Abstract
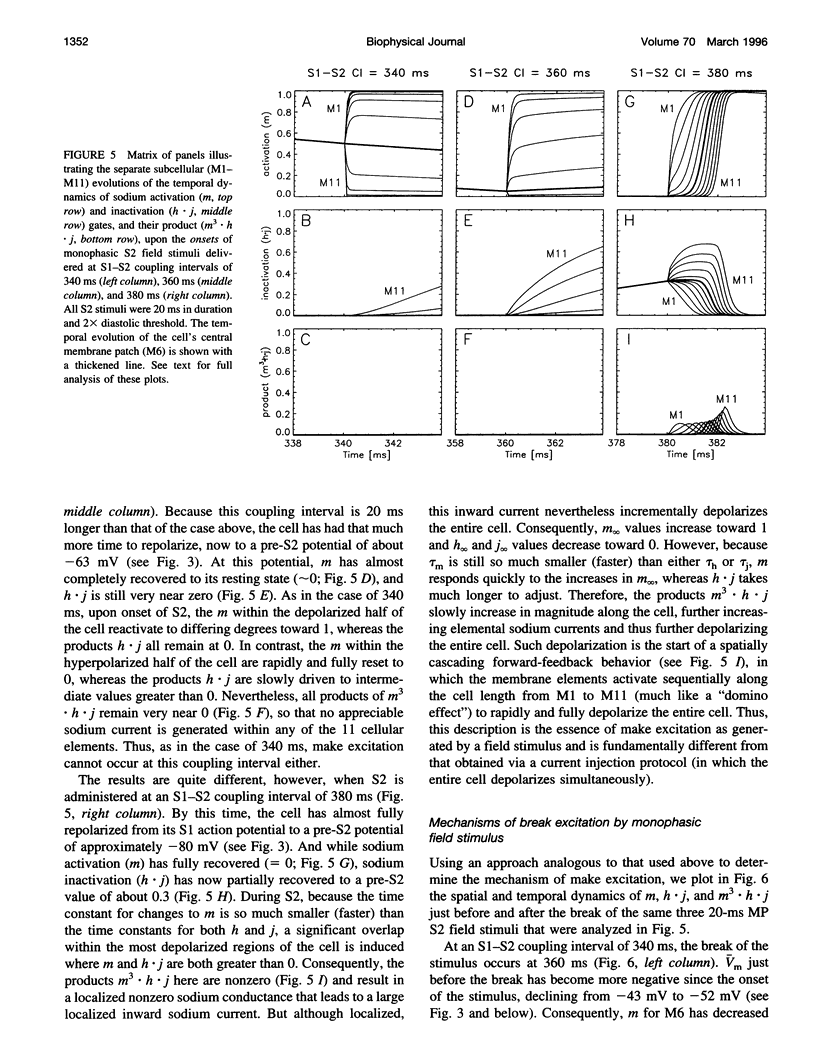
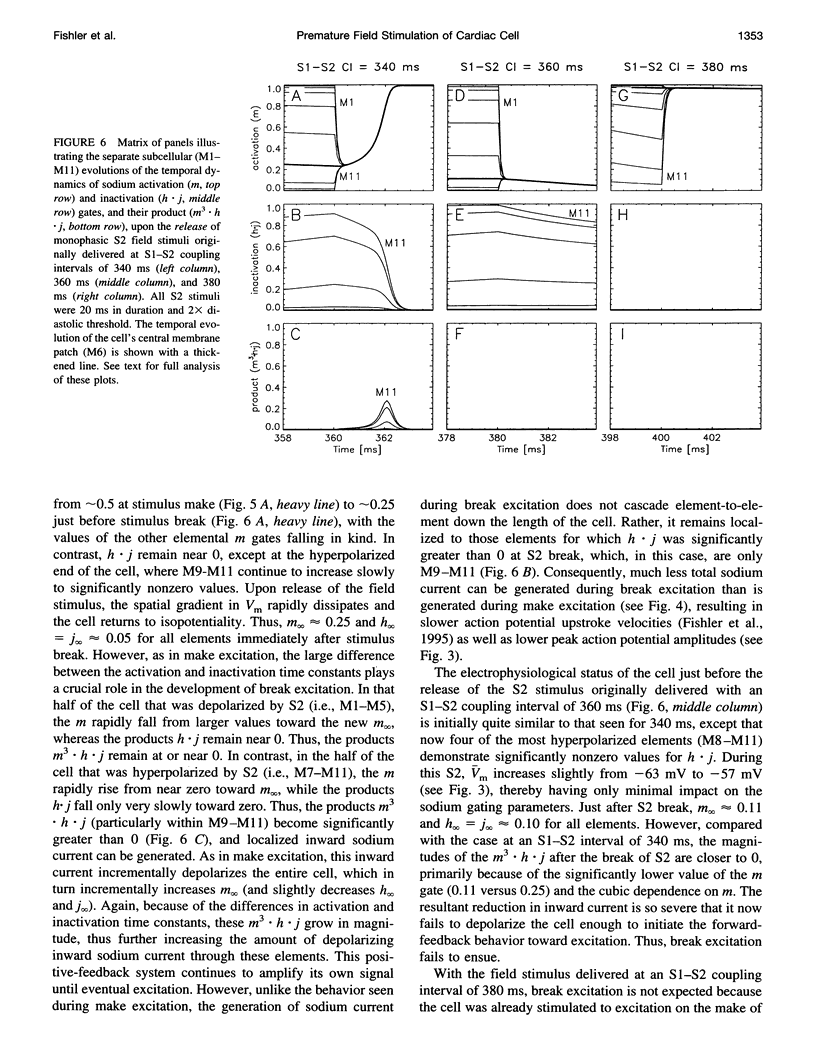
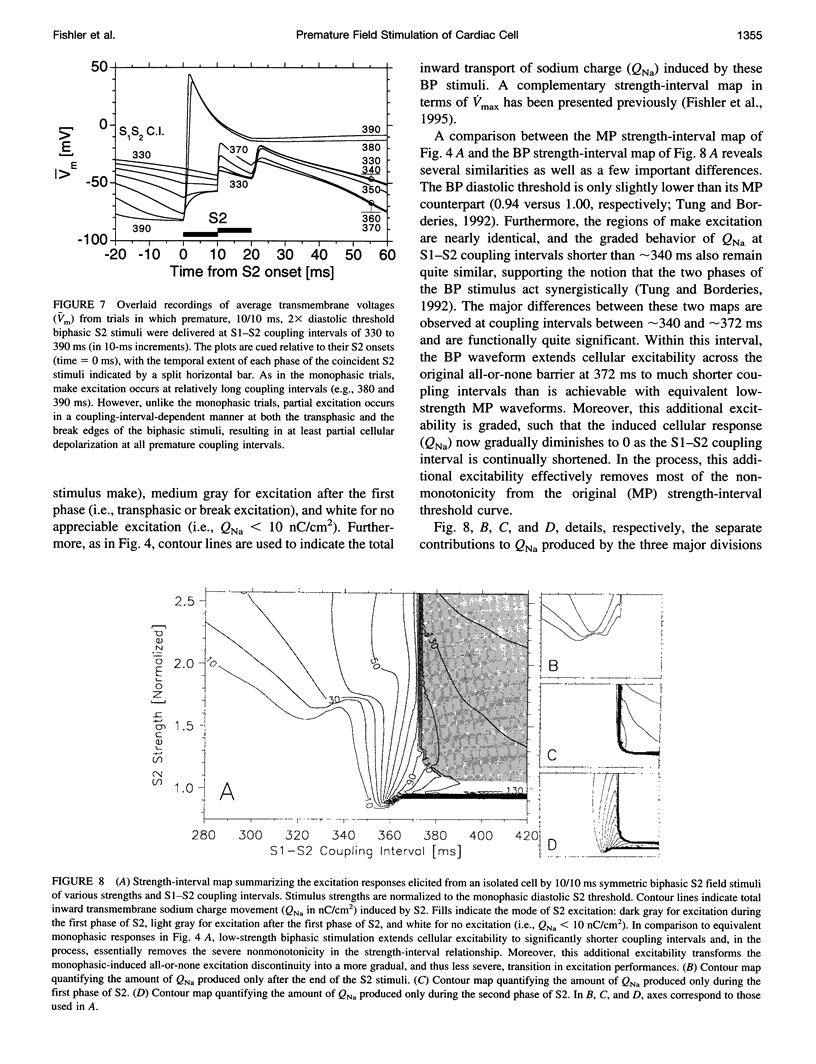
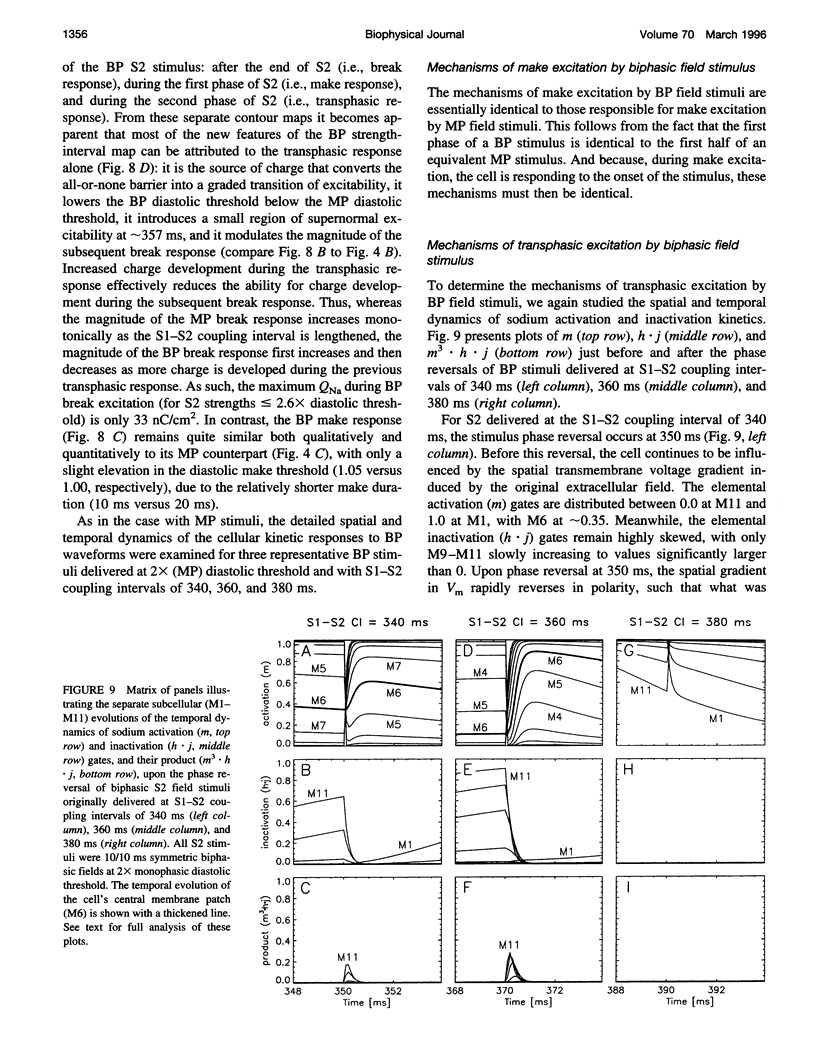
The mechanisms by which extracellular electric field stimuli induce the (re)excitation of cardiac cells in various stages of refractoriness are still not well understood. We modeled the interactions between an isolated cardiac cell and imposed extracellular electric fields to determine the mechanisms by which relatively low-strength uniform monophasic and biphasic field stimuli induce premature reexcitations. An idealized ventricular cell was simulated with 11 subcellular membrane patches, each of which obeyed Luo-Rudy (phase 1) kinetics. Implementing a standard S1-S2 pulse protocol, strength-interval maps of the cellular excitatory responses were generated for rectangular monophasic and symmetric biphasic field stimuli of 2, 5, 10, and 20 ms total duration. In contrast to previously documented current injection studies, our results demonstrate that a cardiac cell exhibits a significantly nonmonotonic excitatory response to premature monophasic and, to a much lesser degree, biphasic field stimuli. Furthermore, for monophasic stimuli at low field strengths, the cell is exquisitely sensitive to the timing of the shock, demonstrating a classic all-or-none depolarizing response. However, at higher field strengths this all-or-none sensitivity reverts to a more gradual transition of excitatory responses with respect to stimulus prematurity. In contrast, biphasic stimuli produce such graded responses at all suprathreshold stimulus strengths. Similar behaviors are demonstrated at all S2 stimulus durations tested. The generation of depolarizing (sodium) currents is triggered by one or more of the sharp field gradient changes produced at the stimulus edges-i.e., make, break, and transphasic (for biphasic stimuli)-with the magnitude of these edge-induced current contributions dependent on both the prematurity and the strength of the applied field. In all cases, however, depolarizing current arises from the partial removal of sodium inactivation from at least part of the cell, because of either the natural process of repolarization or a localized acceleration of this process by the impressed field.