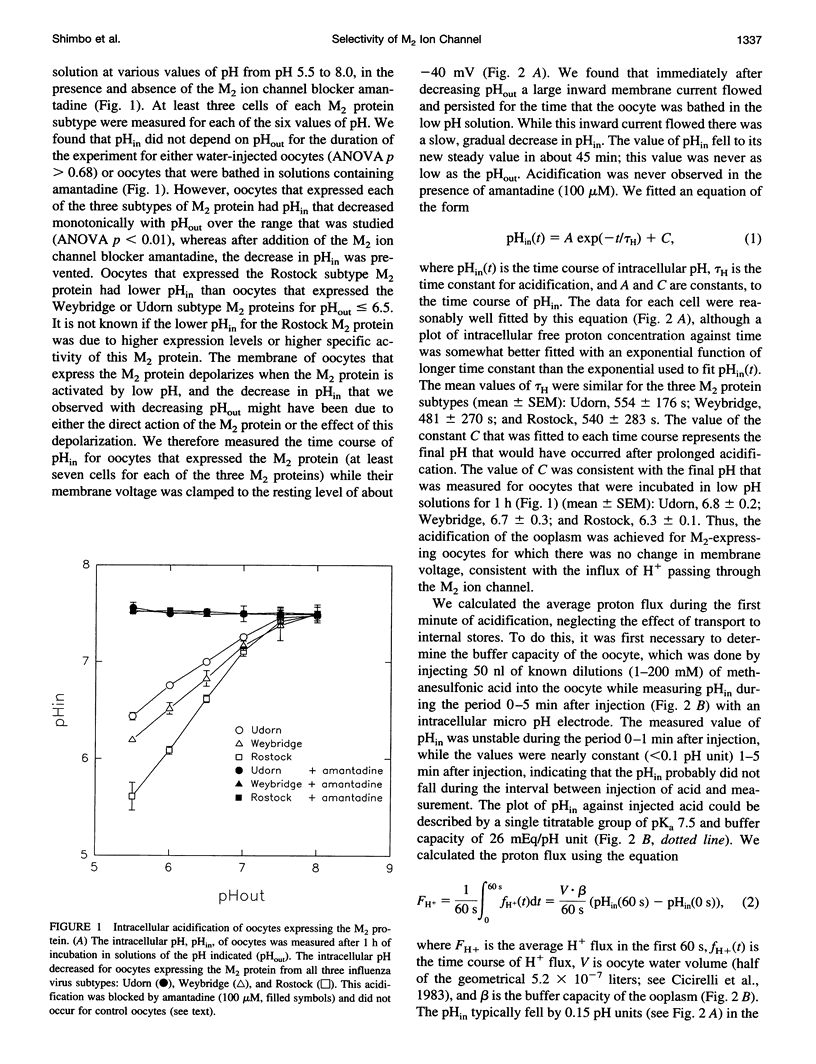
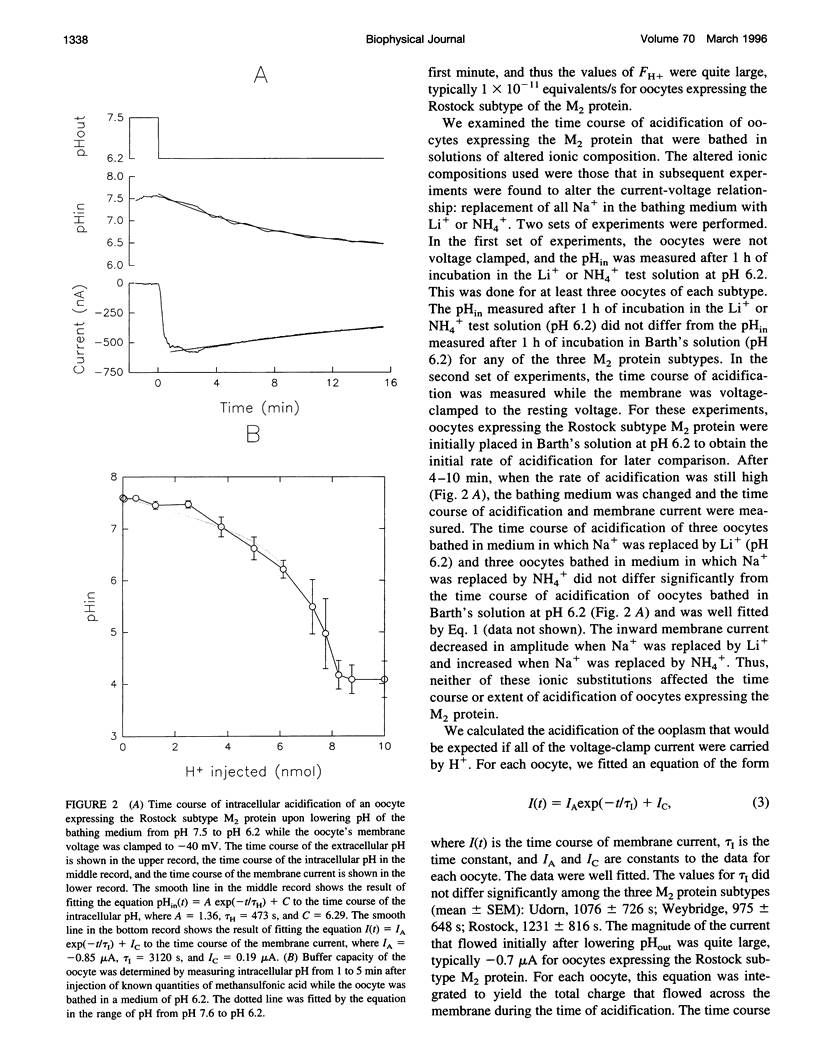
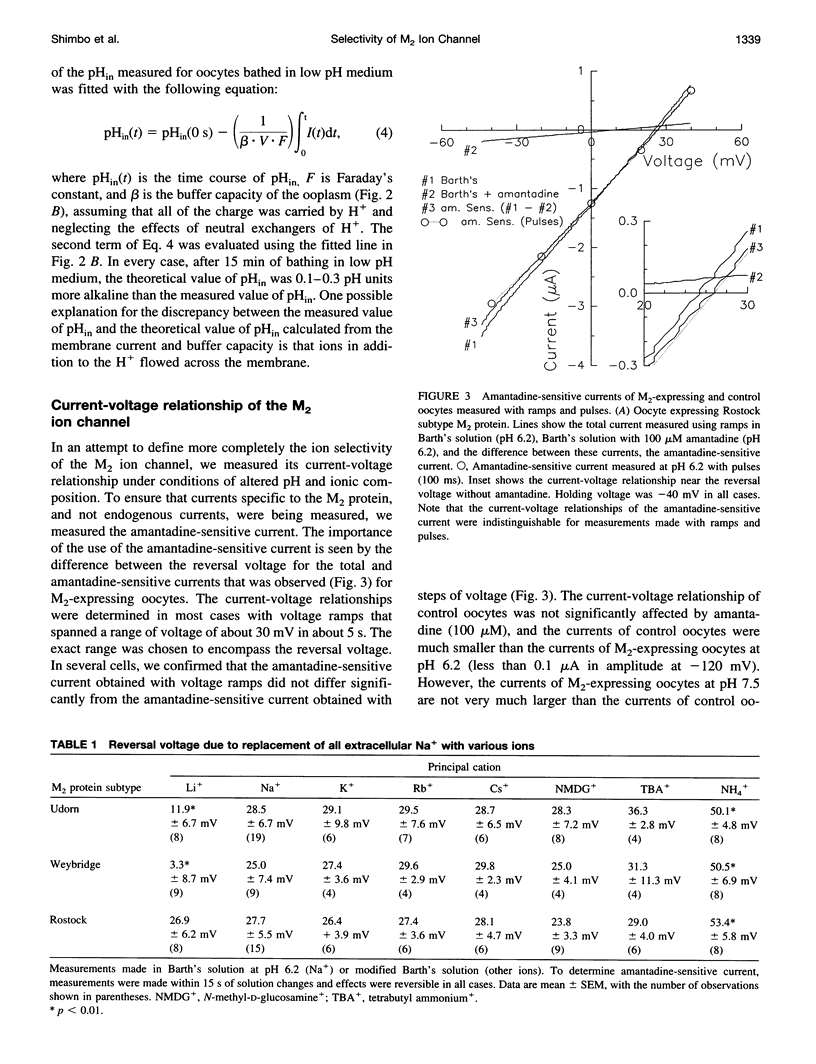
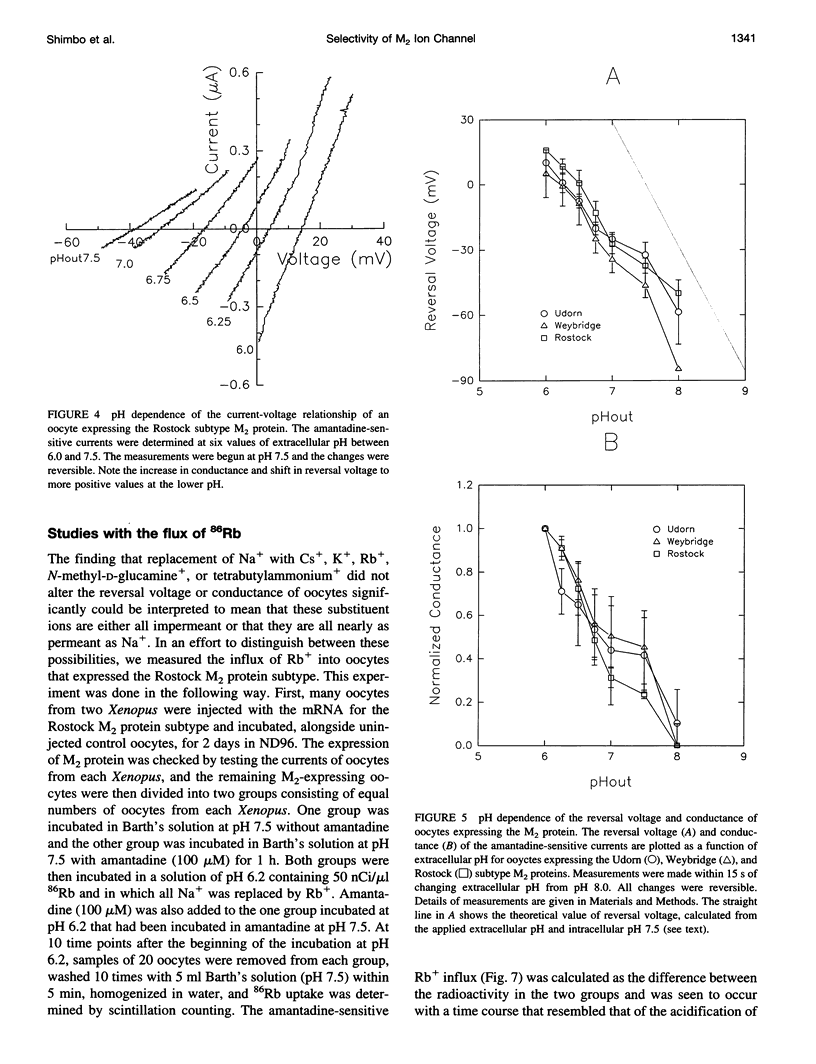
Abstract
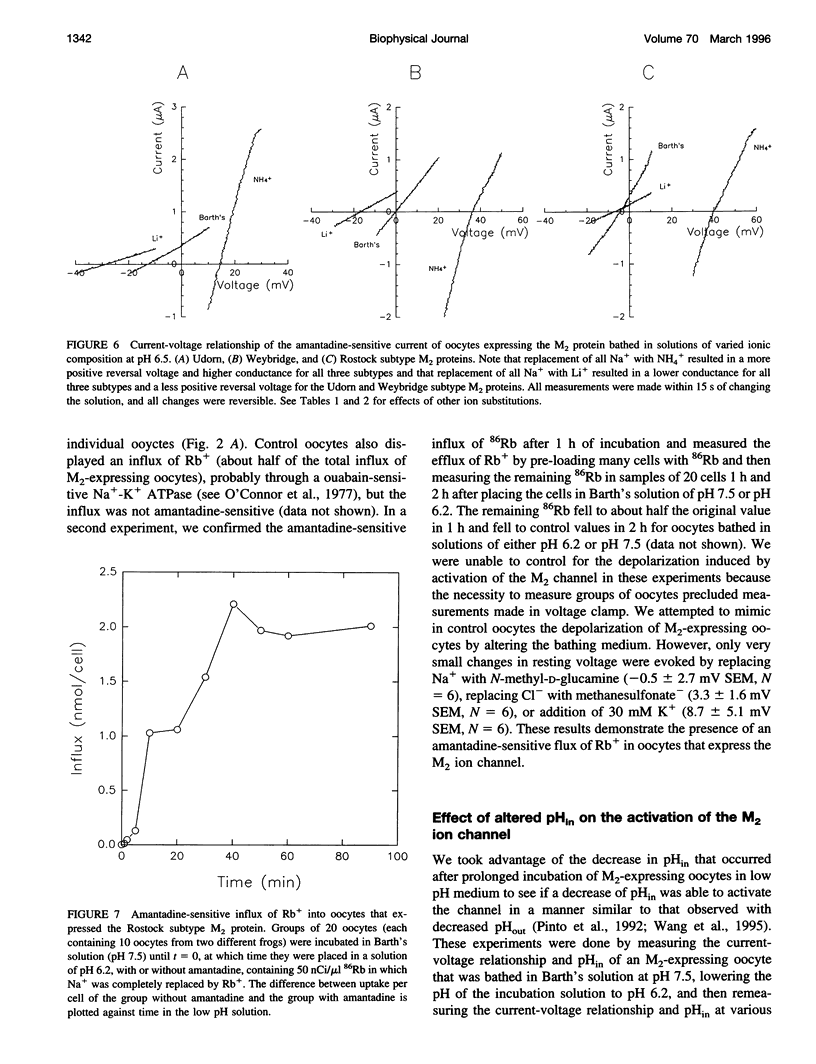
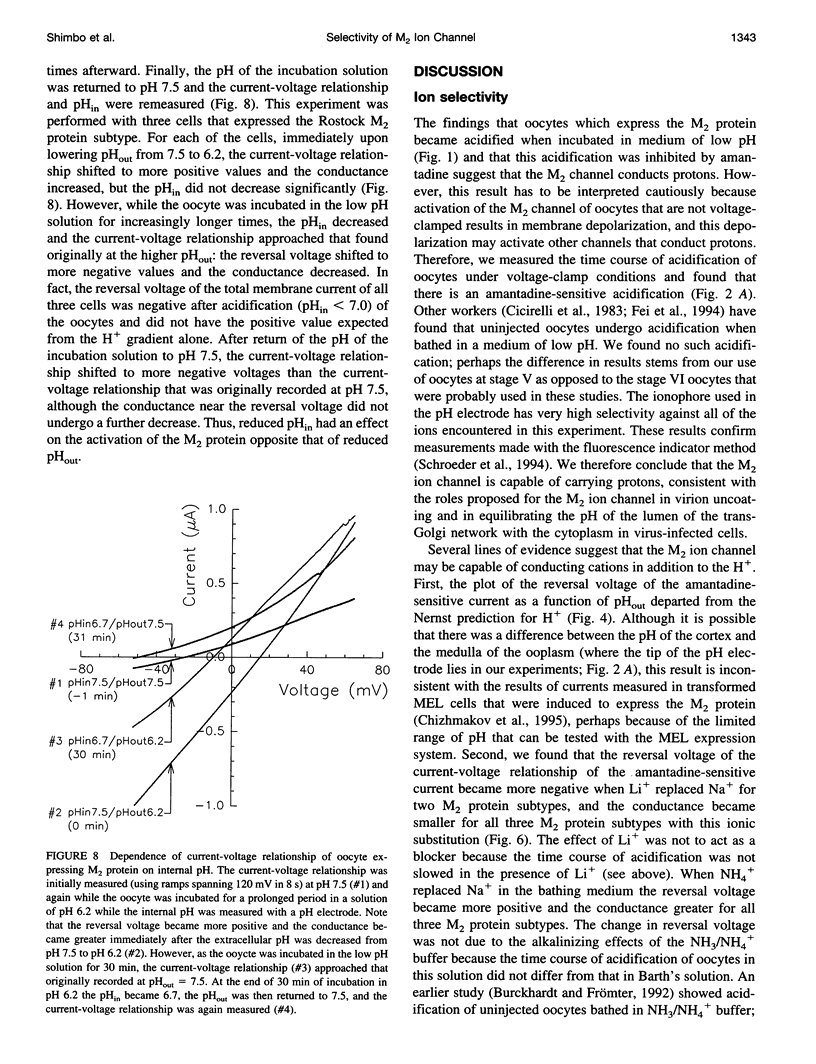
The influenza A virus-associated M2 ion channel is generally believed to function during uncoating of virions in infected cells. On endocytosis of a virion into the lumen of endosomes, the M2 ion channel is thought to cause acidification of the virion interior. In addition, the influenza virus M2 ion channel is thought to function in the exocytic pathway by equilibrating the pH gradient between the acidic lumen of the trans-Golgi network and the neutral cytoplasm. A necessary test of the proposed roles of the influenza virus M2 ion channel in the virus life cycle is to show directly that the M2 ion channel conducts protons. We have measured the ionic selectivity and activation of three subtypes (Udorn, Weybridge, and Rostock) of the M2 ion channel in oocytes of Xenopus laevis by measurement of 1) the intracellular pH (pHin) of voltage-clamped oocytes, 2) the current-voltage relationship in solutions of various pH and ionic composition, and 3) the flux of 86Rb. We took advantage of the low pHin achieved during incubation in low pH medium to study the effects of low pHin on M2 activation. Oocytes expressing each of the three subtypes of the M2 protein a) underwent a slow acidification when incubated in medium of low pH (acidification was blocked by the M2 ion channel inhibitor, amantadine); b) had current-voltage relationships that shifted to more positive