Abstract
Scientific interest to find a treatment for spinal cord injuries has led to the development of numerous experimental strategies to promote axonal regeneration across the spinal cord injury site. Although these strategies have been developed in acute injury paradigms and hold promise for individuals with spinal cord injuries in the future, little is known about their applicability for the vast majority of paralyzed individuals whose injury occurred long ago and who are considered to have a chronic injury. Some studies have shown that the effectiveness of these approaches diminishes dramatically within weeks after injury. Here we investigated the regenerative capacity of rat rubrospinal neurons whose axons were cut in the cervical spinal cord 1 year before. Contrary to earlier reports, we found that rubrospinal neurons do not die after axotomy but, rather, they undergo massive atrophy that can be reversed by applying brain-derived neurotrophic factor to the cell bodies in the midbrain. This administration of neurotrophic factor to the cell body resulted in increased expression of growth-associated protein-43 and Tα1 tubulin, genes thought to be related to axonal regeneration. This treatment promoted the regeneration of these chronically injured rubrospinal axons into peripheral nerve transplants engrafted at the spinal cord injury site. This outcome is a demonstration of the regenerative capacity of spinal cord projection neurons a full year after axotomy.
A variety of experimental strategies are emerging to promote regeneration of the injured spinal cord. These include the application of neurotrophic factors and the transplantation of a variety of cellular substrates such as peripheral nerve, Schwann cells, olfactory ensheathing glial cells, stem cells, fetal tissue, and cell lines genetically augmented to secrete trophic factors. Other strategies target the glial scar, the myelin sheath, or myelin-associated molecules that apparently inhibit axonal regeneration [reviewed by Ramer et al. (1)].
Although the effectiveness of these techniques has been demonstrated in the acute setting, less is known about their applicability in the chronic state. As the postinjury interval increases, some studies have observed a decline in the regenerative ability of central nervous system neurons. For example, although axonal regeneration from supraspinal neurons occurred in peripheral nerve transplants engrafted acutely after cervical spinal cord injury (2), the regeneration of such neurons was absent when the transplantation was delayed by 4 weeks after injury (3). Strategies such as neurotrophic factors and cellular substrates may extend the time window for successful intervention. For example, coerulospinal axons regenerated in response to nerve growth factor-secreting fibroblasts implanted 3 months after the initial injury (4), and brainstem axons regenerated through neurotrophin-3 (NT-3)- or brain-derived neurotrophic factor (BDNF)-supplemented fetal spinal cord transplants inserted 2 and 4 weeks after injury (5). The effectiveness of such regeneration strategies, however, has also been shown to diminish over time (6, 7), in some cases within weeks (8). For example, BDNF-secreting fibroblasts, when implanted acutely into the spinal cord injury site, promoted rubrospinal axonal regeneration through the graft and into the distal spinal cord, and facilitated some functional improvement (9). However, when these fibroblasts were implanted 5 and 6 weeks after injury, the regeneration of serotonergic and rubrospinal axons was less than that demonstrated in the acute treatment paradigm, and extension through the graft and back into the host was no longer observed (10, 11).
The demonstrated decline in the regenerative ability of central nervous system neurons with time may be attributable to events that occur at the cell body level after axotomy. After cervical axotomy, rubrospinal neurons atrophy significantly and approximately 25–40% are reported to die within 4 to 8 weeks (12, 13). Neurotrophic factors may be partially effective in preventing some of this cell loss. For example, ciliary neurotrophic factor (CNTF) prevented some loss of rubrospinal neurons when applied to a refreshed spinal cord lesion site 4 and 8 weeks after injury, but this efficacy was not observed when the application was delayed for 14 and 22 weeks (12). Similarly, we have observed that the delayed application of BDNF at the spinal cord injury site of chronically axotomized rubrospinal neurons is ineffective at rescuing these cells. However, we have reported that, when applied to the cell bodies of rubrospinal neurons acutely after injury, BDNF prevents their atrophy, stimulates their expression of growth associated protein-43 (GAP-43) and Tα1 tubulin, and promotes their regeneration into peripheral nerve transplants (14).
The purpose of this study was to investigate the effectiveness of BDNF application to the cell bodies when initiated as late as 1 year after cervical spinal cord injury. Our results unexpectedly show that, after BDNF infusion, the number of rubrospinal neurons on the injured side is the same as that of the control red nucleus contralaterally, indicating that these neurons do not die after axotomy but rather enter a state of significant atrophy from which they can be rescued. Subsequently, this cell-body treatment with BDNF caused an increase in the expression of regeneration-associated genes thought to be related to axonal regeneration, and the promotion of rubrospinal axonal regeneration even 1 year after injury.
Materials and Methods
Male Sprague–Dawley rats (Charles River Breeding Laboratories), aged 2.5–3.0 months and weighing 200–250 g were used in this study. Eleven animals were evaluated for cell counts, cell size, and regeneration-associated gene expression (by in situ hybridization), and 20 animals were evaluated for the axonal regeneration experiments. All animal procedures were performed in accordance with the guidelines of the Canadian Council for Animal Care and approved by our institution's animal care committee.
Chronic Injury Model and Minipump Implantation for BDNF Administration.
Under ketamine/xylazine anesthesia, 11 rats underwent a transection of the dorsolateral funiculus at the level of the fourth cervical vertebra (C4), unilaterally severing the rubrospinal tract. The lesion was made with a pair of fine iris scissors and then expanded with gentle aspiration to create a rostral-caudal cavity of ≈2–3 mm. The animals were then housed for 6 or 12 months in large, custom-made multitiered cages (approximately 30 × 60 × 50 cm) about which they could move freely. After this period, the infusion cannula of an osmotic minipump was introduced intracranially into the vicinity of the rubrospinal neurons. A 28-gauge cannula connected by silastic tubing to an osmotic minipump (Alzet no. 2001, 1 μl/h; Alzet, Palo Alto, CA) was inserted stereotactically 6.3 mm posterior to bregma, 1.7 mm to the right of midline, and 6.5 mm deep to the dural membrane. This intraparenchymal infusion system and its diffusion characteristics have been described in detail by our laboratory in work on acutely injured rats (14). As before, animals were excluded from further analysis if the cannula placement was determined on subsequent histologic sections to be within 500 μm of the red nucleus (thus avoiding the local cellular inflammatory reaction observed around the cannula). Five animals received 0.5 μg⋅μl−1⋅h−1 of BDNF (gift from Regeneron Pharmaceuticals, Tarrytown, NY) in a vehicle solution of 20 mM sterile PBS, 100 units of Penicillin/Streptomycin, and 0.5% rat serum albumin (Sigma–Aldrich; no. A-6272). Six animals received the vehicle solution alone. Seven days later, the animals received an overdose of chloral hydrate and were perfused transcardially with PBS followed by 4% paraformaldehyde.
