Abstract
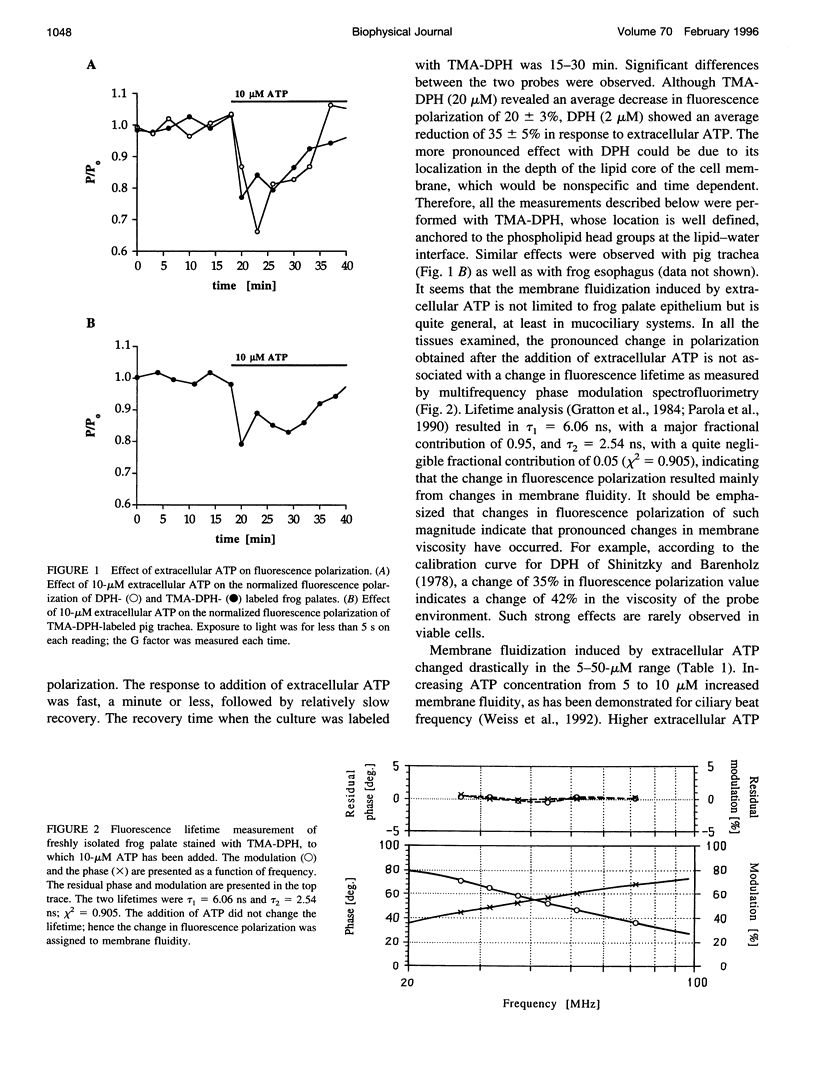
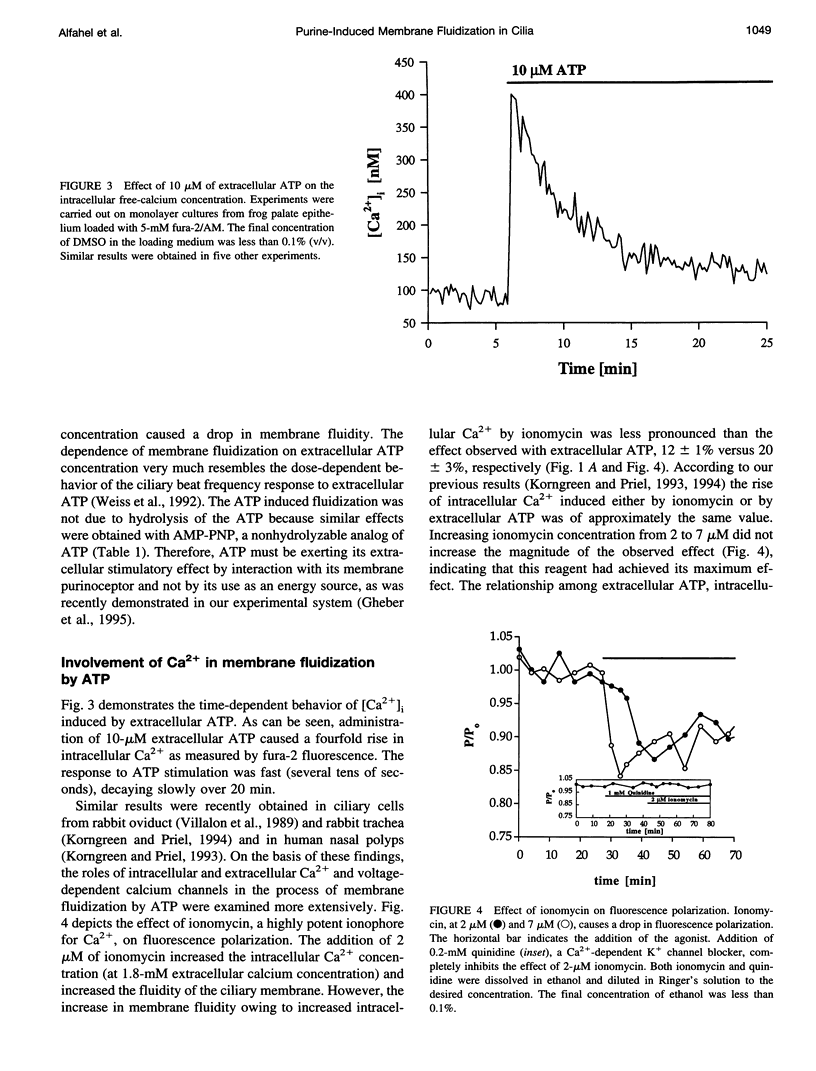
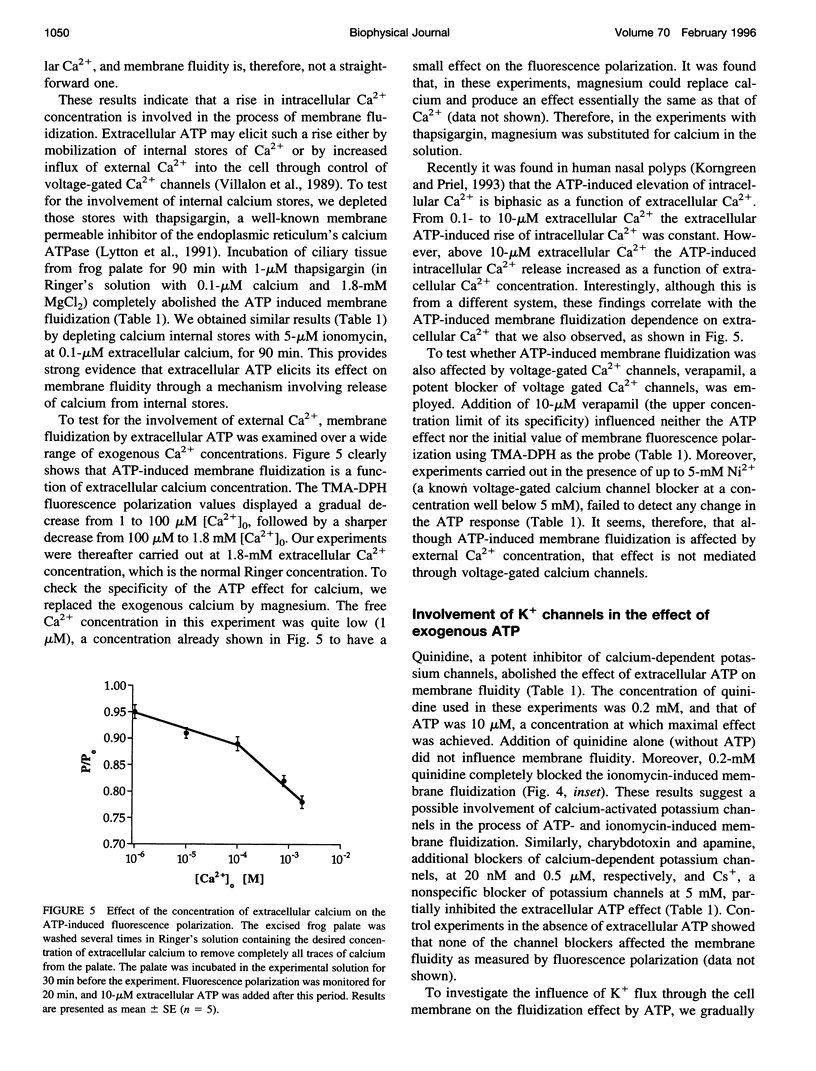
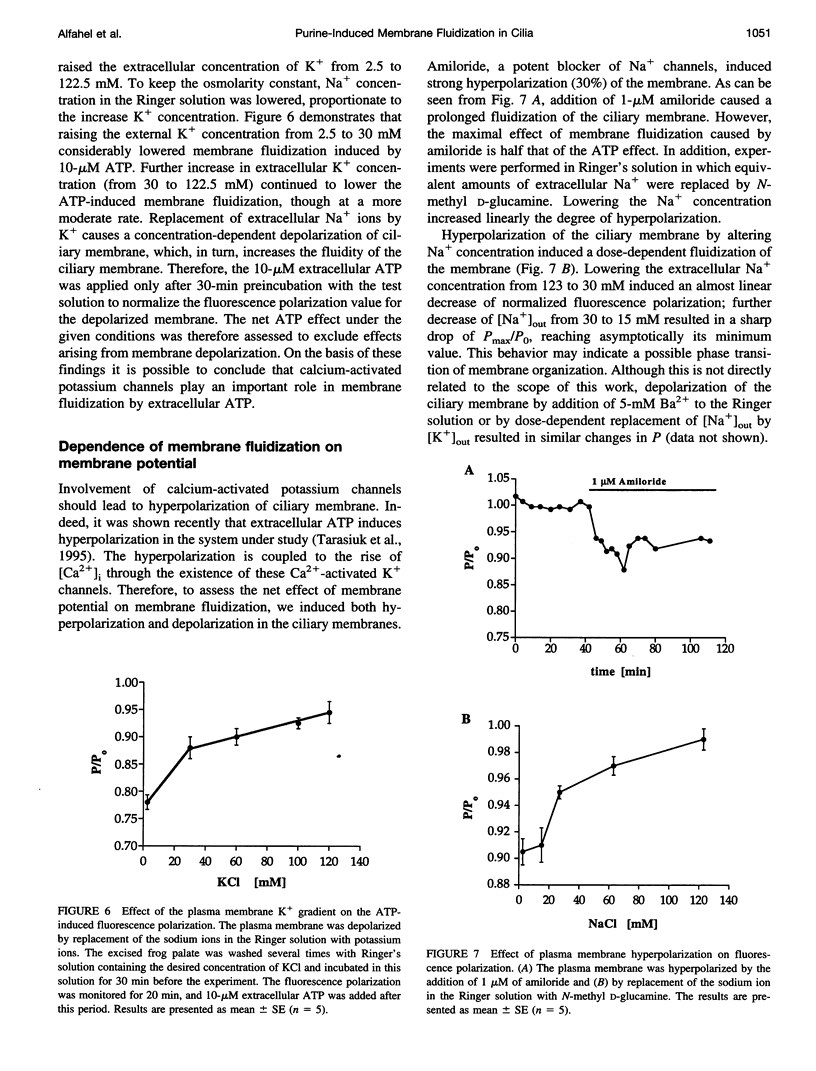
To examine the role of membrane dynamics in transmembrane signal transduction, we studied changes in membrane fluidity in mucociliary tissues from frog palate and esophagus epithelia stimulated by extracellular ATP. Micromolar concentrations of ATP induced strong changes in fluorescence polarization, possibly indicating membrane fluidization. This effect was dosage dependent, reaching a maximum at 10-microM ATP. It was dependent on the presence of extracellular Ca2+ (or Mg2+), though it was insensitive to inhibitors of voltage-gated calcium channels. It was inhibited by thapsigargin and by ionomycin (at low extracellular Ca2+ concentration), both of which deplete Ca2+ stores. It was inhibited by the calcium-activated potassium channel inhibitors quinidine, charybdotoxin, and apamine and was reduced considerably by replacement of extracellular Na+ with K+. Hyperpolarization, or depolarization, of the mucociliary membrane induced membrane fluidization. The degree of membrane fluidization depended on the degree of hyperpolarization or depolarization of the ciliary membrane potential and was considerably lower than the effect induced by extracellular ATP. These results indicate that appreciable membrane fluidization induced by extracellular ATP depends both on an increase in intracellular Ca2+, mainly from its internal stores, and on hyperpolarization of the membrane. Calcium-dependent potassium channels couple the two effects. In light of recent results on the enhancement of ciliary beat frequency, it would appear that extracellular ATP-induced changes both in ciliary beat frequency and in membrane fluidity are triggered by similar signal transduction pathways.