values and had greater conductance when the pHout was lowered (this relationship was modified when Na- was replaced by NH4+ or Li+); c) had an amantadine-sensitive influx of Rb+. The effects on the current-voltage relationship of reduced pHin were opposed to the increased conductance found with reduced pHout. We interpret these results to indicate that the M2 ion channel is capable of conducting H+ and that other ions may also be conducted. Moreover, the channel conductance is reduced by decreased pHin. These findings are consistent with the proposed roles of the M2 protein in the life cycle of influenza A virus.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammann D., Lanter F., Steiner R. A., Schulthess P., Shijo Y., Simon W. Neutral carrier based hydrogen ion selective microelectrode for extra- and intracellular studies. Anal Chem. 1981 Dec;53(14):2267–2269. doi: 10.1021/ac00237a031. [DOI] [PubMed] [Google Scholar]
- Bland R. D., Boyd C. A. Cation transport in lung epithelial cells derived from fetal, newborn, and adult rabbits. J Appl Physiol (1985) 1986 Aug;61(2):507–515. doi: 10.1152/jappl.1986.61.2.507. [DOI] [PubMed] [Google Scholar]
- Bukrinskaya A. G., Vorkunova N. K., Kornilayeva G. V., Narmanbetova R. A., Vorkunova G. K. Influenza virus uncoating in infected cells and effect of rimantadine. J Gen Virol. 1982 May;60(Pt 1):49–59. doi: 10.1099/0022-1317-60-1-49. [DOI] [PubMed] [Google Scholar]
- Burckhardt B. C., Frömter E. Pathways of NH3/NH4+ permeation across Xenopus laevis oocyte cell membrane. Pflugers Arch. 1992 Jan;420(1):83–86. doi: 10.1007/BF00378645. [DOI] [PubMed] [Google Scholar]
