Abstract
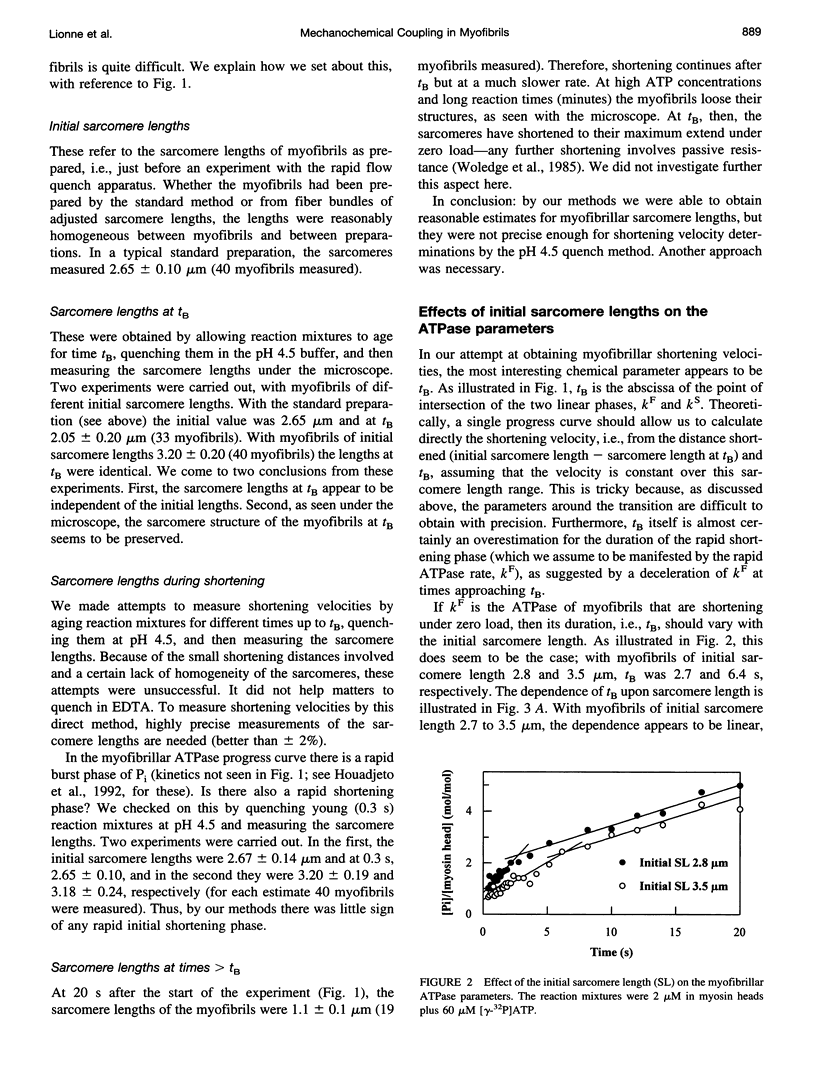
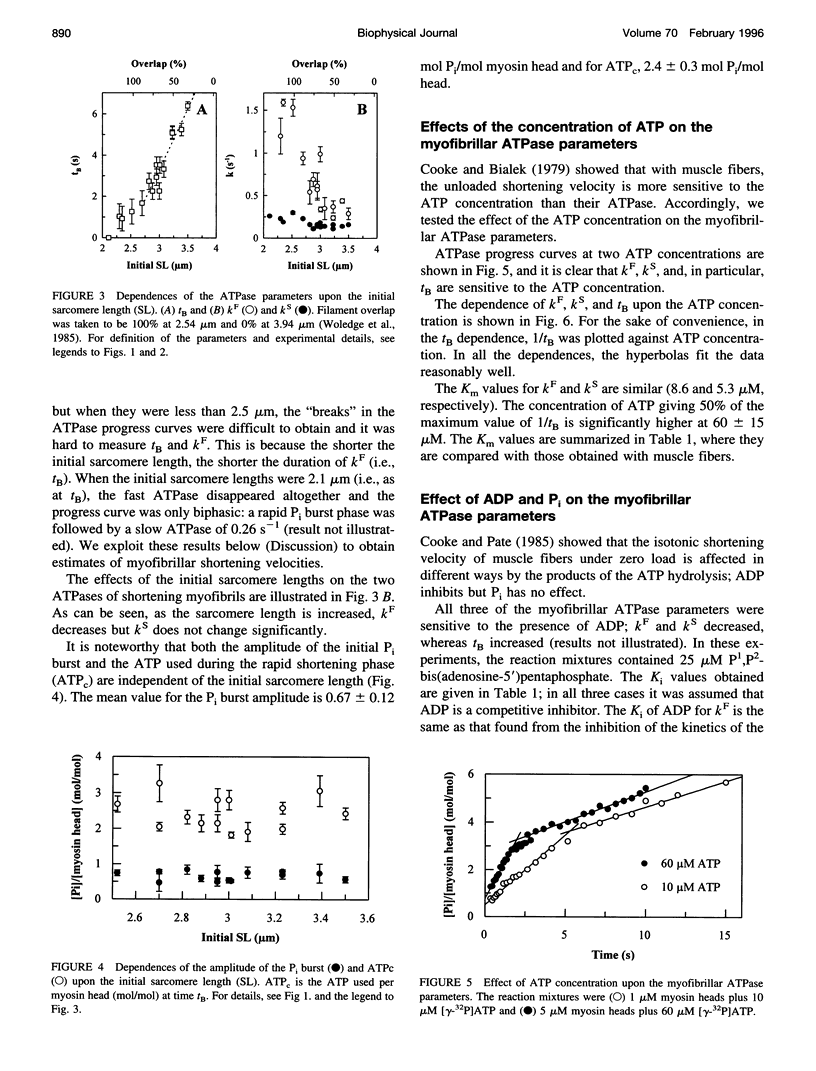
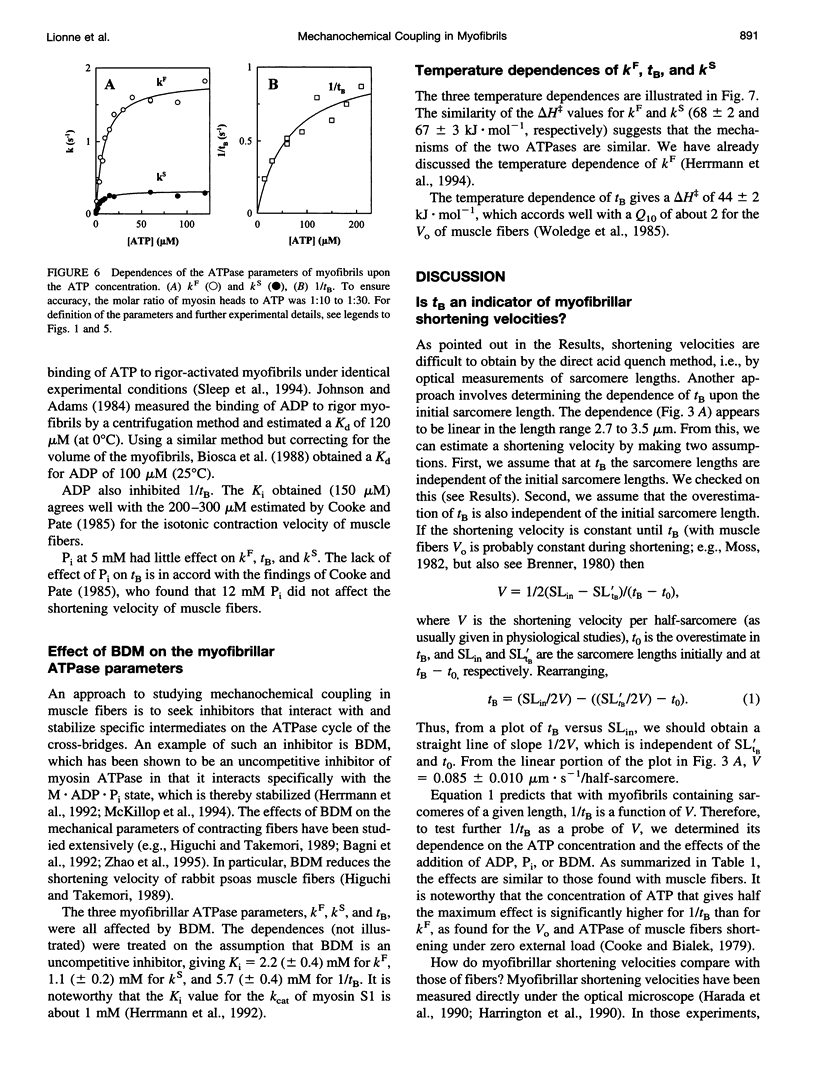
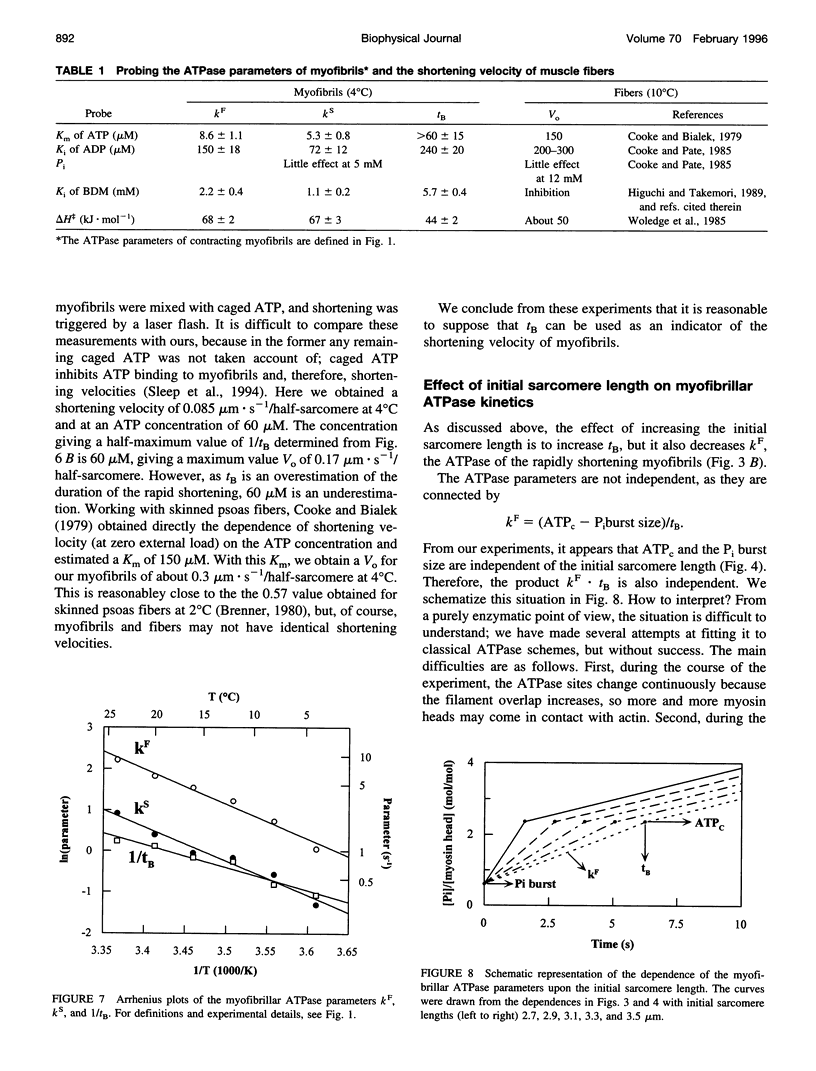
We studied the ATPase of shortening myofibrils at 4 degrees C by the rapid flow quench method. The progress curve has three phases: a P(i) burst, a fast linear phase kF of duration tB, and a deceleration to a slow kS. We propose that kF is the ATPase of myofibrils shortening under zero external load; at tB shortening and ATPase rates are reduced by passive resistance. The total ATP consumed during the rapid shortening is ATPc. Our purpose was to obtain information on the myofibrillar shortening velocity from their ATPase progress curves. We tested tB as an indicator of shortening velocity by determining the effects of different probes upon it and the other ATPase parameters. The dependence of tB upon the initial sarcomere length was linear, giving a shortening velocity close to that of muscle fibres (Vo). The Km of ATP was larger for tB than for kF, as found with fibers for Vo and their ATPase. ADP and 2,3-butanedione monoxime, but not P(i), inhibited tB to the same extent as Vo. The delta H for tB and Vo were similar. ATPc was independent of the sarcomere length, implying that the more the myofibrils shorten, the less ATP expended per myosin head per micron shortened. We propose that tB can be used as an indicator for myofibrillar shortening velocities.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagni M. A., Cecchi G., Colomo F., Garzella P. Effects of 2,3-butanedione monoxime on the crossbridge kinetics in frog single muscle fibres. J Muscle Res Cell Motil. 1992 Oct;13(5):516–522. doi: 10.1007/BF01737994. [DOI] [PubMed] [Google Scholar]
