Abstract
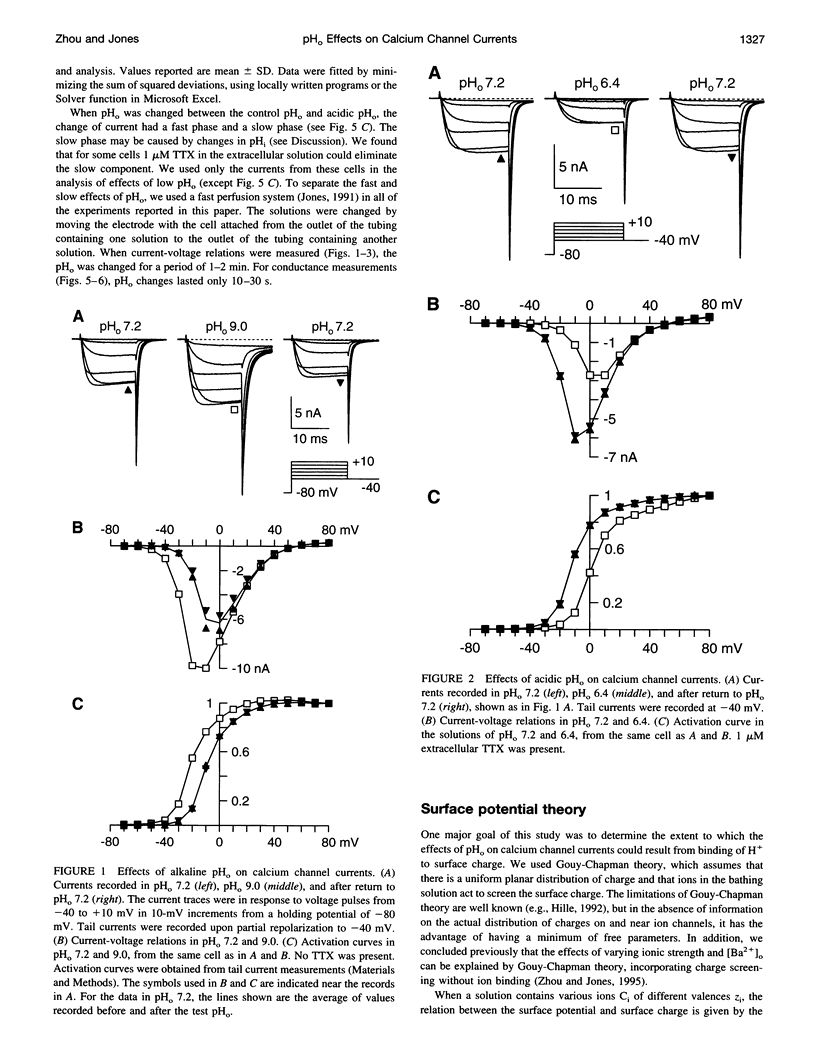
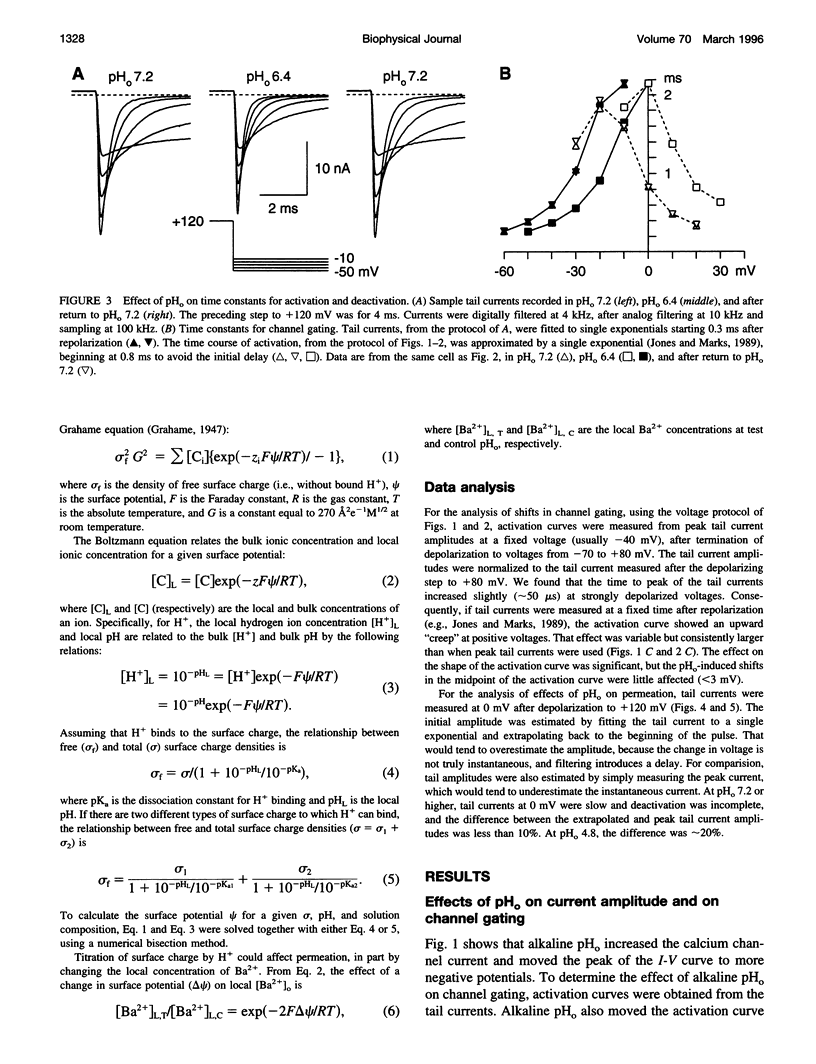
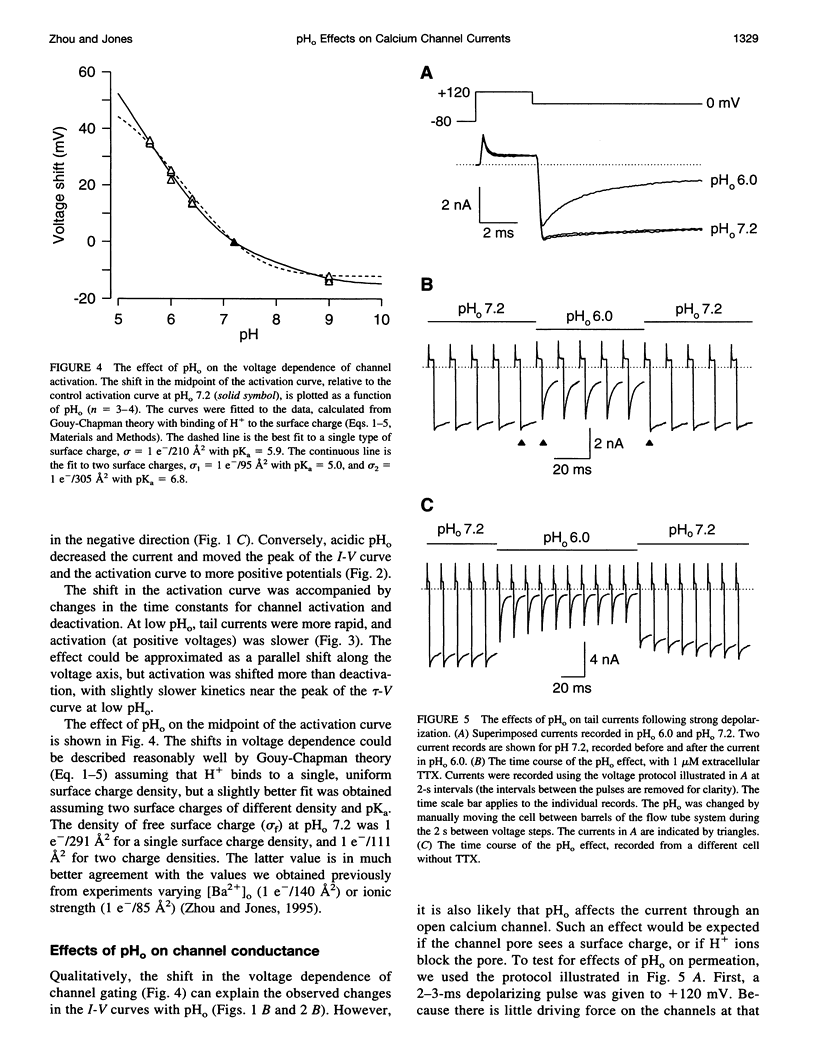
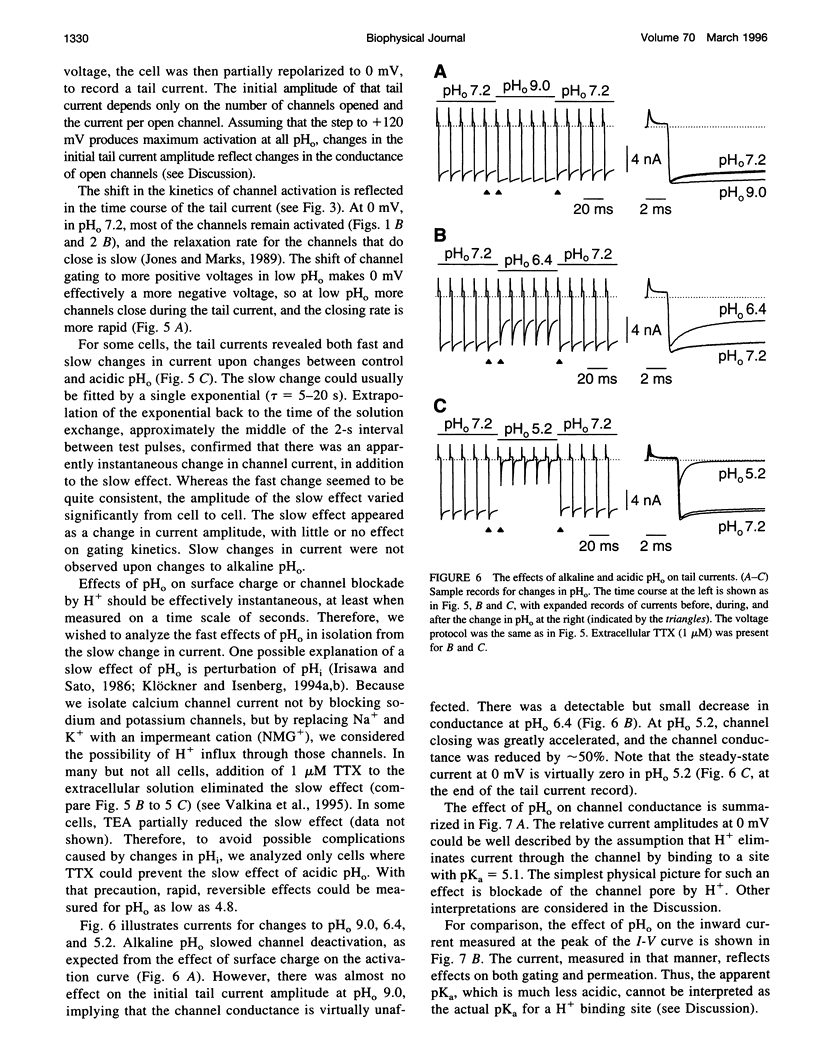
We have investigated the effects of external pH (pHo) on whole-cell calcium channel currents in bullfrog sympathetic neurons. The peak inward current increased at alkaline pHo and decreased at acidic pHo. We used tail currents to distinguish effects of pHo on channel gating and permeation. There were large shifts in the voltage dependence of channel activation (approximately 40 mV between pHo and 9.0 and pHo 5.6), which could be explained by binding of H+ to surface charge according to Gouy-Chapman theory. To examine the effects of pHo on permeation, we measured tail currents at 0 mV, following steps to + 120 mV to maximally activate the channels. Unlike most previous studies, we found only a approximately 10% reduction in channel conductance from pHo 9.0 to pHo 6.4, despite a approximately 25 mV shift of channel activation. At lower pHo the channel conductance did decrease, which could be described by binding of H+ to a site with pKa = 5.1. In some cells, there was a separate slow decrease in conductance at low pHo, possibly because of changes in internal pH. These results suggest that changes in current at pHo > 6.4 result primarily from a shift in the voltage dependence of channel activation. A H(+)-binding site can explain a rapid decrease in channel conductance at lower pHo. The surface charge affecting gating has little effect on the local ion concentration near the pore, or on the channel conductance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989 Jul 13;340(6229):153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- Begenisich T., Danko M. Hydrogen ion block of the sodium pore in squid giant axons. J Gen Physiol. 1983 Nov;82(5):599–618. doi: 10.1085/jgp.82.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T. Magnitude and location of surface charges on Myxicola giant axons. J Gen Physiol. 