Abstract
The vibronic theory of activation and quantum chemical intermediate neglect of differential overlap (INDO) calculations are used to study the activation of carbon monoxide (change of the C-O bond index and force field constant) by the imidazole complex with heme in dependence on the distortion of the porphyrin ring, geometry of the CO coordination, iron-carbon and iron-imidazole distances, iron displacement out of the porphyrin plane, and presence of the charged groups in the heme environment. It is shown that the main contribution to the CO activation stems from the change in the sigma donation from the 5 sigma CO orbital to iron, and back-bonding from the iron to the 2 pi orbital of CO. It follows from the results that none of the studied distortions can explain, by itself, the wide variation of the C-O vibrational frequency in the experimentally studied model compounds and heme proteins. To study the dependence of the properties of the FeCO unit on the presence of charged groups in the heme environment, the latter are simulated by the homogeneous electric field and point charges of different magnitude and location. The results show that charged groups can strongly affect the strength of the C-O bond and its vibrational frequency. It is found that the charges located on the distal side of the heme plane can affect the Fe-C and C-O bond indexes (and, consequently, the Fe-C and C-O vibrational frequencies), both in the same and in opposite directions, depending on their position. The theoretical results allow us to understand the peculiarities of the effect of charged groups on the properties of the FeCO unit both in heme proteins and in their model compounds.
Full text
PDF


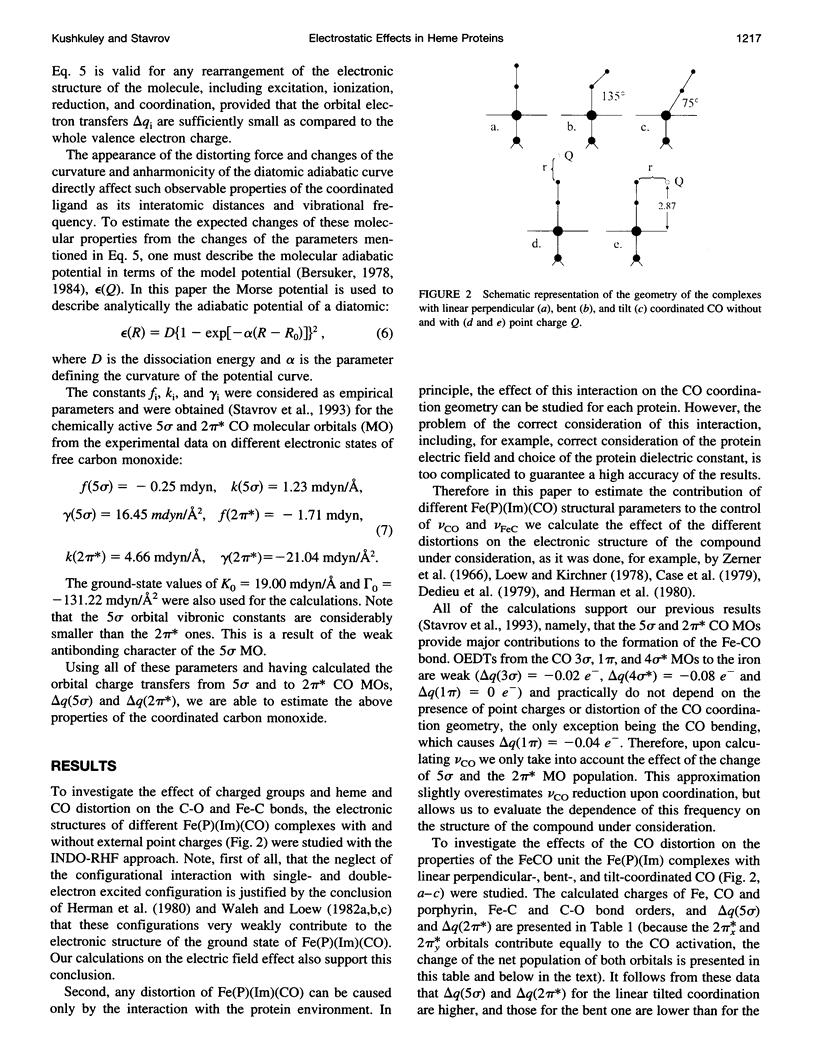
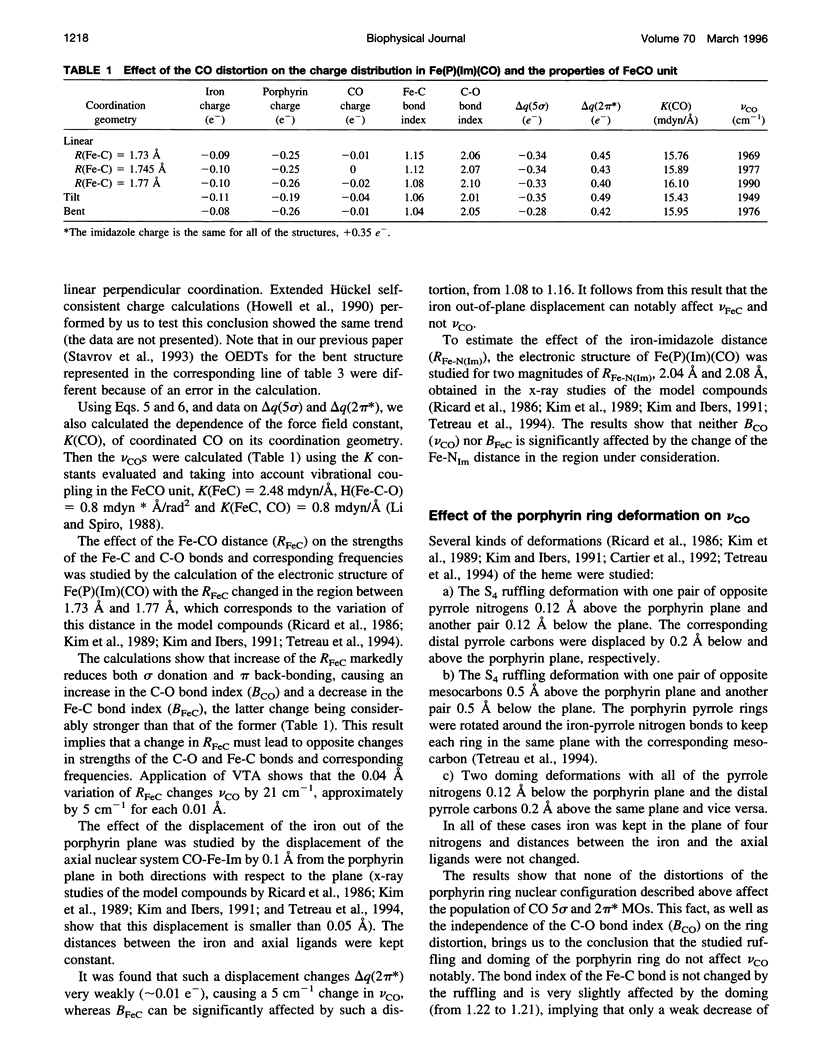
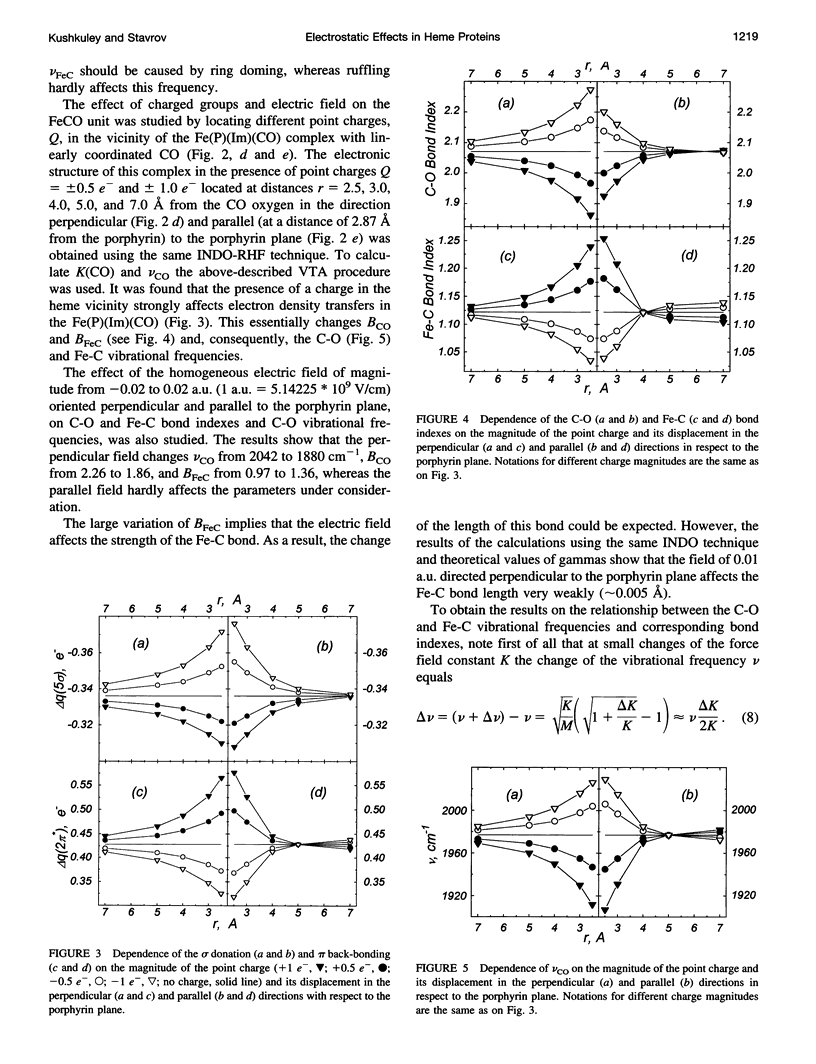








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alben J. O., Beece D., Bowne S. F., Doster W., Eisenstein L., Frauenfelder H., Good D., McDonald J. D., Marden M. C., Moh P. P. Infrared spectroscopy of photodissociated carboxymyoglobin at low temperatures. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3744–3748. doi: 10.1073/pnas.79.12.