Abstract
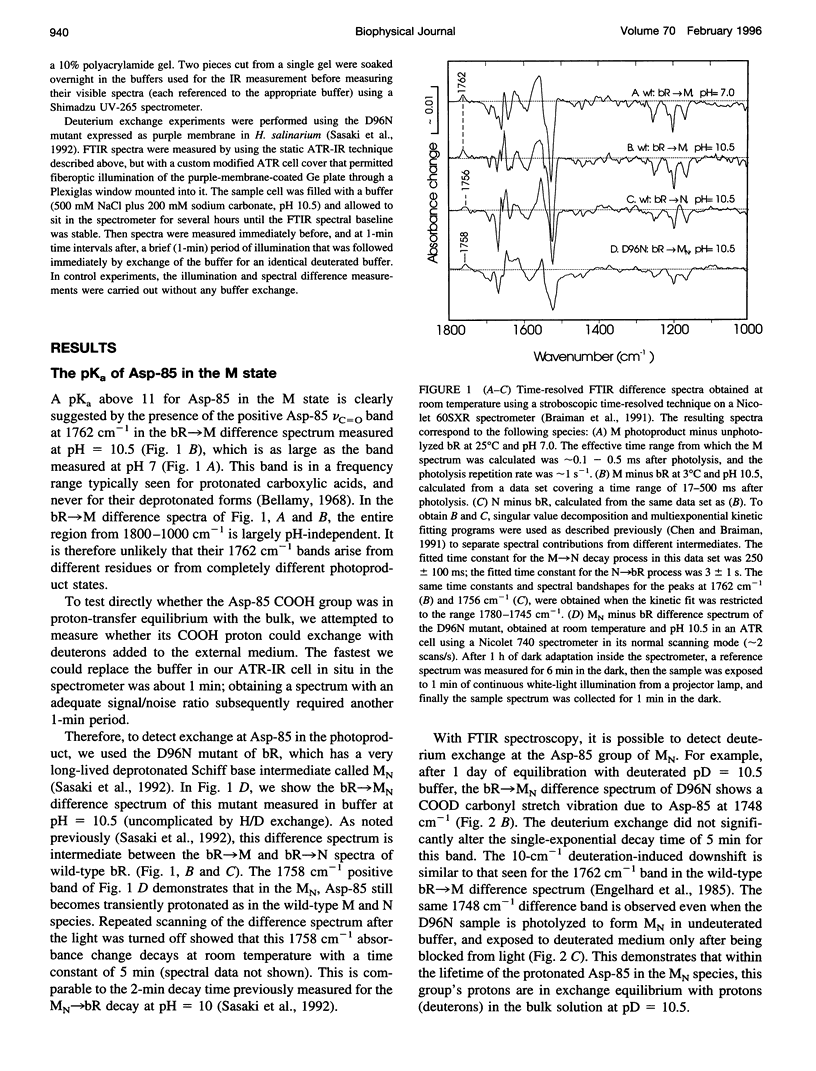
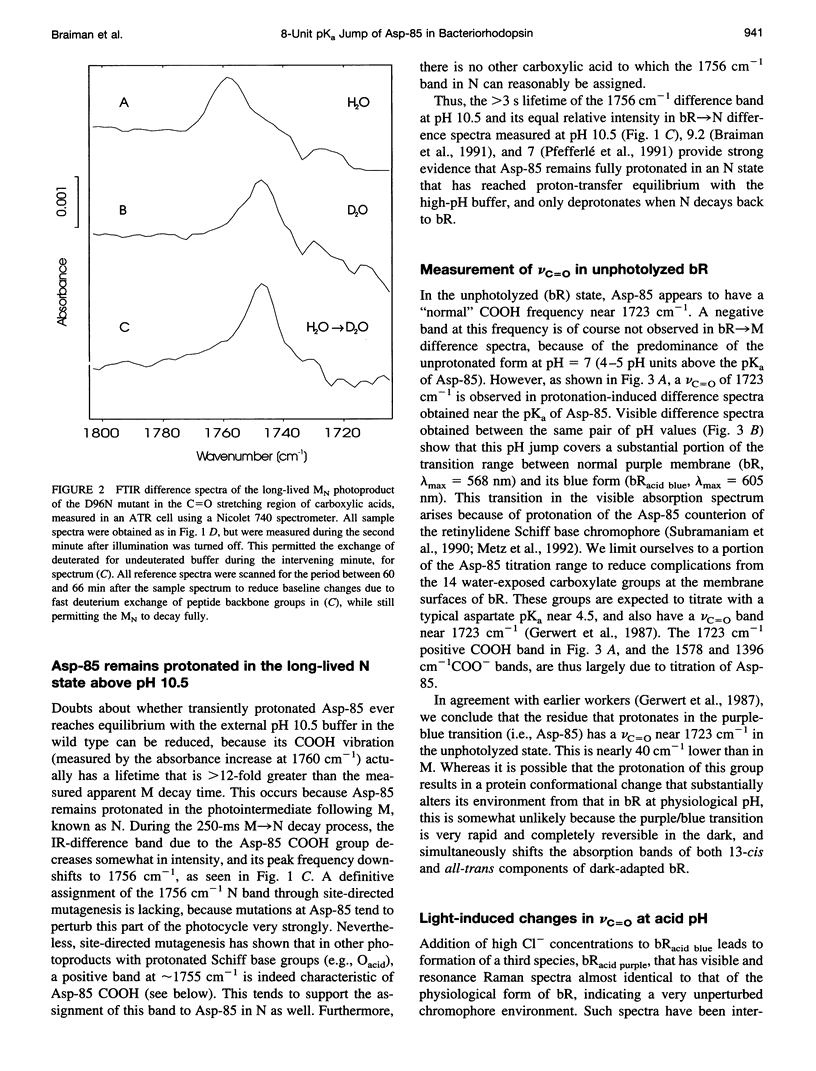
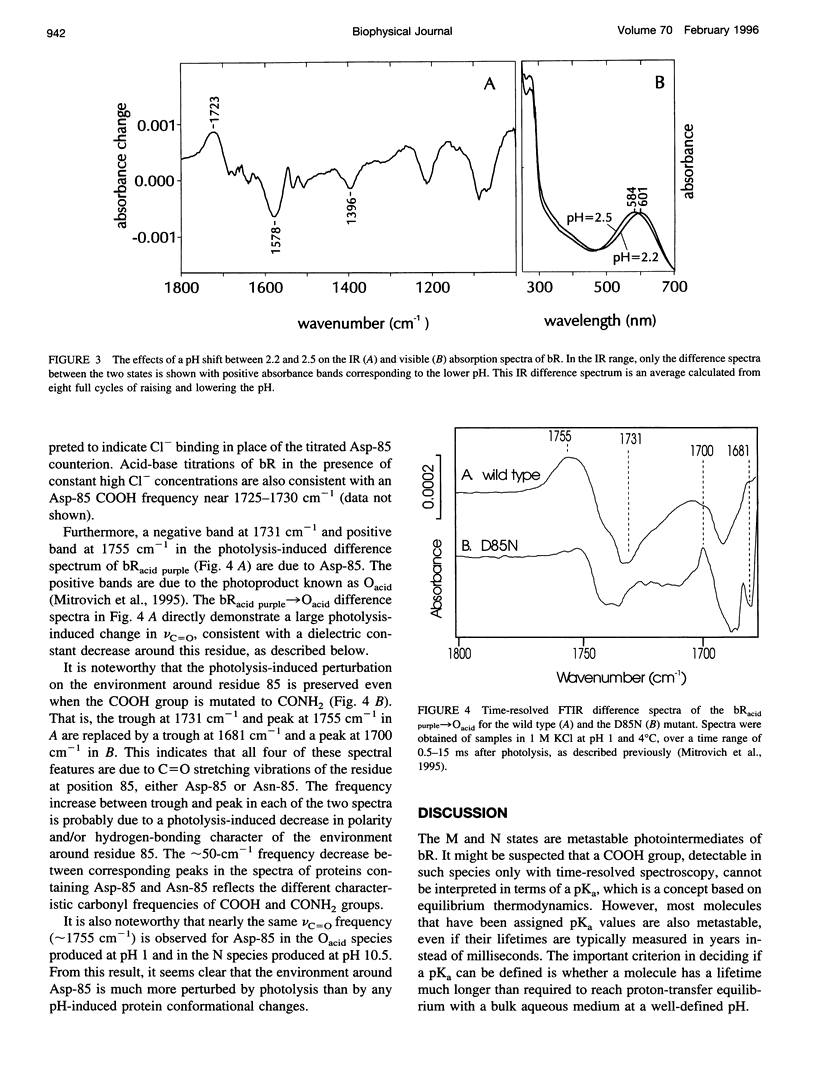
The proton-pumping mechanism of bacteriorhodopsin is dependent on a photolysis-induced transfer of a proton from the retinylidene Schiff base chromophore to the aspartate-85 counterion. Up until now, this transfer was ascribed to a > 7-unit decrease in the pKa of the protonated Schiff base caused by photoisomerization of the retinal. However, a comparably large increase in the pKa of the Asp-85 acceptor also plays a role, as we show here with infrared measurements. Furthermore, the shifted vibrational frequency of the Asp-85 COOH group indicates a transient drop in the effective dielectric constant around Asp-85 to approximately 2 in the M photointermediate. This dielectric decrease would cause a > 40 kJ-mol-1 increase in free energy of the anionic form of Asp-85, fully explaining the observed pK alpha increase. An analogous photolysis-induced destabilization of the Schiff base counterion could initiate anion transport in the related protein, halorhodopsin, in which aspartate-85 is replaced by Cl- and the Schiff base proton is consequently never transferred.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bashford D., Gerwert K. Electrostatic calculations of the pKa values of ionizable groups in bacteriorhodopsin. J Mol Biol. 1992 Mar 20;224(2):473–486. doi: 10.1016/0022-2836(92)91009-e. [DOI] [PubMed] [Google Scholar]
- Bousché O., Braiman M., He Y. W., Marti T., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants. Evidence that ASP-96 deprotonates during the M----N transition. J Biol Chem. 1991 Jun 15;266(17):11063–11067. [PubMed] [Google Scholar]
- Braiman M. S., Bousché O., Rothschild K. J. Protein dynamics in the bacteriorhodopsin photocycle: submillisecond Fourier transform infrared spectra of the L, M, and N photointermediates. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2388–2392. doi: 10.1073/pnas.88.6.