- Ciampor F., Bayley P. M., Nermut M. V., Hirst E. M., Sugrue R. J., Hay A. J. Evidence that the amantadine-induced, M2-mediated conversion of influenza A virus hemagglutinin to the low pH conformation occurs in an acidic trans Golgi compartment. Virology. 1992 May;188(1):14–24. doi: 10.1016/0042-6822(92)90730-d. [DOI] [PubMed] [Google Scholar]
- Ciampor F., Thompson C. A., Grambas S., Hay A. J. Regulation of pH by the M2 protein of influenza A viruses. Virus Res. 1992 Mar;22(3):247–258. doi: 10.1016/0168-1702(92)90056-f. [DOI] [PubMed] [Google Scholar]
- Cicirelli M. F., Robinson K. R., Smith L. D. Internal pH of Xenopus oocytes: a study of the mechanism and role of pH changes during meiotic maturation. Dev Biol. 1983 Nov;100(1):133–146. doi: 10.1016/0012-1606(83)90204-x. [DOI] [PubMed] [Google Scholar]
- DeCoursey T. E., Cherny V. V. Na(+)-H+ antiport detected through hydrogen ion currents in rat alveolar epithelial cells and human neutrophils. J Gen Physiol. 1994 May;103(5):755–785. doi: 10.1085/jgp.103.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Fei Y. J., Kanai Y., Nussberger S., Ganapathy V., Leibach F. H., Romero M. F., Singh S. K., Boron W. F., Hediger M. A. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature. 1994 Apr 7;368(6471):563–566. doi: 10.1038/368563a0. [DOI] [PubMed] [Google Scholar]
- Grambas S., Bennett M. S., Hay A. J. Influence of amantadine resistance mutations on the pH regulatory function of the M2 protein of influenza A viruses. Virology. 1992 Dec;191(2):541–549. doi: 10.1016/0042-6822(92)90229-i. [DOI] [PubMed] [Google Scholar]