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiello E., Kennedy J., Hernandez C. Stimulation of frog ciliated cells in culture by acetylcholine and substance P. Comp Biochem Physiol C. 1991;99(3):497–506. doi: 10.1016/0742-8413(91)90277-z. [DOI] [PubMed] [Google Scholar]
- Andrews D., Nelson D. L. Biochemical studies of the excitable membrane of Paramecium tetraurelia. II. Phospholipids of ciliary and other membranes. Biochim Biophys Acta. 1979 Jan 19;550(2):174–187. doi: 10.1016/0005-2736(79)90205-0. [DOI] [PubMed] [Google Scholar]
- Andrivon C. Membrane control of ciliary movement in ciliates. Biol Cell. 1988;63(2):133–142. doi: 10.1016/0248-4900(88)90052-4. [DOI] [PubMed] [Google Scholar]
- Bloodgood R. A. Directed movements of ciliary and flagellar membrane components: a review. Biol Cell. 1992;76(3):291–301. doi: 10.1016/0248-4900(92)90431-y. [DOI] [PubMed] [Google Scholar]
- Bonini N. M., Nelson D. L. Differential regulation of Paramecium ciliary motility by cAMP and cGMP. J Cell Biol. 1988 May;106(5):1615–1623. doi: 10.1083/jcb.106.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman D., Gómez-Fernández J. C., Goñi F. M. Intrinsic protein--lipid interactions. Physical and biochemical evidence. FEBS Lett. 1979 Feb 15;98(2):211–223. doi: 10.1016/0014-5793(79)80186-6. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993 Sep;265(3 Pt 1):C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Eckert R. Bioelectric control of ciliary activity. Science. 1972 May 5;176(4034):473–481. doi: 10.1126/science.176.4034.473. [DOI] [PubMed] [Google Scholar]
- Edidin M. Rotational and translational diffusion in membranes. Annu Rev Biophys Bioeng. 1974;3(0):179–201. doi: 10.1146/annurev.bb.03.060174.001143. [DOI] [PubMed] [Google Scholar]
