Abstract
Gangliosides have been shown to function as cell surface receptors, as well as participating in cell growth, differentiation, and transformation. In spite of their multiple biological functions, relatively little is known about their structure and physical properties in membrane systems. The thermotropic and structural properties of ganglioside GM1 alone and in a binary system with 1,2-dipalmitoyl phosphatidylcholine (DPPC) have been investigated by differential scanning calorimetry (DSC) and x-ray diffraction. By DSC hydrated GM1 undergoes a broad endothermic transition TM = 26 degrees C (delta H = 1.7 kcal/mol GM1). X-ray diffraction below (-2 degrees C) and above (51 degrees C) this transition indicates a micellar structure with changes occurring only in the wide angle region of the diffraction pattern (relatively sharp reflection at 1/4.12 A-1 at -2 degrees C; more diffuse reflection at 1/4.41 A-1 at 51 degrees C). In hydrated binary mixtures with DPPC, incorporation of GM1 (0-30 mol%; zone 1) decreases the enthalpy of the DPPC pretransition at low molar compositions while increasing the TM of both the pre- and main transitions (limiting values, 39 and 44 degrees C, respectively). X-ray diffraction studies indicate the presence of a single bilayer gel phase in zone 1 that can undergo chain melting to an L alpha bilayer phase. A detailed hydration study of GM1 (5.7 mol %)/DPPC indicated a conversion of the DPPC bilayer gel phase to an infinite swelling system in zone 1 due to the presence of the negatively charged sialic acid moiety of GM1. At 30-61 mol % GM1 (zone 2), two calorimetric transitions are observed at 44 and 47 degrees C, suggesting the presence of two phases. The lower transition reflects the bilayer gel --> L alpha transition (zone 1), whereas the