- Grambas S., Hay A. J. Maturation of influenza A virus hemagglutinin--estimates of the pH encountered during transport and its regulation by the M2 protein. Virology. 1992 Sep;190(1):11–18. doi: 10.1016/0042-6822(92)91187-y. [DOI] [PubMed] [Google Scholar]
- Hay A. J., Wolstenholme A. J., Skehel J. J., Smith M. H. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985 Nov;4(11):3021–3024. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A. Unpacking the incoming influenza virus. Cell. 1992 May 15;69(4):577–578. doi: 10.1016/0092-8674(92)90219-3. [DOI] [PubMed] [Google Scholar]
- Holsinger L. J., Lamb R. A. Influenza virus M2 integral membrane protein is a homotetramer stabilized by formation of disulfide bonds. Virology. 1991 Jul;183(1):32–43. doi: 10.1016/0042-6822(91)90115-r. [DOI] [PubMed] [Google Scholar]
- Holsinger L. J., Nichani D., Pinto L. H., Lamb R. A. Influenza A virus M2 ion channel protein: a structure-function analysis. J Virol. 1994 Mar;68(3):1551–1563. doi: 10.1128/jvi.68.3.1551-1563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsinger L. J., Nichani D., Pinto L. H., Lamb R. A. Influenza A virus M2 ion channel protein: a structure-function analysis. J Virol. 1994 Mar;68(3):1551–1563. doi: 10.1128/jvi.68.3.1551-1563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsinger L. J., Shaughnessy M. A., Micko A., Pinto L. H., Lamb R. A. Analysis of the posttranslational modifications of the influenza virus M2 protein. J Virol. 1995 Feb;69(2):1219–1225. doi: 10.1128/jvi.69.2.1219-1225.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howl J. D., von Sicard N. A., Publicover S. J., Anderson M. A method for the manufacture of single barrel liquid ion-selective microelectrodes: an in situ study of ant venom pH. Pflugers Arch. 1988 Feb;411(2):212–215. doi: 10.1007/BF00582317. [DOI] [PubMed] [Google Scholar]