NeuN Immunohistochemistry.
Immunohistochemistry with the neuronal marker NeuN was used to increase our ability to detect small neurons that might have been missed with the standard cresyl violet staining (Fig. 1). Cryostat sections of 20-μm thickness were washed in 0.01 M PBS, and incubated overnight with 1:100 NeuN primary antibody (Chemicon). This procedure was followed with 5% goat blocking serum and 1:100 secondary goat anti-mouse antibody conjugated to Alexa 488 (Molecular Probes). Cross-reactivity with astrocytes and microglial cells was ruled out with primary antibodies to GFAP (Dakopatts, Carpinteria, CA; Z334) and isolectin B4 (Sigma–Aldrich; L2140), respectively, with 1:200 secondary antibody conjugated with ExtraAvidin Cy3 (Sigma–Aldrich; E-4142) (Fig. 2).
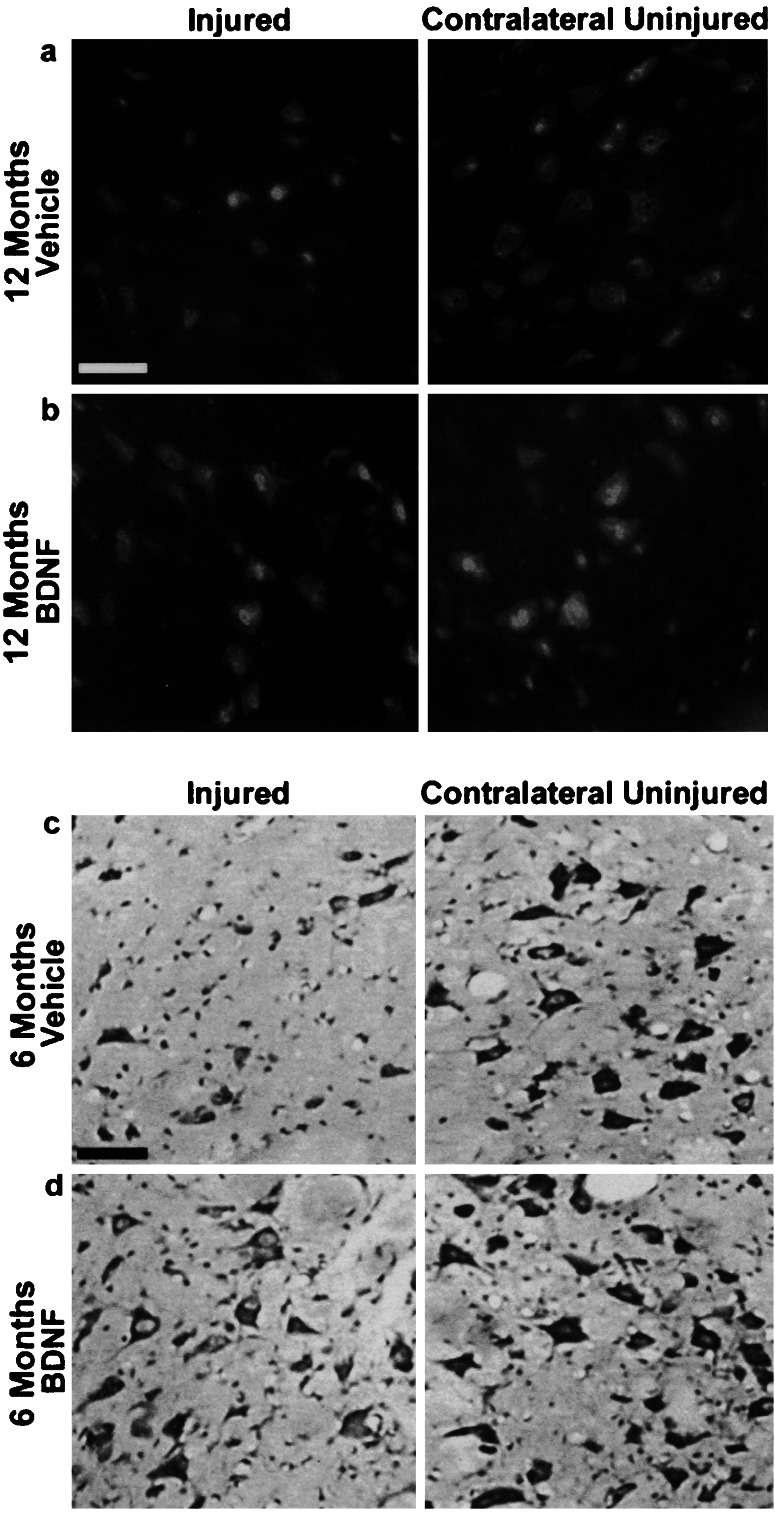
Figure 1.