upper transition appears to be a consequence of the formation of a nonbilayer, micellar or hexagonal phase, although the structure of this phase has not been defined by x-ray diffraction. At > 61 mol % GM1 (zone 3) the calorimetric and phase behavior is dominated by the micelle-forming properties of GM1; the presence of mixed GM1/DPPC micellar phases is predicted.
Full text
PDF

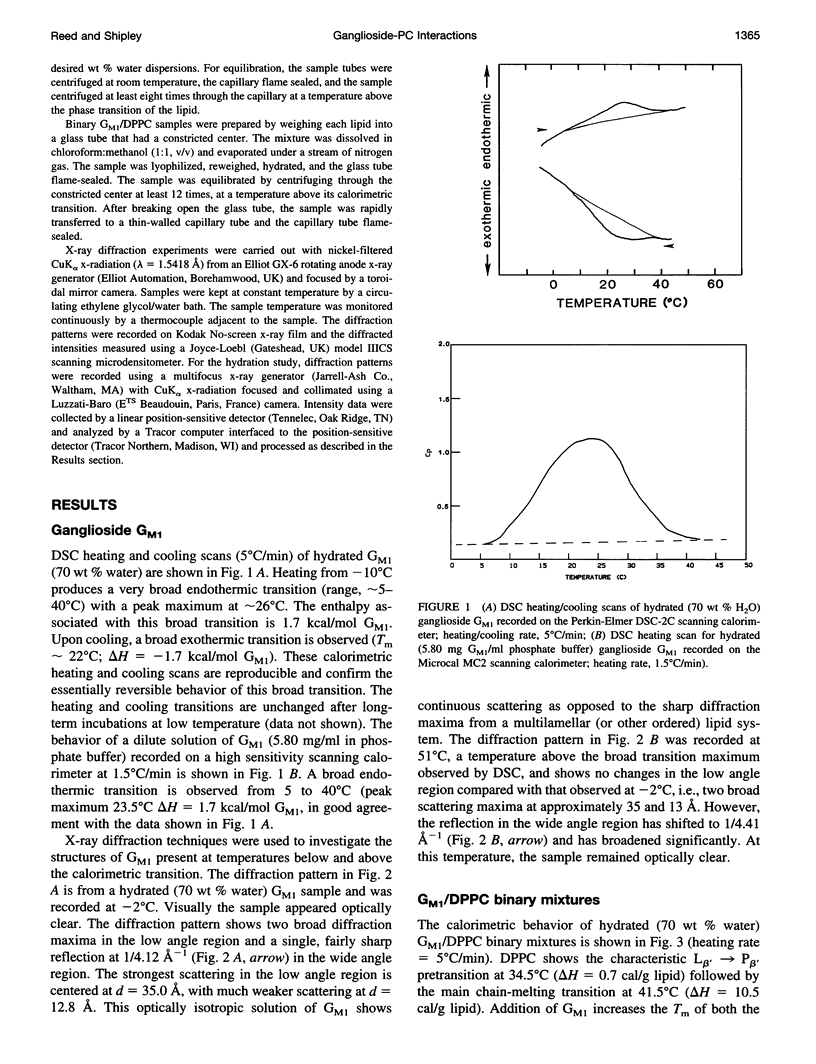
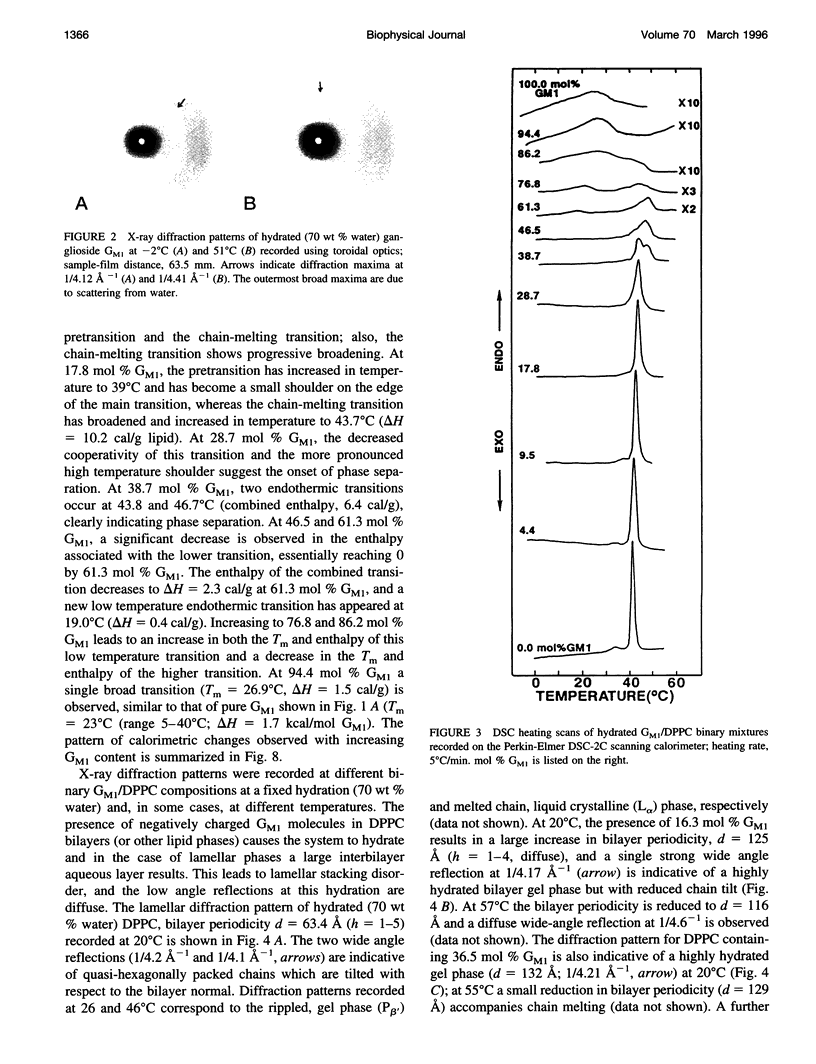
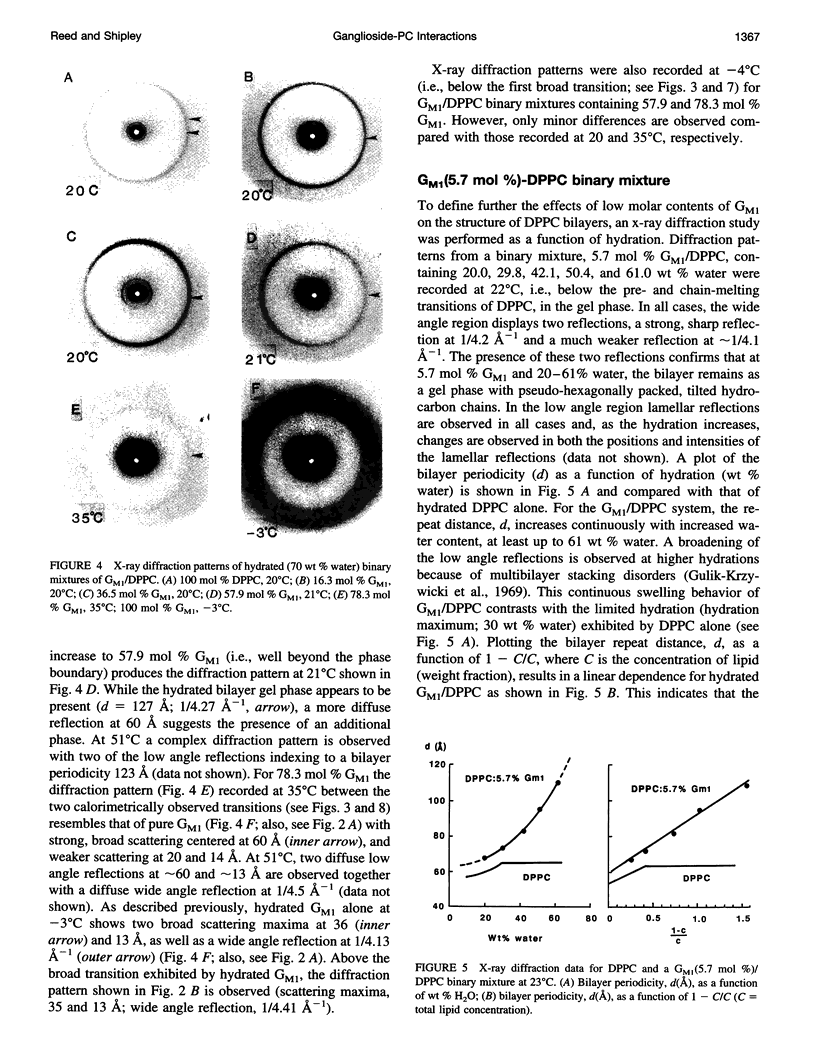
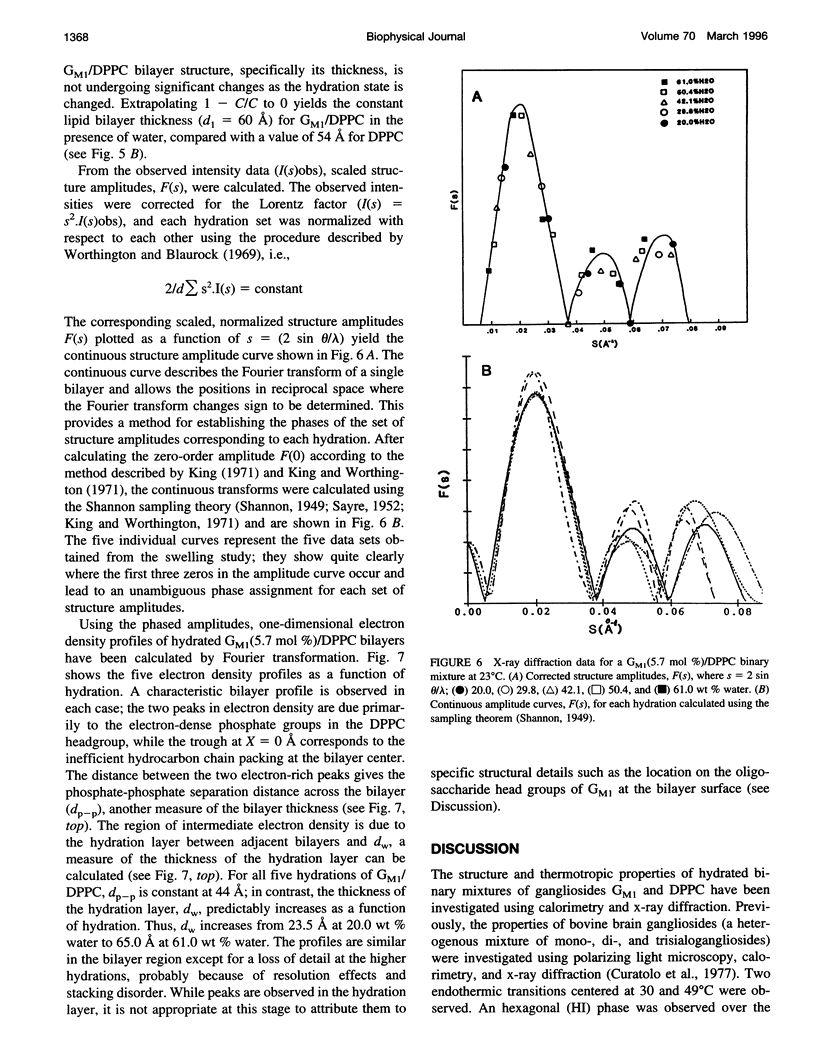
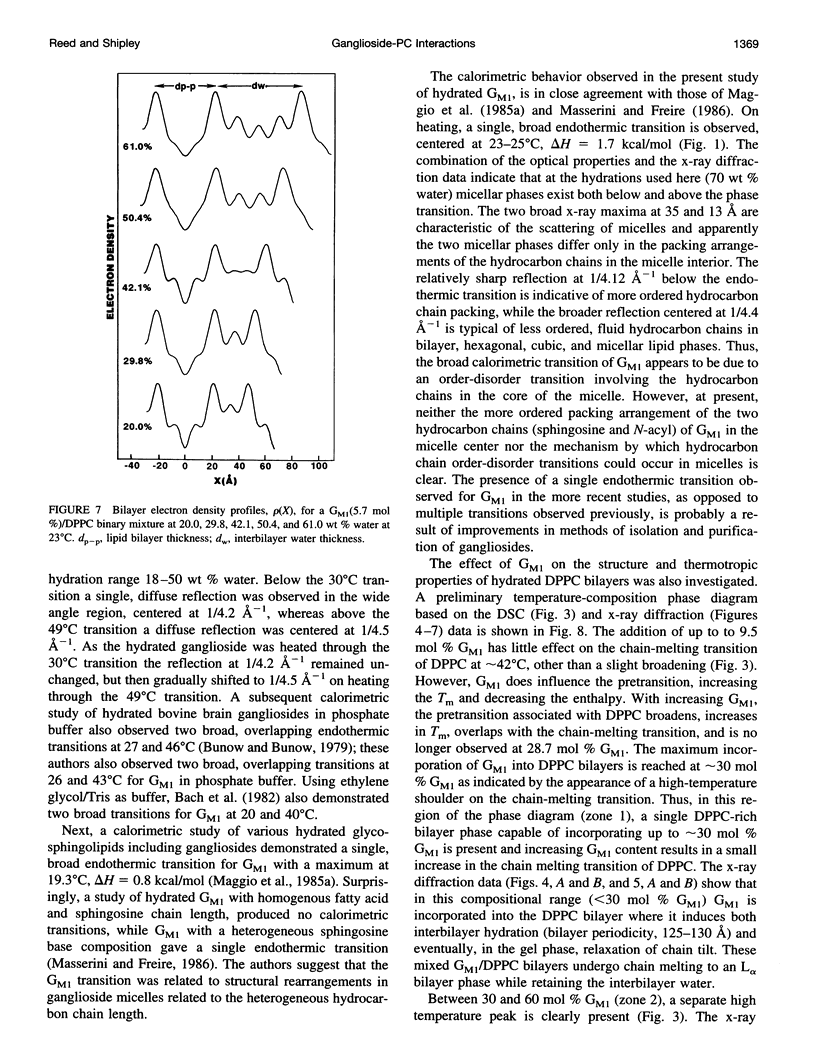