- Kemp P. J., Roberts G. C., Boyd C. A. Identification and properties of pathways for K+ transport in guinea-pig and rat alveolar epithelial type II cells. J Physiol. 1994 Apr 1;476(1):79–88. [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Identification of a second protein (M2) encoded by RNA segment 7 of influenza virus. Virology. 1981 Jul 30;112(2):729–737. doi: 10.1016/0042-6822(81)90317-2. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Zebedee S. L., Richardson C. D. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985 Mar;40(3):627–633. doi: 10.1016/0092-8674(85)90211-9. [DOI] [PubMed] [Google Scholar]
- Marsh M. Keeping the viral coat on. Curr Biol. 1992 Jul;2(7):379–381. doi: 10.1016/0960-9822(92)90080-t. [DOI] [PubMed] [Google Scholar]
- Martin K., Helenius A. Nuclear transport of influenza virus ribonucleoproteins: the viral matrix protein (M1) promotes export and inhibits import. Cell. 1991 Oct 4;67(1):117–130. doi: 10.1016/0092-8674(91)90576-k. [DOI] [PubMed] [Google Scholar]
- O'Connor C. M., Robinson K. R., Smith L. D. Calcium, potassium, and sodium exchange by full-grown and maturing Xenopus laevis oocytes. Dev Biol. 1977 Nov;61(1):28–40. doi: 10.1016/0012-1606(77)90339-6. [DOI] [PubMed] [Google Scholar]
- Pinto L. H., Holsinger L. J., Lamb R. A. Influenza virus M2 protein has ion channel activity. Cell. 1992 May 1;69(3):517–528. doi: 10.1016/0092-8674(92)90452-i. [DOI] [PubMed] [Google Scholar]