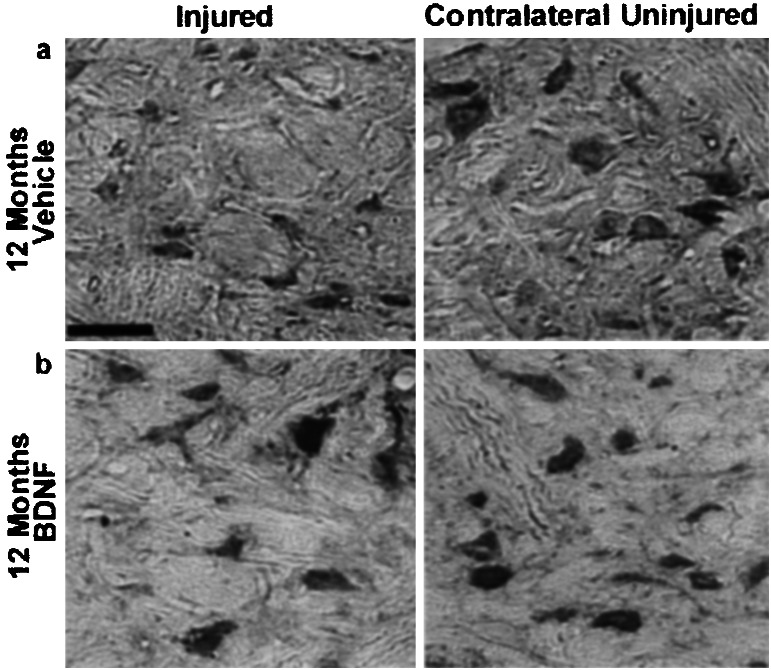
Atrophy of rubrospinal neurons can be reversed by BDNF 6 and 12 months after injury. NeuN immunohistochemistry (a, b) (animals injured 12 months before) and cresol violet staining (c, d) (animals injured 6 months before) both demonstrate numerous atrophic neurons on the injured side treated with vehicle alone. Note the recovery in cell size of the BDNF-treated injured neurons to almost normal size as seen on the contralateral side in both NeuN (b) and cresyl violet staining (d). All sections are taken from comparable areas of the rubrospinal nucleus, approximately 240 μm from the caudal pole. (Bar = 50 μm.)
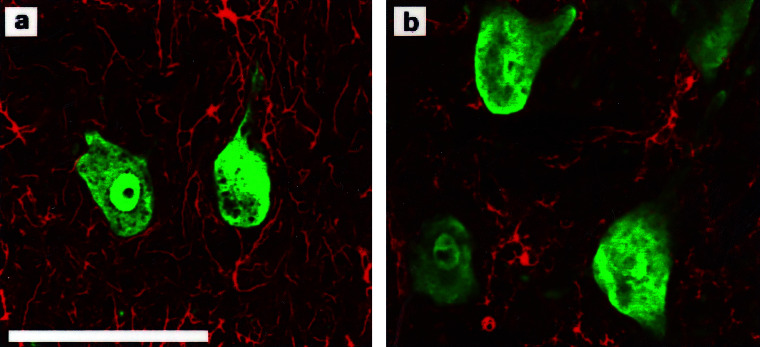
Figure 2.
NeuN immunostaining specifically labels neurons and does not label astrocytes or microglia. (a) NeuN (green) and GFAP (red) immunohistochemistry for neurons and astrocytes, respectively, shows no overlap of labeling. (b) NeuN (green) and isolectin B4 (red) immunohistochemistry for neurons and microglia, respectively, shows no overlap of labeling. (Bar = 50 μm.)
Tyrosine Kinase B Receptor Immunohistochemistry.
Because the rubrospinal cell bodies were the target of our neurotrophic treatment, immunohistochemistry was performed to determine whether the cell bodies expressed full-length tyrosine kinase B (trkB) receptors, which would presumably make them responsive to BDNF. Cryostat sections of 20-μm thickness were incubated overnight with 1:100 rabbit polyclonal anti-trkB (794) antibody (Santa Cruz Biotechnology; sc-12), which binds the carboxy-terminal end of the intracellular tyrosine kinase domain, making it specific for the full-length trkB receptor isoform (15). This procedure was followed by 5% donkey blocking serum, incubation with 1:200 biotinylated donkey anti-rabbit secondary antibody (Jackson ImmunoResearch), and subsequent development with the ABC kit (Elite ABC Kit, Vector Laboratories).
Physical Disector Counting Method.
The principles of stereologic counting methods have been reviewed elsewhere (16). In brief, 20-μm-thick sections of the caudal, magnocellular portion of the red nucleus (approximately 480 μm) were digitized, and adjacent sections were superimposed. Only the neurons in the “sampling section” which disappeared in the adjacent “lookup” section were scored, effectively counting only the tops of the cells and therefore making the analysis irrespective of cell size. On cresyl violet staining, neurons were distinguished from glia by their morphology, staining pattern, and, to some extent, by their size. Glial cells were characterized by their oval shape, homogeneously dark nuclear staining, and consistently small diameter (≈5–8 μm). Neurons were characterized by a stellate shape, a more heterogeneous cytoplasmic staining pattern (Nissl substance), and a dark nucleolus within a lightly stained nucleus. In the sampling section and lookup sections we did, in fact, count cells as neurons if they were large and possessed a stellate shape and a darkly stained cytoplasm, even if their nucleus and nucleolus were not clearly distinguishable (although in most cases they were). The diameter of the smallest cells counted as neurons was approximately 10 μm.
In Situ Hybridization.
The Tα1 tubulin probe was a 50-mer oligonucleotide complementary to the 3′-untranslated sequence of Tα1 tubulin 5′-AAACCCATCAGTGAAGTGGACGGCTCGGG-TCTCTGACAAATCATTCA-3. The GAP-43 probe was complementary to bases 200–270 (17). Probes were end-labeled with 35S-ATP by using deoxynucleotide terminal transferase according to a standard protocol (18). Perfusion-fixed sections of 20-μm thickness were hybridized to 106 cpm of probe for 16–18 h at 43°C [for details, see Giehl and Tetzlaff (19)]. The slides were dipped in Kodak NTB-2 emulsion and exposed for 7 days for GAP-43 and 2 days for Tα1 tubulin. Sense-control probes showed no specific binding (data not shown).
Peripheral Nerve Transplant Paradigm.