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beeler G. W., Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977 Jun;268(1):177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartee L. A., Plonsey R. The transient subthreshold response of spherical and cylindrical cell models to extracellular stimulation. IEEE Trans Biomed Eng. 1992 Jan;39(1):76–85. doi: 10.1109/10.108130. [DOI] [PubMed] [Google Scholar]
- Chen P. S., Shibata N., Dixon E. G., Wolf P. D., Danieley N. D., Sweeney M. B., Smith W. M., Ideker R. E. Activation during ventricular defibrillation in open-chest dogs. Evidence of complete cessation and regeneration of ventricular fibrillation after unsuccessful shocks. J Clin Invest. 1986 Mar;77(3):810–823. doi: 10.1172/JCI112378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P. S., Wolf P. D., Ideker R. E. Mechanism of cardiac defibrillation. A different point of view. Circulation. 1991 Aug;84(2):913–919. doi: 10.1161/01.cir.84.2.913. [DOI] [PubMed] [Google Scholar]
- Dekker E. Direct current make and break thresholds for pacemaker electrodes on the canine ventricle. Circ Res. 1970 Nov;27(5):811–823. doi: 10.1161/01.res.27.5.811. [DOI] [PubMed] [Google Scholar]
- Drouhard J. P., Roberge F. A. Revised formulation of the Hodgkin-Huxley representation of the sodium current in cardiac cells. Comput Biomed Res. 1987 Aug;20(4):333–350. doi: 10.1016/0010-4809(87)90048-6. [DOI] [PubMed] [Google Scholar]
- Earm Y. E., Noble D. A model of the single atrial cell: relation between calcium current and calcium release. Proc R Soc Lond B Biol Sci. 1990 May 22;240(1297):83–96. doi: 10.1098/rspb.1990.0028. [DOI] [PubMed] [Google Scholar]
- Fain E. S., Sweeney M. B., Franz M. R. Improved internal defibrillation efficacy with a biphasic waveform. Am Heart J. 1989 Feb;117(2):358–364. doi: 10.1016/0002-8703(89)90779-5. [DOI] [PubMed] [Google Scholar]