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman M. S., Mogi T., Marti T., Stern L. J., Khorana H. G., Rothschild K. J. Vibrational spectroscopy of bacteriorhodopsin mutants: light-driven proton transport involves protonation changes of aspartic acid residues 85, 96, and 212. Biochemistry. 1988 Nov 15;27(23):8516–8520. doi: 10.1021/bi00423a002. [DOI] [PubMed] [Google Scholar]
- Brown L. S., Bonet L., Needleman R., Lanyi J. K. Estimated acid dissociation constants of the Schiff base, Asp-85, and Arg-82 during the bacteriorhodopsin photocycle. Biophys J. 1993 Jul;65(1):124–130. doi: 10.1016/S0006-3495(93)81064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Váró G., Klinger A. L., Czajkowsky D. M., Braiman M. S., Needleman R., Lanyi J. K. Proton transfer from Asp-96 to the bacteriorhodopsin Schiff base is caused by a decrease of the pKa of Asp-96 which follows a protein backbone conformational change. Biochemistry. 1993 Mar 2;32(8):1981–1990. doi: 10.1021/bi00059a015. [DOI] [PubMed] [Google Scholar]
- Deng H., Huang L., Callender R., Ebrey T. Evidence for a bound water molecule next to the retinal Schiff base in bacteriorhodopsin and rhodopsin: a resonance Raman study of the Schiff base hydrogen/deuterium exchange. Biophys J. 1994 Apr;66(4):1129–1136. doi: 10.1016/S0006-3495(94)80893-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhard M., Gerwert K., Hess B., Kreutz W., Siebert F. Light-driven protonation changes of internal aspartic acids of bacteriorhodopsin: an investigation by static and time-resolved infrared difference spectroscopy using [4-13C]aspartic acid labeled purple membrane. Biochemistry. 1985 Jan 15;24(2):400–407. doi: 10.1021/bi00323a024. [DOI] [PubMed] [Google Scholar]
- Gerwert K., Souvignier G., Hess B. Simultaneous monitoring of light-induced changes in protein side-group protonation, chromophore isomerization, and backbone motion of bacteriorhodopsin by time-resolved Fourier-transform infrared spectroscopy. Proc Natl Acad Sci U S A. 1990 Dec 15;87(24):9774–9778. doi: 10.1073/pnas.87.24.9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindjee R., Balashov S., Ebrey