Under ketamine/xylazine anesthesia, 20 rats received a 100-nl microinjection of 1% fast blue (Sigma–Aldrich) at C7/8 to label retrogradely the rubrospinal neurons (see Fig. 6a). A week later, the left dorsolateral funiculus was cut at C4 in the same fashion as before to sever the rubrospinal tract. The animals were again housed in large multitiered cages for 1 year. One year after axotomy (n = 18) and 18 months after axotomy (n = 2), 200 nl of FluoroGold (Fluorochrome, Englewood, CO) was injected at C8-T1 to ensure that no sparing had occurred, and the right sciatic nerve was cut proximally to generate a predegenerated graft. A week later, the C4 lesion was refreshed by extending it rostrally 2 mm, and a 30- to 35-mm segment of the predegenerated sciatic nerve was engrafted and secured to the dura with 10–0 Prolene sutures (Ethicon, Sommervile, NJ). The osmotic minipump was inserted stereotactically as before, and 14 animals received 0.5 μg⋅μl−1⋅h−1 pegylated-BDNF (provided by Q. Yan, Amgen, under a Materials Transfer Agreement) plus vehicle solution, and six control animals received vehicle alone. To label retrogradely neurons whose axons regenerated to the end of the graft, we applied the carbocyanine dye, DiI (Molecular Probes) or biotin dextran amine (BDA; Molecular Probes) to the free tip of the graft. All peripheral nerve transplants were left in place for 2 months. Two months after transplantation, the animals were killed and the 4% paraformaldehyde-fixed midbrains were processed for histology according to standard protocols.
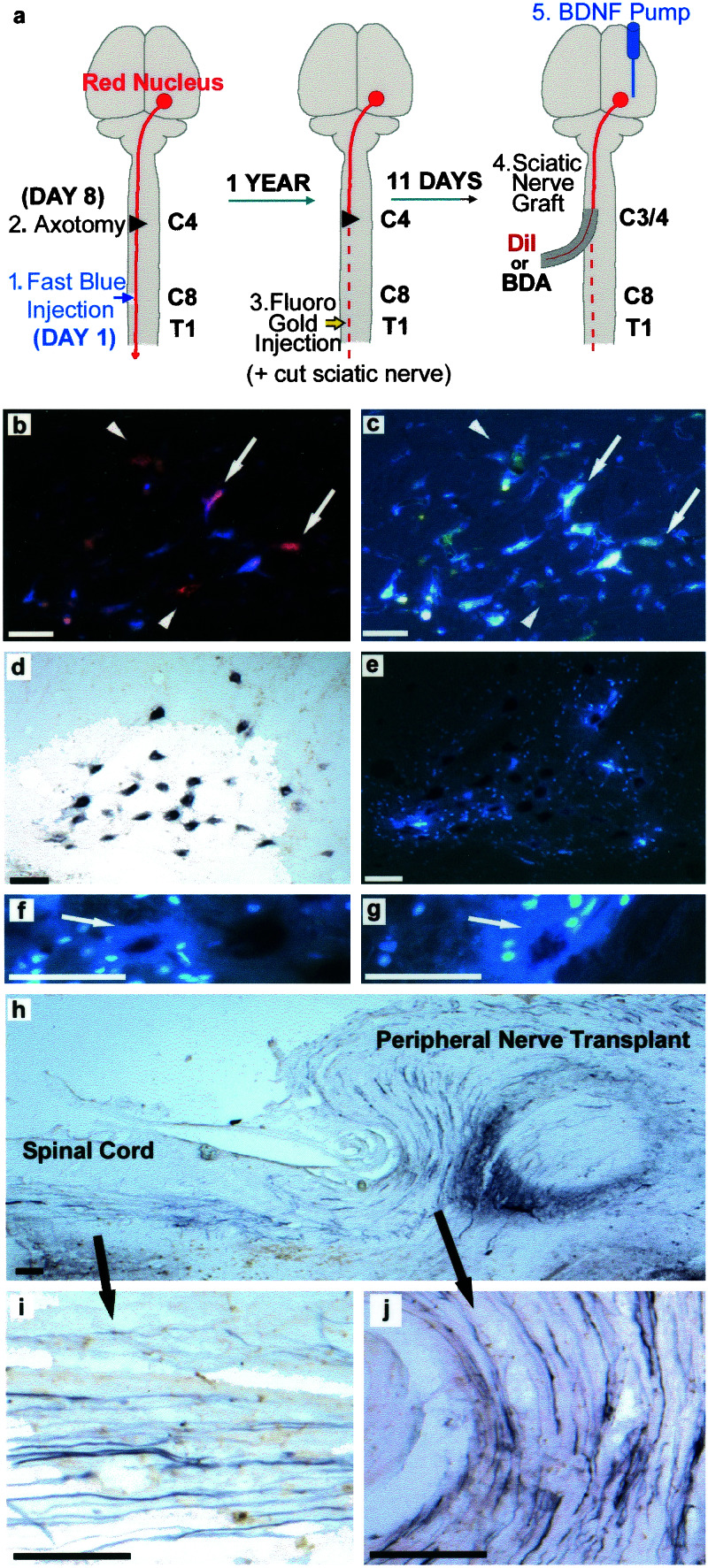
Figure 6.
Regeneration of rubrospinal neurons 12 months after cervical spinal cord injury. (a) Schematic of transplantation procedures. (b) Labeling with DiI and fast blue shows single-labeled cells (arrow heads) and double-labeled cells (arrows). (c) Fast blue labeling of the same section as in b. (d) BDA labeling of regenerating rubrospinal neurons. (e) Same section as in d, demonstrating quenching/washout of the fast blue label caused by the BDA procedure. With BDA staining, double-labeled neurons (arrows) were still visible by an obvious halo of fast blue, with examples in f and g. Surrounding glial cells take up the washed-out fast blue (arrowhead). (h–j) Sagittal sections at the interface between host spinal cord and peripheral nerve graft demonstrate large BDA-labeled axons (arrows) in the position of the rubrospinal tract, which is consistent with the robust regenerative response in this BDNF-treated animal. (Bar = 50 μm.)
Statistics.
Cell counts, cell surface area measurements, and numbers of labeled neurons between and within BDNF- and vehicle-treated groups were analyzed by using either standard or paired t tests. Statistical analysis was performed with Microsoft excel.
Results
Cell Counts and Cross-Sectional Area.