1975 Jul;66(1):47–65. doi: 10.1085/jgp.66.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt M. R. K+ channels of stomatal guard cells. Characteristics of the inward rectifier and its control by pH. J Gen Physiol. 1992 Apr;99(4):615–644. doi: 10.1085/jgp.99.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumas P., Andersen O. S. Proton block of rat brain sodium channels. Evidence for two proton binding sites and multiple occupancy. J Gen Physiol. 1993 Jan;101(1):27–43. doi: 10.1085/jgp.101.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAME D. C. The electrical double layer and the theory of electrocapillarity. Chem Rev. 1947 Dec;41(3):441–501. doi: 10.1021/cr60130a002. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hille B., Woodhull A. M., Shapiro B. I. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Iijima T., Ciani S., Hagiwara S. Effects of the external pH on Ca channels: experimental studies and theoretical considerations using a two-site, two-ion model. Proc Natl Acad Sci U S A. 1986 Feb;83(3):654–658. doi: 10.1073/pnas.83.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irisawa H., Sato R. Intra- and extracellular actions of proton on the calcium current of isolated guinea pig ventricular cells. Circ Res. 1986 Sep;59(3):348–355. doi: 10.1161/01.res.59.3.348. [DOI] [PubMed] [Google Scholar]
- Jones S. W., Elmslie K. S. Separation and modulation of calcium currents in bullfrog sympathetic neurons. Can J Physiol Pharmacol. 1992;70 (Suppl):S56–S63. doi: 10.1139/y92-244. [DOI] [PubMed] [Google Scholar]
- Jones S. W., Marks T. N. Calcium currents in bullfrog sympathetic neurons. I. Activation kinetics and pharmacology. J Gen Physiol. 1989 Jul;94(1):151–167. doi: 10.1085/jgp.94.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W. Time course of receptor-channel coupling in frog sympathetic neurons. Biophys J. 1991 Aug;60(2):502–507. doi: 10.1016/S0006-3495(91)82077-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Calcium channel current of vascular smooth muscle cells: extracellular protons modulate gating and single channel conductance. J Gen Physiol. 