- Eisinger J., Flores J. Front-face fluorometry of liquid samples. Anal Biochem. 1979 Apr 1;94(1):15–21. doi: 10.1016/0003-2697(79)90783-8. [DOI] [PubMed] [Google Scholar]
- Eshel D., Grossman Y., Priel Z. Spectral characterization of ciliary beating: variations of frequency with time. Am J Physiol. 1985 Jul;249(1 Pt 1):C160–C165. doi: 10.1152/ajpcell.1985.249.1.C160. [DOI] [PubMed] [Google Scholar]
- Fuchs P., Parola A., Robbins P. W., Blout E. R. Fluorescence polarization and viscosities of membrane lipids of 3T3 cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3351–3354. doi: 10.1073/pnas.72.9.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheber L., Priel Z., Aflalo C., Shoshan-Barmatz V. Extracellular ATP binding proteins as potential receptors in mucociliary epithelium: characterization using [32P]3'-O-(4-benzoyl)benzoyl ATP, a photoaffinity label. J Membr Biol. 1995 Sep;147(1):83–93. doi: 10.1007/BF00235399. [DOI] [PubMed] [Google Scholar]
- Gheber L., Priel Z. Metachronal activity of cultured mucociliary epithelium under normal and stimulated conditions. Cell Motil Cytoskeleton. 1994;28(4):333–345. doi: 10.1002/cm.970280407. [DOI] [PubMed] [Google Scholar]
- Gratton E., Jameson D. M., Hall R. D. Multifrequency phase and modulation fluorometry. Annu Rev Biophys Bioeng. 1984;13:105–124. doi: 10.1146/annurev.bb.13.060184.000541. [DOI] [PubMed] [Google Scholar]
- Jean T., Klee C. B. Calcium modulation of inositol 1,4,5-trisphosphate-induced calcium release from neuroblastoma x glioma hybrid (NG108-15) microsomes. J Biol Chem. 1986 Dec 15;261(35):16414–16420. [PubMed] [Google Scholar]