T., Oesterhelt D., Steinberg G., Sheves M. Lowering the intrinsic pKa of the chromophore's Schiff base can restore its light-induced deprotonation in the inactive Tyr-57-->Asn mutant of bacteriorhodopsin. J Biol Chem. 1994 May 20;269(20):14353–14354. [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Humphrey W., Logunov I., Schulten K., Sheves M. Molecular dynamics study of bacteriorhodopsin and artificial pigments. Biochemistry. 1994 Mar 29;33(12):3668–3678. doi: 10.1021/bi00178a025. [DOI] [PubMed] [Google Scholar]
- Kalisky O., Ottolenghi M., Honig B., Korenstein R. Environmental effects on formation and photoreaction of the M412 photoproduct of bacteriorhodopsin: implications for the mechanism of proton pumping. Biochemistry. 1981 Feb 3;20(3):649–655. doi: 10.1021/bi00506a031. [DOI] [PubMed] [Google Scholar]
- Krebs M. P., Khorana H. G. Mechanism of light-dependent proton translocation by bacteriorhodopsin. J Bacteriol. 1993 Mar;175(6):1555–1560. doi: 10.1128/jb.175.6.1555-1560.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K. Proton translocation mechanism and energetics in the light-driven pump bacteriorhodopsin. Biochim Biophys Acta. 1993 Dec 7;1183(2):241–261. doi: 10.1016/0005-2728(93)90226-6. [DOI] [PubMed] [Google Scholar]
- Lind C., Höjeberg B., Khorana H. G. Reconstitution of delipidated bacteriorhodopsin with endogenous polar lipids. J Biol Chem. 1981 Aug 25;256(16):8298–8305. [PubMed] [Google Scholar]
- Maeda A., Sasaki J., Yamazaki Y., Needleman R., Lanyi J. K. Interaction of aspartate-85 with a water molecule and the protonated Schiff base in the L intermediate of bacteriorhodopsin: a Fourier-transform infrared spectroscopic study. Biochemistry. 1994 Feb 22;33(7):1713–1717. doi: 10.1021/bi00173a013. [DOI] [PubMed] [Google Scholar]
- Mathies R. A., Lin S. W., Ames J. B., Pollard W. T. From femtoseconds to biology: mechanism of bacteriorhodopsin's light-driven proton pump. Annu Rev Biophys Biophys Chem. 1991;20:491–518. doi: 10.1146/annurev.bb.20.060191.002423. [DOI] [PubMed] [Google Scholar]