- Schroeder C., Ford C. M., Wharton S. A., Hay A. J. Functional reconstitution in lipid vesicles of influenza virus M2 protein expressed by baculovirus: evidence for proton transfer activity. J Gen Virol. 1994 Dec;75(Pt 12):3477–3484. doi: 10.1099/0022-1317-75-12-3477. [DOI] [PubMed] [Google Scholar]
- Shimbo K., Brassard D. L., Lamb R. A., Pinto L. H. Viral and cellular small integral membrane proteins can modify ion channels endogenous to Xenopus oocytes. Biophys J. 1995 Nov;69(5):1819–1829. doi: 10.1016/S0006-3495(95)80052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J., Hay A. J., Armstrong J. A. On the mechanism of inhibition of influenza virus replication by amantadine hydrochloride. J Gen Virol. 1978 Jan;38(1):97–110. doi: 10.1099/0022-1317-38-1-97. [DOI] [PubMed] [Google Scholar]
- Skehel J. J. Influenza virus. Amantadine blocks the channel. Nature. 1992 Jul 9;358(6382):110–111. doi: 10.1038/358110b0. [DOI] [PubMed] [Google Scholar]
- Sugrue R. J., Bahadur G., Zambon M. C., Hall-Smith M., Douglas A. R., Hay A. J. Specific structural alteration of the influenza haemagglutinin by amantadine. EMBO J. 1990 Nov;9(11):3469–3476. doi: 10.1002/j.1460-2075.1990.tb07555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue R. J., Hay A. J. Structural characteristics of the M2 protein of influenza A viruses: evidence that it forms a tetrameric channel. Virology. 1991 Feb;180(2):617–624. doi: 10.1016/0042-6822(91)90075-M. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K., Lamb R. A. Influenza virus M2 protein ion channel activity stabilizes the native form of fowl plague virus hemagglutinin during intracellular transport. J Virol. 