- Kennedy J. R., Ranyard J. R. Morphology and quantitation of ciliated outgrowths from cultured rabbit tracheal explants. Eur J Cell Biol. 1983 Jan;29(2):200–208. [PubMed] [Google Scholar]
- Korngreen A., Priel Z. Simultaneous measurement of ciliary beating and intracellular calcium. Biophys J. 1994 Jul;67(1):377–380. doi: 10.1016/S0006-3495(94)80492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton J., Westlin M., Hanley M. R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991 Sep 15;266(26):17067–17071. [PubMed] [Google Scholar]
- Naito Y., Kaneko H. Reactivated triton-extracted models o paramecium: modification of ciliary movement by calcium ions. Science. 1972 May 5;176(4034):523–524. doi: 10.1126/science.176.4034.523. [DOI] [PubMed] [Google Scholar]
- Nathan I., Fleischer G., Livne A., Dvilansky A., Parola A. H. Membrane microenvironmental changes during activation of human blood platelets by thrombin. A study with a fluorescent probe. J Biol Chem. 1979 Oct 10;254(19):9822–9828. [PubMed] [Google Scholar]
- Ovadyahu D., Eshel D., Priel Z. Intensification of ciliary motility by extracellular ATP. Biorheology. 1988;25(3):489–501. doi: 10.3233/bir-1988-25309. [DOI] [PubMed] [Google Scholar]
- Parola A. H., Ibdah M., Gill D., Zaritsky A. Deviation from homeoviscous adaptation in Escherichia coli membranes. Biophys J. 1990 Mar;57(3):621–626. doi: 10.1016/S0006-3495(90)82578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola A. H., Kaplan J. H., Lockwood S. H., Uzgiris E. E. Activation of human lymphocytes by concanavalin A or purified protein derivative results in no alteration of fluorescence polarization of lipid probes although the electrophoretic mobility of the cells is changed. Biochim Biophys Acta. 1981 Dec 21;649(3):616–624. doi: 10.1016/0005-2736(81)90166-8. [DOI] [PubMed] [Google Scholar]
