016/S0006-3495(92)81688-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullerits T., Visscher K. J., Hess S., Sundström V., Freiberg A., Timpmann K., van Grondelle R. Energy transfer in the inhomogeneously broadened core antenna of purple bacteria: a simultaneous fit of low-intensity picosecond absorption and fluorescence kinetics. Biophys J. 1994 Jan;66(1):236–248. doi: 10.1016/S0006-3495(94)80770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark W., Kühlbrandt W., Wildhaber I., Wehrli E., Mühlethaler K. The structure of the photoreceptor unit of Rhodopseudomonas viridis. EMBO J. 1984 Apr;3(4):777–783. doi: 10.1002/j.1460-2075.1984.tb01884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VREDENBERG W. J., DUYSENS L. N. Transfer of energy from bacteriochlorophyll to a reaction centre during bacterial photosynthesis. Nature. 1963 Jan 26;197:355–357. doi: 10.1038/197355a0. [DOI] [PubMed] [Google Scholar]
- Valkunas L., Trinkunas G., Liuolia V., van Grondelle R. Nonlinear annihilation of excitations in photosynthetic systems. Biophys J. 1995 Sep;69(3):1117–1129. doi: 10.1016/S0006-3495(95)79986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visschers R. W., Chang M. C., van Mourik F., Parkes-Loach P. S., Heller B. A., Loach P. A., van Grondelle R. Fluorescence polarization and low-temperature absorption spectroscopy of a subunit form of light-harvesting complex I from purple photosynthetic bacteria. Biochemistry. 1991 Jun 11;30(23):5734–5742. doi: 10.1021/bi00237a015. [DOI] [PubMed] [Google Scholar]
- Visser H. M., Somsen O. J., van Mourik F., Lin S., van Stokkum I. H., van Grondelle R. Direct observation of sub-picosecond equilibration of excitation energy in the light-harvesting antenna of Rhodospirillum rubrum. Biophys J. 1995 Sep;69(3):1083–1099. doi: 10.1016/S0006-3495(95)79982-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W., Lin S., Taguchi A. K., Woodbury N. W. Femtosecond pump-probe analysis of energy and electron transfer in photosynthetic membranes of Rhodobacter capsulatus. Biochemistry. 1994 Jul 12;33(27):8313–8322. doi: 10.1021/bi00193a019. [DOI] [PubMed] [Google Scholar]