1994 Feb;68(2):911–919. doi: 10.1128/jvi.68.2.911-919.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosteson M. T., Pinto L. H., Holsinger L. J., Lamb R. A. Reconstitution of the influenza virus M2 ion channel in lipid bilayers. J Membr Biol. 1994 Oct;142(1):117–126. doi: 10.1007/BF00233389. [DOI] [PubMed] [Google Scholar]
- Wang C., Lamb R. A., Pinto L. H. Activation of the M2 ion channel of influenza virus: a role for the transmembrane domain histidine residue. Biophys J. 1995 Oct;69(4):1363–1371. doi: 10.1016/S0006-3495(95)80003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Lamb R. A., Pinto L. H. Direct measurement of the influenza A virus M2 protein ion channel activity in mammalian cells. Virology. 1994 Nov 15;205(1):133–140. doi: 10.1006/viro.1994.1628. [DOI] [PubMed] [Google Scholar]
- Wang C., Takeuchi K., Pinto L. H., Lamb R. A. Ion channel activity of influenza A virus M2 protein: characterization of the amantadine block. J Virol. 1993 Sep;67(9):5585–5594. doi: 10.1128/jvi.67.9.5585-5594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebedee S. L., Lamb R. A. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J Virol. 1988 Aug;62(8):2762–2772. doi: 10.1128/jvi.62.8.2762-2772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebedee S. L., Richardson C. D., Lamb R. A. Characterization of the influenza virus M2 integral membrane protein and expression at the infected-cell surface from cloned cDNA. J Virol. 1985 Nov;56(2):502–511. doi: 10.1128/jvi.56.2.502-511.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