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S., Lambright D. G., Boxer S. G. Perturbations of the distal heme pocket in human myoglobin mutants probed by infrared spectroscopy of bound CO: correlation with ligand binding kinetics. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4718–4722. doi: 10.1073/pnas.90.10.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantley R. E., Jr, Smerdon S. J., Wilkinson A. J., Singleton E. W., Olson J. S. The mechanism of autooxidation of myoglobin. J Biol Chem. 1993 Apr 5;268(10):6995–7010. [PubMed] [Google Scholar]
- Braunstein D. P., Chu K., Egeberg K. D., Frauenfelder H., Mourant J. R., Nienhaus G. U., Ormos P., Sligar S. G., Springer B. A., Young R. D. Ligand binding to heme proteins: III. FTIR studies of His-E7 and Val-E11 mutants of carbonmonoxymyoglobin. Biophys J. 1993 Dec;65(6):2447–2454. doi: 10.1016/S0006-3495(93)81310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron A. D., Smerdon S. J., Wilkinson A. J., Habash J., Helliwell J. R., Li T., Olson J. S. Distal pocket polarity in ligand binding to myoglobin: deoxy and carbonmonoxy forms of a threonine68(E11) mutant investigated by X-ray crystallography and infrared spectroscopy. Biochemistry. 1993 Dec 7;32(48):13061–13070. doi: 10.1021/bi00211a016. [DOI] [PubMed] [Google Scholar]
- Campbell B. F., Chance M. R., Friedman J. M. Ligand binding channels reflected in the resonance Raman spectra of cryogenically trapped species of myoglobin. J Biol Chem. 1987 Nov 5;262(31):14885–14890. [PubMed] [Google Scholar]
- Cartier C., Momenteau M., Dartyge E., Fontaine A., Tourillon G., Bianconi A., Verdaguer M. X-ray absorption spectroscopy of carbonyl basket handle Fe(II) porphyrins: the distortion of the tetrapyrrolic macrocycle. Biochim Biophys Acta. 1992 Feb 26;1119(2):169–174. doi: 10.1016/0167-4838(92)90387-s. [DOI] [PubMed] [Google Scholar]
- Case D. A., Karplus M. Stereochemistry of carbon monoxide binding to myoglobin and hemoglobin. J Mol Biol. 1978 Aug 25;123(4):697–701. doi: 10.1016/0022-2836(78)90214-0. [DOI] [PubMed] [Google Scholar]
- Cheng X. D., Schoenborn B. P. Neutron diffraction study of carbonmonoxymyoglobin. J Mol Biol. 1991 Jul 20;220(2):381–399. doi: 10.1016/0022-2836(91)90020-7. [DOI] [PubMed] [Google Scholar]