- Feeser S. A., Tang A. S., Kavanagh K. M., Rollins D. L., Smith W. M., Wolf P. D., Ideker R. E. Strength-duration and probability of success curves for defibrillation with biphasic waveforms. Circulation. 1990 Dec;82(6):2128–2141. doi: 10.1161/01.cir.82.6.2128. [DOI] [PubMed] [Google Scholar]
- Fishler M. G., Sobie E. A., Tung L., Thakor N. V. Cardiac responses to premature monophasic and biphasic field stimuli. Results from cell and tissue modeling studies. J Electrocardiol. 1995;28 (Suppl):174–179. doi: 10.1016/s0022-0736(95)80052-2. [DOI] [PubMed] [Google Scholar]
- Flaker G. C., Schuder J. C., McDaniel W. C., Stoeckle H., Dbeis M. Superiority of biphasic shocks in the defibrillation of dogs by epicardial patches and catheter electrodes. Am Heart J. 1989 Aug;118(2):288–291. doi: 10.1016/0002-8703(89)90187-7. [DOI] [PubMed] [Google Scholar]
- Gross D., Loew L. M., Webb W. W. Optical imaging of cell membrane potential changes induced by applied electric fields. Biophys J. 1986 Aug;50(2):339–348. doi: 10.1016/S0006-3495(86)83467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOSHI R., MATSUDA K. Excitability cycle of cardiac muscle examined by intracellular stimulation. Jpn J Physiol. 1962 Aug 15;12:433–446. doi: 10.2170/jjphysiol.12.433. [DOI] [PubMed] [Google Scholar]
- Jones J. L., Jones R. E., Balasky G. Improved cardiac cell excitation with symmetrical biphasic defibrillator waveforms. Am J Physiol. 1987 Dec;253(6 Pt 2):H1418–H1424. doi: 10.1152/ajpheart.1987.253.6.H1418. [DOI] [PubMed] [Google Scholar]
- Jones J. L., Jones R. E., Milne K. B. Refractory period prolongation by biphasic defibrillator waveforms is associated with enhanced sodium current in a computer model of the ventricular action potential. IEEE Trans Biomed Eng. 1994 Jan;41(1):60–68. doi: 10.1109/10.277272. [DOI] [PubMed] [Google Scholar]
- Jones J. L., Jones R. E. Threshold reduction with biphasic defibrillator waveforms. Role of excitation channel recovery in a computer model of the ventricular action potential. J Electrocardiol. 1990;23 (Suppl):30–35. doi: 10.1016/0022-0736(90)90071-9. [DOI] [PubMed] [Google Scholar]