1994 Apr;103(4):665–678. doi: 10.1085/jgp.103.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klöckner U., Isenberg G. Intracellular pH modulates the availability of vascular L-type Ca2+ channels. J Gen Physiol. 1994 Apr;103(4):647–663. doi: 10.1085/jgp.103.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafte D. S., Kass R. S. Hydrogen ion modulation of Ca channel current in cardiac ventricular cells. Evidence for multiple mechanisms. J Gen Physiol. 1988 May;91(5):641–657. doi: 10.1085/jgp.91.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler S. W., Sejnowski T. J. Peptidergic and muscarinic excitation at amphibian sympathetic synapses. J Physiol. 1983 Aug;341:257–278. doi: 10.1113/jphysiol.1983.sp014805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan Y. W., Kass R. S. Interactions between H+ and Ca2+ near cardiac L-type calcium channels: evidence for independent channel-associated binding sites. Biophys J. 1993 Sep;65(3):1188–1195. doi: 10.1016/S0006-3495(93)81152-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R., Latorre R., Miller C. Role of surface electrostatics in the operation of a high-conductance Ca2+-activated K+ channel. Biochemistry. 1989 Oct 3;28(20):8092–8099. doi: 10.1021/bi00446a020. [DOI] [PubMed] [Google Scholar]
- Ohmori H., Yoshii M. Surface potential reflected in both gating and permeation mechanisms of sodium and calcium channels of the tunicate egg cell membrane. J Physiol. 1977 May;267(2):429–463. doi: 10.1113/jphysiol.1977.sp011821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobon D., Prod'hom B., Hess P. Interactions of protons with single open L-type calcium channels. pH dependence of proton-induced current fluctuations with Cs+, K+, and Na+ as permeant ions. J Gen Physiol. 1989 Jul;94(1):1–21. doi: 10.1085/jgp.94.