For each animal, the number of neurons on the side of the injured red nucleus was counted and compared with the number of neurons found on the contralateral uninjured red nucleus; the number of injured neurons is therefore represented as a percentage of the presumably normal number of neurons counted on the uninjured side (Fig. 3a). In the BDNF-treated animals, by using NeuN immunohistochemistry, we counted similar numbers of neurons (101.8 ± 1.3%, P = 0.11) in the injured red nucleus compared with the uninjured red nucleus. Similarly, in the vehicle-treated animals, equal numbers of neurons were counted between injured and uninjured red nuclei (99.5 ± 4.2%, P = 0.47) by using the NeuN marker. With cresyl violet staining, in the BDNF-treated animals we again counted similar numbers of neurons in the injured red nucleus compared with the uninjured red nucleus (106.8 ± 18.5%, P = 0.42). For the vehicle-treated animals we counted 89.0 ± 7.6% as many neurons in the injured red nucleus as in the uninjured red nucleus (P = 0.10). These neuronal counts of BDNF- and vehicle-treated animals performed with cresyl violet staining were not significantly different (P = 0.18). Blinded analysis of the sections demonstrated several atrophic NeuN-positive neurons in the vehicle-treated group that did not stain effectively with cresyl violet, which suggests that atrophic, chronically injured neurons are difficult to identify and/or distinguish from the surrounding glial cells with the standard cresyl violet staining until the application of BDNF restored their cell size.
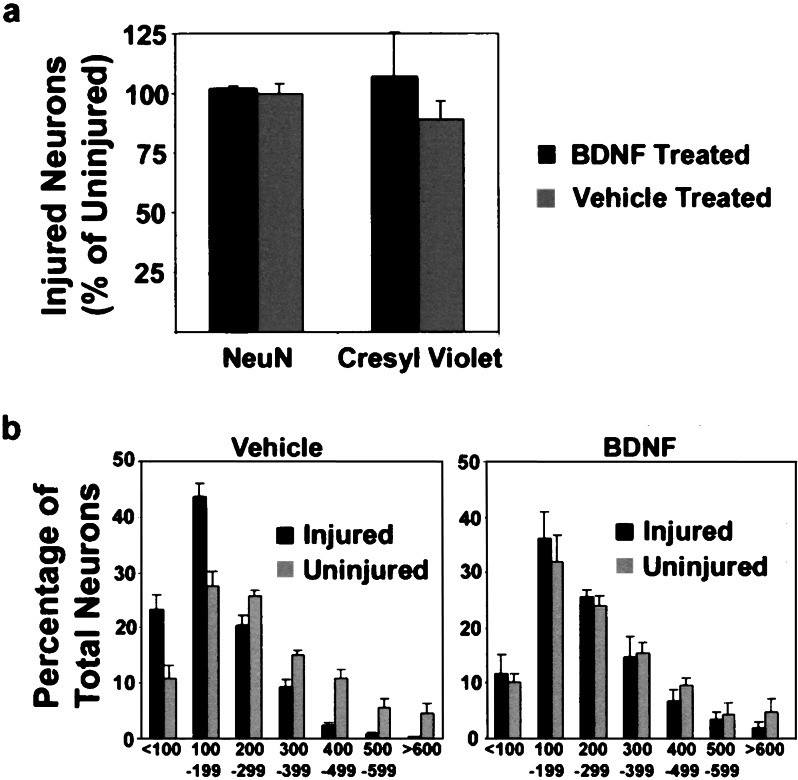
Figure 3.
Neuronal counts and cell cross-sectional area measurements. (a) The number of injured neurons is represented as a percentage of the number of contralateral uninjured neurons. Note that with BDNF treatment, the number of injured neurons counted is approximately 100% of the uninjured, in both NeuN and cresyl violet staining. With cresyl violet staining only, 89% of the number of uninjured neurons was detected in the vehicle treatment group. For the counts of uninjured neurons, 100% was 870 ± 68 neurons. (b) Histogram of cross-sectional area plotting neurons in 100-μm2 increments demonstrates a normalization of the distribution of cell sizes with the BDNF treatment. Note the predominance of small neurons in the vehicle-treated group. (Bar = 50 μm.)
The cross-sectional area of the rubrospinal neurons on cresyl violet staining was then measured in a blinded fashion (Fig. 3b). In control animals, significant atrophy of the injured neurons occurred compared with the contralateral uninjured neurons (178.6 ± 5.8 μm2 vs. 277.0 ± 23.2 μm2, P < 0.001). The mean cross-sectional area of injured neurons in BDNF-treated animals was significantly larger than the injured neurons of the control, vehicle-treated animals (240.0 ± 26.8 μm2 vs. 178.6 ± 5.8 μm2, P = 0.009), which represented a near complete restoration in cell size when compared with the contralateral uninjured neurons (240.0 ± 26.8 μm2 vs. 266.6 ± 30.7 μm2, P = 0.074). No significant difference in size occurred between uninjured neurons of the control and BDNF-treated animals (P = 0.395).
Expression of Regeneration-Associated Genes.