- Klee M., Plonsey R. Stimulation of spheroidal cells--the role of cell shape. IEEE Trans Biomed Eng. 1976 Jul;23(4):347–354. doi: 10.1109/tbme.1976.324597. [DOI] [PubMed] [Google Scholar]
- Knisley S. B., Blitchington T. F., Hill B. C., Grant A. O., Smith W. M., Pilkington T. C., Ideker R. E. Optical measurements of transmembrane potential changes during electric field stimulation of ventricular cells. Circ Res. 1993 Feb;72(2):255–270. doi: 10.1161/01.res.72.2.255. [DOI] [PubMed] [Google Scholar]
- Knisley S. B., Hill B. C., Ideker R. E. Virtual electrode effects in myocardial fibers. Biophys J. 1994 Mar;66(3 Pt 1):719–728. doi: 10.1016/s0006-3495(94)80846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knisley S. B., Hill B. C. Optical recordings of the effect of electrical stimulation on action potential repolarization and the induction of reentry in two-dimensional perfused rabbit epicardium. Circulation. 1993 Nov;88(5 Pt 1):2402–2414. doi: 10.1161/01.cir.88.5.2402. [DOI] [PubMed] [Google Scholar]
- Knisley S. B., Smith W. M., Ideker R. E. Effect of field stimulation on cellular repolarization in rabbit myocardium. Implications for reentry induction. Circ Res. 1992 Apr;70(4):707–715. doi: 10.1161/01.res.70.4.707. [DOI] [PubMed] [Google Scholar]
- Krassowska W., Cabo C., Knisley S. B., Ideker R. E. Propagation versus delayed activation during field stimulation of cardiac muscle. Pacing Clin Electrophysiol. 1992 Feb;15(2):197–210. doi: 10.1111/j.1540-8159.1992.tb03064.x. [DOI] [PubMed] [Google Scholar]
- Krassowska W., Neu J. C. Response of a single cell to an external electric field. Biophys J. 1994 Jun;66(6):1768–1776. doi: 10.1016/S0006-3495(94)80971-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krassowska W., Pilkington T. C., Ideker R. E. Periodic conductivity as a mechanism for cardiac stimulation and defibrillation. IEEE Trans Biomed Eng. 1987 Jul;34(7):555–560. doi: 10.1109/tbme.1987.325986. [DOI] [PubMed] [Google Scholar]
- Leon L. J., Roberge F. A. A model study of extracellular stimulation of cardiac cells. IEEE Trans Biomed Eng. 1993 Dec;40(12):1307–1319. doi: 10.1109/10.250586. [DOI] [PubMed] [Google Scholar]