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prod'hom B., Pietrobon D., Hess P. Interactions of protons with single open L-type calcium channels. Location of protonation site and dependence of proton-induced current fluctuations on concentration and species of permeant ion. J Gen Physiol. 1989 Jul;94(1):23–42. doi: 10.1085/jgp.94.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root M. J., MacKinnon R. Two identical noninteracting sites in an ion channel revealed by proton transfer. Science. 1994 Sep 23;265(5180):1852–1856. doi: 10.1126/science.7522344. [DOI] [PubMed] [Google Scholar]
- Tytgat J., Nilius B., Carmeliet E. Modulation of the T-type cardiac Ca channel by changes in proton concentration. J Gen Physiol. 1990 Nov;96(5):973–990. doi: 10.1085/jgp.96.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkina O. N., Vergun O. V., Turovetsky V. B., Khodorov B. I. Changes of cytoplasmic pH in frog nerve fibers during K(+)-induced membrane depolarization. FEBS Lett. 1995 Mar 20;361(2-3):145–148. doi: 10.1016/0014-5793(95)00143-w. [DOI] [PubMed] [Google Scholar]
- West G. A., Leppla D. C., Simard J. M. Effects of external pH on ionic currents in smooth muscle cells from the basilar artery of the guinea pig. Circ Res. 1992 Jul;71(1):201–209. doi: 10.1161/01.res.71.1.201. [DOI] [PubMed] [Google Scholar]
- Wilson D. L., Morimoto K., Tsuda Y., Brown A. M. Interaction between calcium ions and surface charge as it relates to calcium currents. J Membr Biol. 1983;72(1-2):117–130. doi: 10.1007/BF01870319. [DOI] [PubMed] [Google Scholar]
- Yatani A., Brown A. M., Akaike N. Effect of extracellular pH on sodium current in isolated, single rat ventricular cells. J Membr Biol. 1984;78(2):163–168. doi: 10.1007/BF01869203. [DOI] [PubMed] [Google Scholar]
- Zhou W., Jones S. W. Surface charge and calcium channel saturation in bullfrog sympathetic neurons. J Gen Physiol. 1995 Apr;105(4):441–462. doi: 10.1085/jgp.105.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]