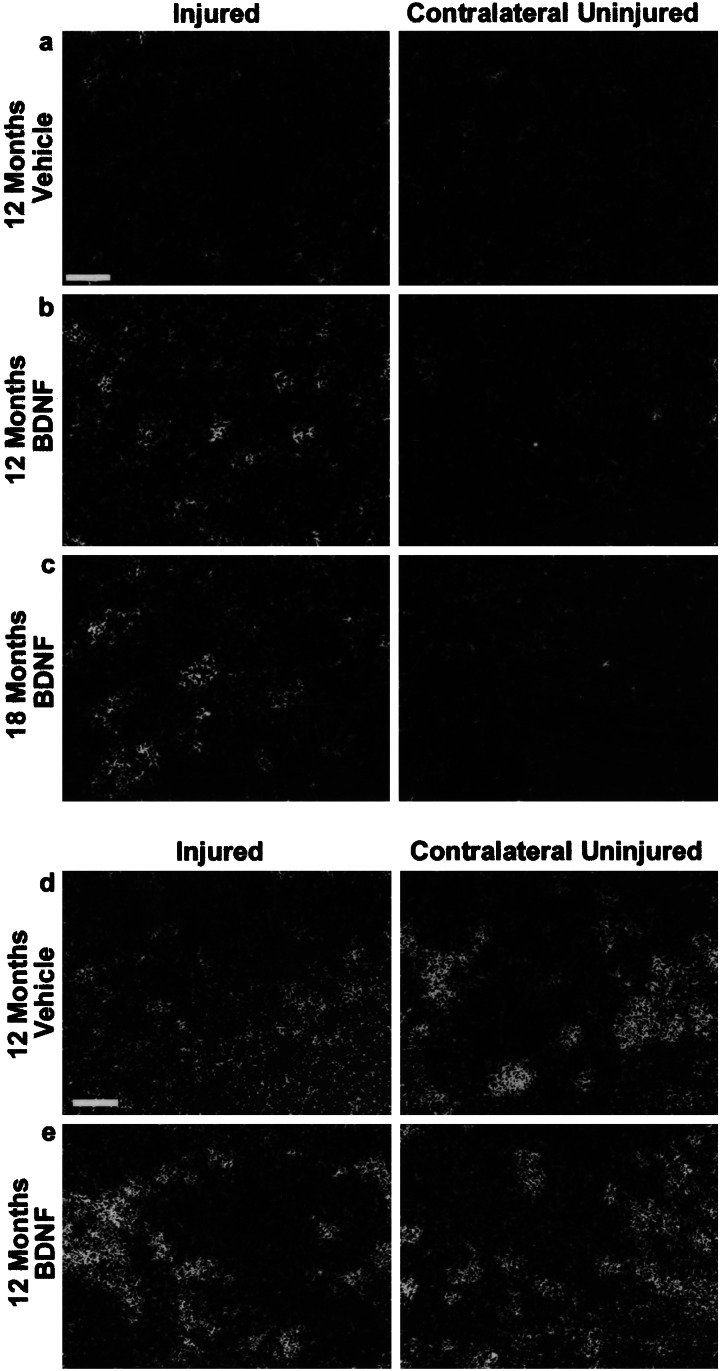
In situ hybridization was performed to determine the effect of BDNF treatment on the expression of GAP-43 and Tα1 tubulin. Twelve months after axotomy the in situ hybridization signal for GAP-43 and Tα1 tubulin mRNA in vehicle-treated animals was found to be low (Fig. 4 a and d). The BDNF infusion initiated 12 months after injury induced high levels of GAP-43 expression (Fig. 4b) and restored Tα1 tubulin expression to levels similar to that of the contralateral uninjured nucleus (Fig. 4d). For GAP-43, we were able to elicit this effect as late as 18 months after axotomy (Fig. 4c). BDNF application to uninjured rubrospinal neurons did not induce GAP-43 expression (data not shown).
Figure 4.
In situ hybridization signals for GAP-43 and Tα1 tubulin mRNA. (a) At 12 months after injury, the GAP-43 signal in the injured vehicle-treated neurons is similar to background levels on the contralateral uninjured side. (b) The GAP-43 signal is greatly enhanced by BDNF treatment initiated 12 months after initial injury, an effect which is still achievable even 18 months after the injury (c). (d) Tα1 tubulin signal is low in injured neurons 12 months after axotomy, but is restored by BDNF treatment (e). (Bar = 50 μm.)
TrkB Immunohistochemistry.
Immunohistochemistry for the full-length isoform of trkB receptors in vehicle-treated animals demonstrated that 1 year after axotomy, these atrophic neurons still demonstrate trkB receptor immunoreactivity in their cell bodies (Fig. 5a). Similarly, full-length trkB receptors were localized to the cell bodies of BDNF-treated rubrospinal neurons (Fig. 5b). Negative control slides were performed with secondary antibody only and in all cases demonstrated no nonspecific staining (data not shown).
Figure 5.
Full-length trkB receptor immunohistochemistry. (a) Axotomized rubrospinal neurons treated with vehicle solution only are very atrophic, 12 months after injury, yet maintain immunoreactivity for full-length trkB. (b) Axotomized rubrospinal neurons treated with BDNF 12 months after injury also maintain full-length trkB expression. Note the hypertrophy of some of the BDNF-treated rubrospinal neurons. (Bar = 50 μm.)
Regeneration into Peripheral Nerve Transplant.
The regenerative capacity of these neurons was tested 1 year after injury by using the peripheral nerve transplant paradigm (2, 3, 20). FluoroGold was injected distal to the lesion site (C8-T1) 1 year after injury. (This retrograde tracer would have entered spared rubrospinal axons en passage and then would be transported back to the cell body.) In all animals, FluoroGold was not observed in the red nucleus, confirming the completeness of the axotomy. By using serial sections to avoid double counting, the number of labeled neurons was counted, again by an observer blinded to treatment (Fig. 6 b–g). In the vehicle-treated animals (n = 6), the mean number of single-labeled (BDA or DiI positive) and double-labeled (BDA plus fast blue or DiI plus fast blue) neurons per nucleus was 22.2 ± 6.6 and 11.0 ± 4.4, respectively. In the BDNF-treated animals (n = 14), a significantly greater number of single (71.1 ± 18.8)- and double (28.6 ± 8.3)-labeled neurons (P < 0.05 for both) was counted.
Discussion
These findings demonstrate the regenerative capacity of rubrospinal neurons a full year after axotomy, and substantially extends the potential window of opportunity for therapeutic intervention reported in other studies of chronic injury. Our observation of neuronal size and number restoration is somewhat at odds with other authors who have reported the death of a significant proportion of rubrospinal neurons after axotomy. The response of rubrospinal neurons to axotomy is an issue that has been examined extensively, in rats predominantly (12, 21), but also in mice (22) and opposums (23). Although neuronal atrophy seems to occur universally after axotomy, different rates of cell loss have been reported between and among species (12, 21, 22). Determining whether these represent true intra- and interspecies differences is made difficult by variations in histologic technique (e.g., retrograde tracing of neurons vs. cresyl violet staining) and counting methods (e.g., stereologic vs. nonstereologic counts). Our findings are based on the application of stereologic counting methods combined with the use of a neuronal specific marker (NeuN) to assist in the identification of atrophic neurons. Although we recognize that significant controversy surrounds the subject of counting techniques (24), we felt that, in the face of such significant neuronal atrophy, a stereologic counting method (such as the disector method) in which cell counts independent of cell size are produced, was an appropriate choice. And although our NeuN immunohistochemistry proved useful for identifying atrophic neurons, perhaps the more striking observations were made on routine cresyl violet staining, where equal cell numbers were counted in injured and uninjured red nuclei after the BDNF-induced restoration in neuronal size. This restoration of cell size facilitated the identification of previously atrophic axotomized neurons, which, in studies that have reported significant cell death after injury, could have shrunk below the threshold of detection and thus be lost.