- Luo C. H., Rudy Y. A dynamic model of the cardiac ventricular action potential. I. Simulations of ionic currents and concentration changes. Circ Res. 1994 Jun;74(6):1071–1096. doi: 10.1161/01.res.74.6.1071. [DOI] [PubMed] [Google Scholar]
- Luo C. H., Rudy Y. A model of the ventricular cardiac action potential. Depolarization, repolarization, and their interaction. Circ Res. 1991 Jun;68(6):1501–1526. doi: 10.1161/01.res.68.6.1501. [DOI] [PubMed] [Google Scholar]
- Mirowski M. The automatic implantable cardioverter-defibrillator: an overview. J Am Coll Cardiol. 1985 Aug;6(2):461–466. doi: 10.1016/s0735-1097(85)80186-8. [DOI] [PubMed] [Google Scholar]
- Neunlist M., Tung L. Spatial distribution of cardiac transmembrane potentials around an extracellular electrode: dependence on fiber orientation. Biophys J. 1995 Jun;68(6):2310–2322. doi: 10.1016/S0006-3495(95)80413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORIAS O., BROOKS C. M., SUCKLING E. E., GILBERT J. L., SIEBENS A. A. Excitability of the mammalian ventricle throughout the cardiac cycle. Am J Physiol. 1950 Nov;163(2):272–282. doi: 10.1152/ajplegacy.1950.163.2.272. [DOI] [PubMed] [Google Scholar]
- Plonsey R., Barr R. C. Effect of microscopic and macroscopic discontinuities on the response of cardiac tissue to defibrillating (stimulating) currents. Med Biol Eng Comput. 1986 Mar;24(2):130–136. doi: 10.1007/BF02443925. [DOI] [PubMed] [Google Scholar]
- Plonsey R., Barr R. C. Inclusion of junction elements in a linear cardiac model through secondary sources: application to defibrillation. Med Biol Eng Comput. 1986 Mar;24(2):137–144. doi: 10.1007/BF02443926. [DOI] [PubMed] [Google Scholar]
- Roth B. J., Wikswo J. P., Jr Electrical stimulation of cardiac tissue: a bidomain model with active membrane properties. IEEE Trans Biomed Eng. 1994 Mar;41(3):232–240. doi: 10.1109/10.284941. [DOI] [PubMed] [Google Scholar]
- Schuder J. C., Gold J. H., Stoeckle H., McDaniel W. C., Cheung K. N. Transthoracic ventricular defibrillation in the 100 kg calf with symmetrical one-cycle bidirectional rectangular wave stimuli. IEEE Trans Biomed Eng. 1983 Jul;30(7):415–422. doi: 10.1109/TBME.1980.326689. [DOI] [PubMed] [Google Scholar]