- Barman T. E., Travers F. The rapid-flow-quench method in the study of fast reactions in biochemistry: extension to subzero conditions. Methods Biochem Anal. 1985;31:1–59. doi: 10.1002/9780470110522.ch1. [DOI] [PubMed] [Google Scholar]
- Bartoo M. L., Popov V. I., Fearn L. A., Pollack G. H. Active tension generation in isolated skeletal myofibrils. J Muscle Res Cell Motil. 1993 Oct;14(5):498–510. doi: 10.1007/BF00297212. [DOI] [PubMed] [Google Scholar]
- Biosca J. A., Barman T. E., Travers F. Transient kinetics of the binding of ATP to actomyosin subfragment 1: evidence that the dissociation of actomyosin subfragment 1 by ATP leads to a new conformation of subfragment 1. Biochemistry. 1984 May 22;23(11):2428–2436. doi: 10.1021/bi00306a017. [DOI] [PubMed] [Google Scholar]
- Biosca J. A., Greene L. E., Eisenberg E. Binding of ADP and 5'-adenylyl imidodiphosphate to rabbit muscle myofibrils. J Biol Chem. 1988 Oct 5;263(28):14231–14235. [PubMed] [Google Scholar]
- Brune M., Hunter J. L., Corrie J. E., Webb M. R. Direct, real-time measurement of rapid inorganic phosphate release using a novel fluorescent probe and its application to actomyosin subfragment 1 ATPase. Biochemistry. 1994 Jul 12;33(27):8262–8271. doi: 10.1021/bi00193a013. [DOI] [PubMed] [Google Scholar]
- Cooke R., Bialek W. Contraction of glycerinated muscle fibers as a function of the ATP concentration. Biophys J. 1979 Nov;28(2):241–258. doi: 10.1016/S0006-3495(79)85174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985 Nov;48(5):789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., White H., Pate E. A model of the release of myosin heads from actin in rapidly contracting muscle fibers. Biophys J. 1994 Mar;66(3 Pt 1):778–788. doi: 10.1016/s0006-3495(94)80854-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman K. A. The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J Physiol. 