- Metz G., Siebert F., Engelhard M. Asp85 is the only internal aspartic acid that gets protonated in the M intermediate and the purple-to-blue transition of bacteriorhodopsin. A solid-state 13C CP-MAS NMR investigation. FEBS Lett. 1992 Jun 1;303(2-3):237–241. doi: 10.1016/0014-5793(92)80528-o. [DOI] [PubMed] [Google Scholar]
- Mitrovich Q. M., Victor K. G., Braiman M. S. Differences between the photocycles of halorhodopsin and the acid purple form of bacteriorhodopsin analyzed with millisecond time-resolved FTIR spectroscopy. Biophys Chem. 1995 Sep-Oct;56(1-2):121–127. doi: 10.1016/0301-4622(95)00023-q. [DOI] [PubMed] [Google Scholar]
- Pfefferlé J. M., Maeda A., Sasaki J., Yoshizawa T. Fourier transform infrared study of the N intermediate of bacteriorhodopsin. Biochemistry. 1991 Jul 2;30(26):6548–6556. doi: 10.1021/bi00240a027. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J. FTIR difference spectroscopy of bacteriorhodopsin: toward a molecular model. J Bioenerg Biomembr. 1992 Apr;24(2):147–167. doi: 10.1007/BF00762674. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Zagaeski M., Cantore W. A. Conformational changes of bacteriorhodopsin detected by Fourier transform infrared difference spectroscopy. Biochem Biophys Res Commun. 1981 Nov 30;103(2):483–489. doi: 10.1016/0006-291x(81)90478-2. [DOI] [PubMed] [Google Scholar]
- Sampogna R. V., Honig B. Environmental effects on the protonation states of active site residues in bacteriorhodopsin. Biophys J. 1994 May;66(5):1341–1352. doi: 10.1016/S0006-3495(94)80925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki J., Lanyi J. K., Needleman R., Yoshizawa T., Maeda A. Complete identification of C = O stretching vibrational bands of protonated aspartic acid residues in the difference infrared spectra of M and N intermediates versus bacteriorhodopsin. Biochemistry. 1994 Mar 22;33(11):3178–3184. doi: 10.1021/bi00177a006. [DOI] [PubMed] [Google Scholar]