- Rimon G., Hanski E., Braun S., Levitzki A. Mode of coupling between hormone receptors and adenylate cyclase elucidated by modulation of membrane fluidity. Nature. 1978 Nov 23;276(5686):394–396. doi: 10.1038/276394a0. [DOI] [PubMed] [Google Scholar]
- Sanderson M. J., Charles A. C., Dirksen E. R. Mechanical stimulation and intercellular communication increases intracellular Ca2+ in epithelial cells. Cell Regul. 1990 Jul;1(8):585–596. doi: 10.1091/mbc.1.8.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J. E., Klumpp S., Benz R., Schürhoff-Goeters W. J., Schmid A. Regulation of adenylyl cyclase from Paramecium by an intrinsic potassium conductance. Science. 1992 Jan 31;255(5044):600–603. doi: 10.1126/science.1371017. [DOI] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta. 1978 Dec 15;515(4):367–394. doi: 10.1016/0304-4157(78)90010-2. [DOI] [PubMed] [Google Scholar]
- Sleigh M. A., Blake J. R., Liron N. The propulsion of mucus by cilia. Am Rev Respir Dis. 1988 Mar;137(3):726–741. doi: 10.1164/ajrccm/137.3.726. [DOI] [PubMed] [Google Scholar]
- Stubbs C. D., Smith A. D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984 Jan 27;779(1):89–137. doi: 10.1016/0304-4157(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Tarasiuk A., Bar-Shimon M., Gheber L., Korngreen A., Grossman Y., Priel Z. Extracellular ATP induces hyperpolarization and motility stimulation of ciliary cells. Biophys J. 1995 Mar;68(3):1163–1169. doi: 10.1016/S0006-3495(95)80292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G. A., Jr, Nozawa Y. Tetrahymena: a system for studying dynamic membrane alterations within the eukaryotic cell. Biochim Biophys Acta. 1977 May 31;472(1):55–92. doi: 10.1016/0304-4157(77)90014-4. [DOI] [PubMed] [Google Scholar]