1979 Jun;291:143–159. doi: 10.1113/jphysiol.1979.sp012804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer J. T., Simmons R. M., Spudich J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature. 1994 Mar 10;368(6467):113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- Goldman Y. E. Kinetics of the actomyosin ATPase in muscle fibers. Annu Rev Physiol. 1987;49:637–654. doi: 10.1146/annurev.ph.49.030187.003225. [DOI] [PubMed] [Google Scholar]
- Harada Y., Sakurada K., Aoki T., Thomas D. D., Yanagida T. Mechanochemical coupling in actomyosin energy transduction studied by in vitro movement assay. J Mol Biol. 1990 Nov 5;216(1):49–68. doi: 10.1016/S0022-2836(05)80060-9. [DOI] [PubMed] [Google Scholar]
- Harrington W. F., Karr T., Busa W. B., Lovell S. J. Contraction of myofibrils in the presence of antibodies to myosin subfragment 2. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7453–7456. doi: 10.1073/pnas.87.19.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Tonomura Y. Dependence of activity of myofibrillar ATPase on sarcomere length and calcium ion concentration. J Biochem. 1968 Jan;63(1):101–118. doi: 10.1093/oxfordjournals.jbchem.a128736. [DOI] [PubMed] [Google Scholar]
- Herrmann C., Lionne C., Travers F., Barman T. Correlation of ActoS1, myofibrillar, and muscle fiber ATPases. Biochemistry. 1994 Apr 12;33(14):4148–4154. doi: 10.1021/bi00180a007. [DOI] [PubMed] [Google Scholar]