- Decatur S. M., Boxer S. G. A test of the role of electrostatic interactions in determining the CO stretch frequency in carbonmonoxymyoglobin. Biochem Biophys Res Commun. 1995 Jul 6;212(1):159–164. doi: 10.1006/bbrc.1995.1950. [DOI] [PubMed] [Google Scholar]
- Du P., Loew G. H. Theoretical study of model compound I complexes of horseradish peroxidase and catalase. Biophys J. 1995 Jan;68(1):69–80. doi: 10.1016/S0006-3495(95)80160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchsman W. H., Appleby C. A. CO and O2 complexes of soybean leghemoglobins: pH effects upon infrared and visible spectra. Comparisons with CO and O2 complexes of myoglobin and hemoglobin. Biochemistry. 1979 Apr 3;18(7):1309–1321. doi: 10.1021/bi00574a030. [DOI] [PubMed] [Google Scholar]
- Hanson J. C., Schoenborn B. P. Real space refinement of neutron diffraction data from sperm whale carbonmonoxymyoglobin. J Mol Biol. 1981 Nov 25;153(1):117–146. doi: 10.1016/0022-2836(81)90530-1. [DOI] [PubMed] [Google Scholar]
- Jewsbury P., Kitagawa T. Distal residue-CO interaction in carbonmonoxy myoglobins: a molecular dynamics study of three distal mutants. Biophys J. 1995 Apr;68(4):1283–1294. doi: 10.1016/S0006-3495(95)80302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewsbury P., Kitagawa T. The distal residue-CO interaction in carbonmonoxy myoglobins: a molecular dynamics study of two distal histidine tautomers. Biophys J. 1994 Dec;67(6):2236–2250. doi: 10.1016/S0006-3495(94)80708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr E. A., Mackin H. C., Yu N. T. Resonance Raman studies of carbon monoxide binding to iron "picket fence" porphyrin with unhindered and hindered axial bases. An inverse relationship between binding affinity and the strength of iron-carbon bond. Biochemistry. 1983 Sep 13;22(19):4373–4379. doi: 10.1021/bi00288a005. [DOI] [PubMed] [Google Scholar]
- Kuriyan J., Wilz S., Karplus M., Petsko G. A. X-ray structure and refinement of carbon-monoxy (Fe II)-myoglobin at 1.5 A resolution. J Mol Biol. 1986 Nov 5;192(1):133–154. doi: 10.1016/0022-2836(86)90470-5. [DOI] [PubMed] [Google Scholar]
- Li T., Quillin M. L., Phillips G. N., Jr, Olson J. S. Structural determinants of the stretching frequency of CO bound to myoglobin. Biochemistry. 1994 Feb 15;33(6):1433–1446. doi: 10.1021/bi00172a021. [DOI] [PubMed] [Google Scholar]