- Toplak H., Batchiulis V., Hermetter A., Hunziker T., Honegger U. E., Wiesmann U. N. Effects of culture and incubation conditions on membrane fluidity in monolayers of cultured cells measured as fluorescence anisotropy using trimethylammoniumdiphenylhexatriene (TMA-DPH). Biochim Biophys Acta. 1990 Sep 21;1028(1):67–72. doi: 10.1016/0005-2736(90)90266-q. [DOI] [PubMed] [Google Scholar]
- VORHAUS E. F., DEYRUP I. J. The effect of adenosinetriphosphate on the cilia of the pharyngeal mucosa of the frog. Science. 1953 Nov 6;118(3071):553–554. doi: 10.1126/science.118.3071.553. [DOI] [PubMed] [Google Scholar]
- Villalón M., Hinds T. R., Verdugo P. Stimulus-response coupling in mammalian ciliated cells. Demonstration of two mechanisms of control for cytosolic [Ca2+]. Biophys J. 1989 Dec;56(6):1255–1258. doi: 10.1016/S0006-3495(89)82772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T., Gheber L., Shoshan-Barmatz V., Priel Z. Possible mechanism of ciliary stimulation by extracellular ATP: involvement of calcium-dependent potassium channels and exogenous Ca2+. J Membr Biol. 1992 May;127(3):185–193. doi: 10.1007/BF00231506. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. Electrolyte transport by airway epithelia. Physiol Rev. 1987 Oct;67(4):1143–1184. doi: 10.1152/physrev.1987.67.4.1143. [DOI] [PubMed] [Google Scholar]
- Wong L. B., Yeates D. B. Luminal purinergic regulatory mechanisms of tracheal ciliary beat frequency. Am J Respir Cell Mol Biol. 1992 Oct;7(4):447–454. doi: 10.1165/ajrcmb/7.4.447. [DOI] [PubMed] [Google Scholar]
- el-Moatassim C., Dornand J., Mani J. C. Extracellular ATP and cell signalling. Biochim Biophys Acta. 1992 Feb 19;1134(1):31–45. doi: 10.1016/0167-4889(92)90025-7. [DOI] [PubMed] [Google Scholar]