- Herrmann C., Sleep J., Chaussepied P., Travers F., Barman T. A structural and kinetic study on myofibrils prevented from shortening by chemical cross-linking. Biochemistry. 1993 Jul 20;32(28):7255–7263. doi: 10.1021/bi00079a023. [DOI] [PubMed] [Google Scholar]
- Herrmann C., Wray J., Travers F., Barman T. Effect of 2,3-butanedione monoxime on myosin and myofibrillar ATPases. An example of an uncompetitive inhibitor. Biochemistry. 1992 Dec 8;31(48):12227–12232. doi: 10.1021/bi00163a036. [DOI] [PubMed] [Google Scholar]
- Higuchi H., Goldman Y. E. Sliding distance between actin and myosin filaments per ATP molecule hydrolysed in skinned muscle fibres. Nature. 1991 Jul 25;352(6333):352–354. doi: 10.1038/352352a0. [DOI] [PubMed] [Google Scholar]
- Higuchi H., Goldman Y. E. Sliding distance per ATP molecule hydrolyzed by myosin heads during isotonic shortening of skinned muscle fibers. Biophys J. 1995 Oct;69(4):1491–1507. doi: 10.1016/S0006-3495(95)80020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi H., Takemori S. Butanedione monoxime suppresses contraction and ATPase activity of rabbit skeletal muscle. J Biochem. 1989 Apr;105(4):638–643. doi: 10.1093/oxfordjournals.jbchem.a122717. [DOI] [PubMed] [Google Scholar]
- Homsher E. Muscle enthalpy production and its relationship to actomyosin ATPase. Annu Rev Physiol. 1987;49:673–690. doi: 10.1146/annurev.ph.49.030187.003325. [DOI] [PubMed] [Google Scholar]
- Houadjeto M., Barman T., Travers F. What is the true ATPase activity of contracting myofibrils? FEBS Lett. 1991 Apr 9;281(1-2):105–107. doi: 10.1016/0014-5793(91)80369-e. [DOI] [PubMed] [Google Scholar]
- Houadjeto M., Travers F., Barman T. Ca(2+)-activated myofibrillar ATPase: transient kinetics and the titration of its active sites. Biochemistry. 1992 Feb 11;31(5):1564–1569. doi: 10.1021/bi00120a038. [DOI] [PubMed] [Google Scholar]