- Lian T., Locke B., Kitagawa T., Nagai M., Hochstrasser R. M. Determination of Fe-CO geometry in the subunits of carbonmonoxy hemoglobin M Boston using femtosecond infrared spectroscopy. Biochemistry. 1993 Jun 8;32(22):5809–5814. doi: 10.1021/bi00073a013. [DOI] [PubMed] [Google Scholar]
- Lim M., Jackson T. A., Anfinrud P. A. Binding of CO to myoglobin from a heme pocket docking site to form nearly linear Fe-C-O. Science. 1995 Aug 18;269(5226):962–966. doi: 10.1126/science.7638619. [DOI] [PubMed] [Google Scholar]
- Makinen M. W., Houtchens R. A., Caughey W. S. Structure of carboxymyoglobin in crystals and in solution. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6042–6046. doi: 10.1073/pnas.76.12.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell J. C., Caughey W. S. An infrared study of NO bonding to heme B and hemoglobin A. Evidence for inositol hexaphosphate induced cleavage of proximal histidine to iron bonds. Biochemistry. 1976 Jan 27;15(2):388–396. doi: 10.1021/bi00647a023. [DOI] [PubMed] [Google Scholar]
- Morikis D., Champion P. M., Springer B. A., Sligar S. G. Resonance raman investigations of site-directed mutants of myoglobin: effects of distal histidine replacement. Biochemistry. 1989 May 30;28(11):4791–4800. doi: 10.1021/bi00437a041. [DOI] [PubMed] [Google Scholar]
- Mourant J. R., Braunstein D. P., Chu K., Frauenfelder H., Nienhaus G. U., Ormos P., Young R. D. Ligand binding to heme proteins: II. Transitions in the heme pocket of myoglobin. Biophys J. 1993 Oct;65(4):1496–1507. doi: 10.1016/S0006-3495(93)81218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M., Yoneyama Y., Kitagawa T. Unusual CO bonding geometry in abnormal subunits of hemoglobin M Boston and hemoglobin M Saskatoon. Biochemistry. 1991 Jul 2;30(26):6495–6503. doi: 10.1021/bi00240a021. [DOI] [PubMed] [Google Scholar]
- Park K. D., Guo K. M., Adebodun F., Chiu M. L., Sligar S. G., Oldfield E. Distal and proximal ligand interactions in heme proteins: correlations between C-O and Fe-C vibrational frequencies, oxygen-17 and carbon-13 nuclear magnetic resonance chemical shifts, and oxygen-17 nuclear quadrupole coupling constants in C17O- and 13CO-labeled species. Biochemistry. 1991 Mar 5;30(9):2333–2347. doi: 10.1021/bi00223a007. [DOI] [PubMed] [Google Scholar]