- Shibata N., Chen P. S., Dixon E. G., Wolf P. D., Danieley N. D., Smith W. M., Ideker R. E. Epicardial activation after unsuccessful defibrillation shocks in dogs. Am J Physiol. 1988 Oct;255(4 Pt 2):H902–H909. doi: 10.1152/ajpheart.1988.255.4.H902. [DOI] [PubMed] [Google Scholar]
- Tang A. S., Yabe S., Wharton J. M., Dolker M., Smith W. M., Ideker R. E. Ventricular defibrillation using biphasic waveforms: the importance of phasic duration. J Am Coll Cardiol. 1989 Jan;13(1):207–214. doi: 10.1016/0735-1097(89)90572-x. [DOI] [PubMed] [Google Scholar]
- Trayanova N., Pilkington T. C. A bidomain model with periodic intracellular junctions: a one-dimensional analysis. IEEE Trans Biomed Eng. 1993 May;40(5):424–433. doi: 10.1109/10.243419. [DOI] [PubMed] [Google Scholar]
- Tung L., Borderies J. R. Analysis of electric field stimulation of single cardiac muscle cells. Biophys J. 1992 Aug;63(2):371–386. doi: 10.1016/S0006-3495(92)81632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung L., Sliz N., Mulligan M. R. Influence of electrical axis of stimulation on excitation of cardiac muscle cells. Circ Res. 1991 Sep;69(3):722–730. doi: 10.1161/01.res.69.3.722. [DOI] [PubMed] [Google Scholar]
- Wharton J. M., Richard V. J., Murry C. E., Dixon E. G., Reimer K. A., Meador J., Smith W. M., Ideker R. E. Electrophysiological effects of monophasic and biphasic stimuli in normal and infarcted dogs. Pacing Clin Electrophysiol. 1990 Sep;13(9):1158–1172. doi: 10.1111/j.1540-8159.1990.tb02174.x. [DOI] [PubMed] [Google Scholar]
- Winkle R. A., Mead R. H., Ruder M. A., Gaudiani V., Buch W. S., Pless B., Sweeney M., Schmidt P. Improved low energy defibrillation efficacy in man with the use of a biphasic truncated exponential waveform. Am Heart J. 1989 Jan;117(1):122–127. doi: 10.1016/0002-8703(89)90665-0. [DOI] [PubMed] [Google Scholar]
- Zhou X., Daubert J. P., Wolf P. D., Smith W. M., Ideker R. E. Epicardial mapping of ventricular defibrillation with monophasic and biphasic shocks in dogs. Circ Res. 1993 Jan;72(1):145–160. doi: 10.1161/01.res.72.1.145. [DOI] [PubMed] [Google Scholar]