- Irving M. Motor proteins. Biomechanics goes quantum. Nature. 1991 Jul 25;352(6333):284–286. doi: 10.1038/352284a0. [DOI] [PubMed] [Google Scholar]
- Johnson R. E., Adams P. H. ADP binds similarly to rigor muscle myofibrils and to actomyosin-subfragment one. FEBS Lett. 1984 Aug 20;174(1):11–14. doi: 10.1016/0014-5793(84)81067-4. [DOI] [PubMed] [Google Scholar]
- Knight P. J., Trinick J. A. Preparation of myofibrils. Methods Enzymol. 1982;85(Pt B):9–12. doi: 10.1016/0076-6879(82)85004-0. [DOI] [PubMed] [Google Scholar]
- Lionne C., Brune M., Webb M. R., Travers F., Barman T. Time resolved measurements show that phosphate release is the rate limiting step on myofibrillar ATPases. FEBS Lett. 1995 May 1;364(1):59–62. doi: 10.1016/0014-5793(95)00356-e. [DOI] [PubMed] [Google Scholar]
- Lionne C., Herrmann C., Travers F., Barman T. The myofibril as a model for muscle fiber ATPase. Biophys J. 1995 Apr;68(4 Suppl):217S–217S. [PMC free article] [PubMed] [Google Scholar]
- Lombardi V., Piazzesi G., Linari M. Rapid regeneration of the actin-myosin power stroke in contracting muscle. Nature. 1992 Feb 13;355(6361):638–641. doi: 10.1038/355638a0. [DOI] [PubMed] [Google Scholar]
- Ma Y. Z., Taylor E. W. Kinetic mechanism of myofibril ATPase. Biophys J. 1994 May;66(5):1542–1553. doi: 10.1016/S0006-3495(94)80945-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKillop D. F., Fortune N. S., Ranatunga K. W., Geeves M. A. The influence of 2,3-butanedione 2-monoxime (BDM) on the interaction between actin and myosin in solution and in skinned muscle fibres. J Muscle Res Cell Motil. 1994 Jun;15(3):309–318. doi: 10.1007/BF00123483. [DOI] [PubMed] [Google Scholar]