- Paul J., Smith M. L., Paul K. G. The vibrational bands of carbon monoxide bound to hemes or metal surfaces. Biochim Biophys Acta. 1985 Dec 20;832(3):257–264. doi: 10.1016/0167-4838(85)90258-4. [DOI] [PubMed] [Google Scholar]
- Potter W. T., Hazzard J. H., Choc M. G., Tucker M. P., Caughey W. S. Infrared spectra of carbonyl hemoglobins: characterization of dynamic heme pocket conformers. Biochemistry. 1990 Jul 3;29(26):6283–6295. doi: 10.1021/bi00478a025. [DOI] [PubMed] [Google Scholar]
- Quillin M. L., Arduini R. M., Olson J. S., Phillips G. N., Jr High-resolution crystal structures of distal histidine mutants of sperm whale myoglobin. J Mol Biol. 1993 Nov 5;234(1):140–155. doi: 10.1006/jmbi.1993.1569. [DOI] [PubMed] [Google Scholar]
- Quillin M. L., Li T., Olson J. S., Phillips G. N., Jr, Dou Y., Ikeda-Saito M., Regan R., Carlson M., Gibson Q. H., Li H. Structural and functional effects of apolar mutations of the distal valine in myoglobin. J Mol Biol. 1995 Jan 27;245(4):416–436. doi: 10.1006/jmbi.1994.0034. [DOI] [PubMed] [Google Scholar]
- Ramsden J., Spiro T. G. Resonance Raman evidence that distal histidine protonation removes the steric hindrance to upright binding of carbon monoxide by myoglobin. Biochemistry. 1989 Apr 18;28(8):3125–3128. doi: 10.1021/bi00434a001. [DOI] [PubMed] [Google Scholar]
- Sakan Y., Ogura T., Kitagawa T., Fraunfelter F. A., Mattera R., Ikeda-Saito M. Time-resolved resonance Raman study on the binding of carbon monoxide to recombinant human myoglobin and its distal histidine mutants. Biochemistry. 1993 Jun 8;32(22):5815–5824. doi: 10.1021/bi00073a014. [DOI] [PubMed] [Google Scholar]
- Ullrich V. Cytochrome P450 and biological hydroxylation reactions. Top Curr Chem. 1979;83:67–104. doi: 10.1007/BFb0019663. [DOI] [PubMed] [Google Scholar]
- Yu N. T., Kerr E. A., Ward B., Chang C. K. Resonance Raman detection of Fe-CO stretching and Fe-C-O bending vibrations in sterically hindered carbonmonoxy "strapped hemes". A structural probe of Fe-C-O distortion. Biochemistry. 1983 Sep 13;22(19):4534–4540. doi: 10.1021/bi00288a028. [DOI] [PubMed] [Google Scholar]