- Sasaki J., Shichida Y., Lanyi J. K., Maeda A. Protein changes associated with reprotonation of the Schiff base in the photocycle of Asp96-->Asn bacteriorhodopsin. The MN intermediate with unprotonated Schiff base but N-like protein structure. J Biol Chem. 1992 Oct 15;267(29):20782–20786. [PubMed] [Google Scholar]
- Sharp K. A., Honig B. Electrostatic interactions in macromolecules: theory and applications. Annu Rev Biophys Biophys Chem. 1990;19:301–332. doi: 10.1146/annurev.bb.19.060190.001505. [DOI] [PubMed] [Google Scholar]
- Sheves M., Albeck A., Friedman N., Ottolenghi M. Controlling the pKa of the bacteriorhodopsin Schiff base by use of artificial retinal analogues. Proc Natl Acad Sci U S A. 1986 May;83(10):3262–3266. doi: 10.1073/pnas.83.10.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S., Marti T., Khorana H. G. Protonation state of Asp (Glu)-85 regulates the purple-to-blue transition in bacteriorhodopsin mutants Arg-82----Ala and Asp-85----Glu: the blue form is inactive in proton translocation. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1013–1017. doi: 10.1073/pnas.87.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Száraz S., Oesterhelt D., Ormos P. pH-induced structural changes in bacteriorhodopsin studied by Fourier transform infrared spectroscopy. Biophys J. 1994 Oct;67(4):1706–1712. doi: 10.1016/S0006-3495(94)80644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Gowda D. C., Peng S., Parker T. M., Jing N., Harris R. D. Nanometric design of extraordinary hydrophobic-induced pKa shifts for aspartic acid: relevance to protein mechanisms. Biopolymers. 1994 Jul;34(7):889–896. doi: 10.1002/bip.360340708. [DOI] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Kinetic and spectroscopic evidence for an irreversible step between deprotonation and reprotonation of the Schiff base in the bacteriorhodopsin photocycle. Biochemistry. 1991 May 21;30(20):5008–5015. doi: 10.1021/bi00234a024. [DOI] [PubMed] [Google Scholar]
- Wilson N. A., Barbar E., Fuchs J. A., Woodward C. Aspartic acid 26 in reduced Escherichia coli thioredoxin has a pKa > 9. Biochemistry. 1995 Jul 18;34(28):8931–8939. doi: 10.1021/bi00028a001. [DOI] [PubMed] [Google Scholar]