The GAP-43 and Tα1 tubulin expression observed in the BDNF-treated animals represents merely two members of an ensemble of regeneration-associated genes that is thought to be required for axonal growth. The prerequisite rather than associative role of multiple genes acting in concert was recently illustrated in vitro by the promotion of axonal outgrowth with the combined overexpression of GAP-43 and CAP-23 in contrast to the negligible regeneration observed when each gene was overexpressed alone (25).
In our regeneration studies, we were somewhat surprised to observe the presence of double-labeled rubrospinal neurons in our control, vehicle-treated animals, in light of the finding by Ye and Houle (26), who demonstrated that these neurons essentially cease to regenerate 1 month after injury. Clearly, our model of vehicle or BDNF delivery by direct infusion into the brain parenchyma represents a local “injury.” To minimize the effect of this local injury on the rubrospinal neurons, animals were excluded if the cannula was found to be placed within 500 μm of the red nucleus, as we had done in other work (14). Although we have not yet undertaken a comprehensive characterization of this local injury response, it is conceptually possible that cytokines generated in the inflammatory reaction to the cannula may have had a direct or indirect trophic and/or tropic effect on the rubrospinal neurons (hence, the small number of double-labeled neurons observed in the vehicle-treated group). In any event, with the low number (on average, fewer than a dozen) of double-labeled neurons in our vehicle-treated animals, we share a similarly pessimistic view of the regenerative competence of untreated, chronically injured rubrospinal neurons as Houle and colleagues.
The effectiveness of BDNF factor administration at the cell body versus the axon may be explained by differences in the expression of the trkB receptor through which BDNF acts. Here we have demonstrated in both vehicle- and BDNF-treated animals that rubrospinal neurons retain immunoreactivity for full-length trkB receptors at the level of the cell bodies, 12 months after axotomy. Liebl and colleagues also showed in a rat contusion model that the expression of full-length trkB receptors at the cell bodies of the rubrospinal neurons remained intact 42 days after injury, but it was absent at the injury site and replaced by truncated trkB receptors (27). Similarly, Lu and colleagues demonstrated the persistent trkB receptor immunoreactivity on the cell bodies of corticospinal neurons after axotomy, but the absence of these receptors on the projecting axons (28). These observations support the rationale of applying neurotrophins to the cell body rather than to the injured axon in the chronic state. The administration of neurotrophic factors to promote axonal regeneration after spinal cord injury will likely achieve higher levels of sophistication with the further advances in the use of viral vectors (29) or cell lines genetically altered to secrete neurotrophic factors (9, 30), potentially combining the temporal and quantitative control of such gene expression that has been demonstrated with tetracycline- (31) or tamoxifen-regulated systems (32).
Although we observed, in our current study, a statistically significant increase in the regeneration of chronically injured (double-labeled) neurons, the number of neurons was still quite small (28.6 ± 8.3) and only ≈3.3% of the total number of neurons counted (870 ± 68). It is difficult to know how much functional recovery could be achieved with such regeneration, although it should be noted that numerous authors have demonstrated that substantial neurologic function after incomplete spinal cord injury can be mediated by a small fraction (ranging from 1.4 to 12%) of the total number of axons in the normal cord (33–35). Clearly, further study into this important issue of functional recovery in the chronic injury setting is warranted.
Finally, our experimental paradigm was designed to ask a basic question: are rubrospinal neurons capable of responding to BDNF applied to the cell body long after they have been axotomized? With the restoration in cell size we found that they were not undergoing massive cell death after atrophy, and that indeed the BDNF could induce them to enter a “regeneration-capable” state. Further study is necessary to determine whether such cell-body treatment to enhance the neurons' regenerative competence can effect functional recovery by promoting localized sprouting and/or long-distance axonal regeneration around or through the chronically established injury site. Such treatment would likely require a combinatorial approach with a cellular bridging substrate and/or strategies to overcome the inhibitory epitopes within the myelin and glial scar. Like all promising experimental therapies being developed in animal models of spinal cord injury, the applicability of our current findings to the human population is uncertain, because it depends on neurophysiologic responses to injury and to treatment being similar between animals and humans. Nonetheless, if neuronal atrophy and the failure to express the appropriate genes to mount a regenerative response are factors that contribute to the permanence of neurologic disability in humans with chronic spinal cord injuries, then the findings of this study do provide some encouragement for sufferers of this devastating condition. Conceptually, our work suggests that patients with chronic spinal cord injuries might benefit from therapeutic strategies that combine a restoration in regenerative competence at the cell-body level with other approaches that are being developed in the acute spinal cord injury context.