- Moss R. L. The effect of calcium on the maximum velocity of shortening in skinned skeletal muscle fibres of the rabbit. J Muscle Res Cell Motil. 1982 Sep;3(3):295–311. doi: 10.1007/BF00713039. [DOI] [PubMed] [Google Scholar]
- Ohno T., Kodama T. Kinetics of adenosine triphosphate hydrolysis by shortening myofibrils from rabbit psoas muscle. J Physiol. 1991 Sep;441:685–702. doi: 10.1113/jphysiol.1991.sp018773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann E. M., Umfleet R. A. Selective precipitation of 32Pi onto filter papers. Application to ATPase and cyclic AMP phosphodiesterase determination. Biochim Biophys Acta. 1978 Apr 12;523(2):516–521. doi: 10.1016/0005-2744(78)90054-2. [DOI] [PubMed] [Google Scholar]
- Sleep J., Herrmann C., Barman T., Travers F. Inhibition of ATP binding to myofibrils and acto-myosin subfragment 1 by caged ATP. Biochemistry. 1994 May 24;33(20):6038–6042. doi: 10.1021/bi00186a002. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Stewart A. W., Wilson G. J. Dissociation of force from myofibrillar MgATPase and stiffness at short sarcomere lengths in rat and toad skeletal muscle. J Physiol. 1989 Mar;410:351–366. doi: 10.1113/jphysiol.1989.sp017537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woledge R. C., Curtin N. A., Homsher E. Energetic aspects of muscle contraction. Monogr Physiol Soc. 1985;41:1–357. [PubMed] [Google Scholar]
- Zhao L., Naber N., Cooke R. Muscle cross-bridges bound to actin are disordered in the presence of 2,3-butanedione monoxime. Biophys J. 1995 May;68(5):1980–1990. doi: 10.1016/S0006-3495(95)80375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