Acknowledgments
We acknowledge the support of the Canadian Institutes for Health Research, the British Columbia Neurotrauma Initiative, the NeuroScience Canada Foundation, and the National Science and Engineering Research Council. B.K.K. is the Gowan and Michele Guest NeuroScience Canada Foundation/Canadian Institutes for Health Research Research Fellow. W.T. is the Rick Hansen Man in Motion Chair in Spinal Cord Research at the University of British Columbia.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- TrkB
tyrosine kinase B
- GAP-43
growth associated protein-43
- NT-3
neurotrophin-3
- CNTF
ciliary neurotrophic factor
- BDA
biotin dextran amine
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Ramer M S, Harper G P, Bradbury E J. Spinal Cord. 2000;38:449–472. doi: 10.1038/sj.sc.3101055. [DOI] [PubMed] [Google Scholar]
- 2.Richardson P M, Issa V M, Aguayo A J. J Neurocytol. 1984;13:165–182. doi: 10.1007/BF01148324. [DOI] [PubMed] [Google Scholar]
- 3.Houle J D. Exp Neurol. 1991;113:1–9. doi: 10.1016/0014-4886(91)90139-4. [DOI] [PubMed] [Google Scholar]
- 4.Grill R J, Blesch A, Tuszynski M H. Exp Neurol. 1997;148:444–452. doi: 10.1006/exnr.1997.6704. [DOI] [PubMed] [Google Scholar]
- 5.Coumans J V, Lin T T, Dai H N, MacArthur L, McAtee M, Nash C, Bregman B S. J Neurosci. 2001;21:9334–9344. doi: 10.1523/JNEUROSCI.21-23-09334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Meyenburg J, Brosamle C, Metz G A, Schwab M E. Exp Neurol. 1998;154:583–594. doi: 10.1006/exnr.1998.6912. [DOI] [PubMed] [Google Scholar]
- 7.Houle J D, Ye J H. NeuroReport. 1997;8:751–755. doi: 10.1097/00001756-199702100-00034. [DOI] [PubMed] [Google Scholar]
- 8.Decherchi P, Gauthier P. Neuroscience. 2000;101:197–210. doi: 10.1016/s0306-4522(00)00343-2. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Kim D, Himes B T, Chow S Y, Schallert T, Murray M, Tessler A, Fischer I. J Neurosci. 1999;19:4370–4387. doi: 10.1523/JNEUROSCI.19-11-04370.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin Y, Tessler A, Fischer I, Houle J D. Neurorehabil Neural Repair. 2000;14:311–317. doi: 10.1177/154596830001400407. [DOI] [PubMed] [Google Scholar]
- 11.Tobias C A, Shibata M, Shumsky J S, Tuszynski M H, Fischer I, Tessler A, Murray M. Soc Neurosci Abstr. 2001;369:8. [Google Scholar]
- 12.Houle J D, Ye J H. Neuroscience. 1999;94:929–936. doi: 10.1016/s0306-4522(99)00359-0. [DOI] [PubMed] [Google Scholar]
- 13.Mori F, Himes B T, Kowada M, Murray M, Tessler A. Exp Neurol. 1997;143:45–60. doi: 10.1006/exnr.1996.6318. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi N R, Fan D P, Giehl K M, Bedard A M, Wiegand S J, Tetzlaff W. J Neurosci. 1997;17:9583–9595. doi: 10.1523/JNEUROSCI.17-24-09583.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasika S, Alvarez-Buylla A, Nottebohm F. Neuron. 1999;22:53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 16.West M J. Trends Neurosci. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- 17.Basi G S, Jacobson R D, Virag I, Schilling J, Skene J H. Cell. 1987;49:785–791. doi: 10.1016/0092-8674(87)90616-7. [DOI] [PubMed] [Google Scholar]
- 18.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith K A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1987. [Google Scholar]
- 19.Giehl K M, Tetzlaff W. Eur J Neurosci. 1996;8:1167–1175. doi: 10.1111/j.1460-9568.1996.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 20.Fernandes K J, Fan D P, Tsui B J, Cassar S L, Tetzlaff W. J Comp Neurol. 1999;414:495–510. doi: 10.1002/(sici)1096-9861(19991129)414:4<495::aid-cne6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 21.Novikova L N, Novikov L N, Kellerth J O. Eur J Neurosci. 2000;12:776–780. doi: 10.1046/j.1460-9568.2000.00978.x. [DOI] [PubMed] [Google Scholar]
- 22.Zhou L, Connors T, Chen D F, Murray M, Tessler A, Kambin P, Saavedra R A. NeuroReport. 1999;10:3417–3421. doi: 10.1097/00001756-199911080-00029. [DOI] [PubMed] [Google Scholar]
- 23.Xu X M, Martin G F. Exp Neurol. 1990;108:46–54. doi: 10.1016/0014-4886(90)90006-e. [DOI] [PubMed] [Google Scholar]
- 24.Benes F M, Lange N. Trends Neurosci. 2001;24:11–17. doi: 10.1016/s0166-2236(00)01660-x. [DOI] [PubMed] [Google Scholar]
- 25.Bomze H M, Bulsara K R, Iskandar B J, Caroni P, Pate Skene J H. Nat Neurosci. 2001;4:38–43. doi: 10.1038/82881. [DOI] [PubMed] [Google Scholar]
- 26.Ye J H, Houle J D. Exp Neurol. 1997;143:70–81. doi: 10.1006/exnr.1996.6353. [DOI] [PubMed] [Google Scholar]
- 27.Liebl D J, Huang W, Young Y, Parada L F. Exp Neurol. 2001;167:15–26. doi: 10.1006/exnr.2000.7548. [DOI] [PubMed] [Google Scholar]
- 28.Lu P, Blesch A, Tuszynski M H. J Comp Neurol. 2001;436:456–470. doi: 10.1002/cne.1080. [DOI] [PubMed] [Google Scholar]
- 29.Huber A B, Ehrengruber M U, Schwab M E, Brosamle C. Eur J Neurosci. 2000;12:3437–3442. doi: 10.1046/j.1460-9568.2000.00255.x. [DOI] [PubMed] [Google Scholar]
- 30.Tuszynski M H, Peterson D A, Ray J, Baird A, Nakahara Y, Gage F H. Exp Neurol. 1994;126:1–14. doi: 10.1006/exnr.1994.1037. [DOI] [PubMed] [Google Scholar]
- 31.Blesch A, Conner J M, Tuszynski M H. Gene Ther. 2001;8:954–960. doi: 10.1038/sj.gt.3301480. [DOI] [PubMed] [Google Scholar]
- 32.Vallier L, Mancip J, Markossian S, Lukaszewicz A, Dehay C, Metzger D, Chambon P, Samarut J, Savatier P. Proc Natl Acad Sci USA. 2001;98:2467–2472. doi: 10.1073/pnas.041617198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fehlings M G, Tator C H. Exp Neurol. 1995;132:220–228. doi: 10.1016/0014-4886(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 34.Eidelberg E, Straehley D, Erspamer R, Watkins C J. Exp Neurol. 1977;56:312–322. doi: 10.1016/0014-4886(77)90350-8. [DOI] [PubMed] [Google Scholar]
- 35.Blight A R. Neuroscience. 1983;10:521–543. doi: 10.1016/0306-4522(83)90150-1. [DOI] [PubMed] [Google Scholar]