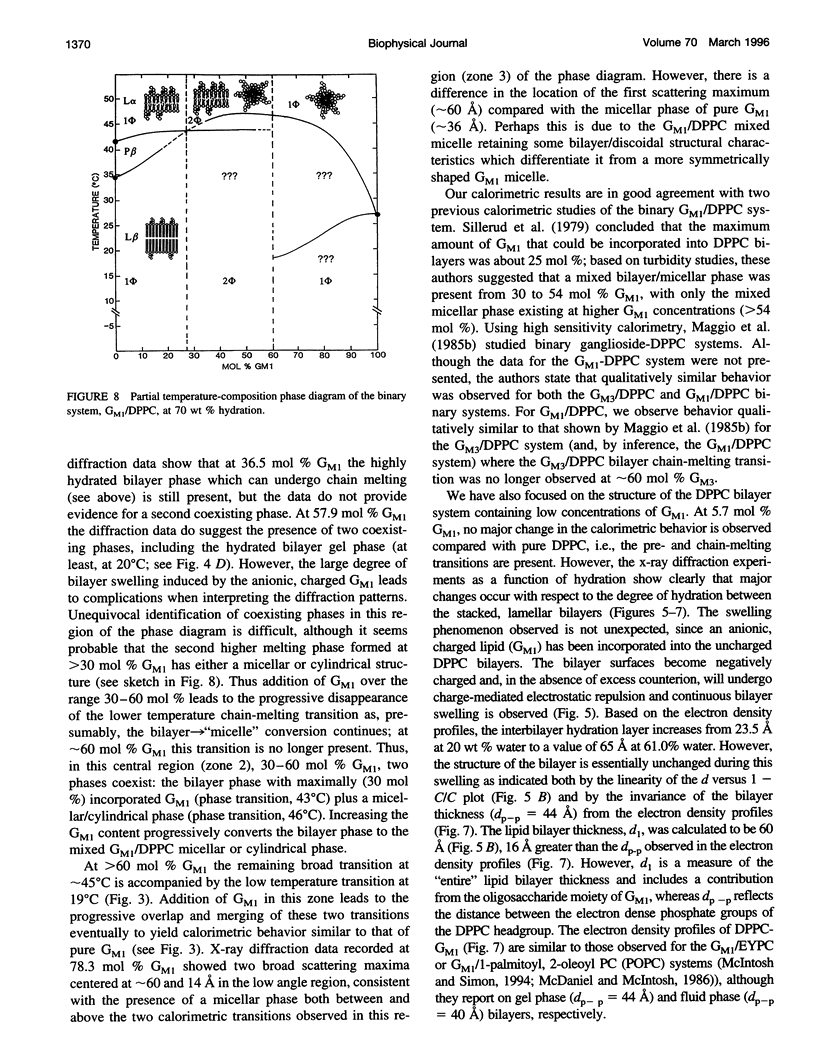


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach D., Miller I. R., Sela B. A. Calorimetric studies on various gangliosides and ganglioside-lipid interactions. Biochim Biophys Acta. 1982 Apr 7;686(2):233–239. doi: 10.1016/0005-2736(82)90117-1. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Rose J. K. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992 Feb 7;68(3):533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Bunow M. R., Bunow B. Phase behavior of ganglioside-lecithin mixtures. Relation to dispersion of gangliosides in membranes. Biophys J. 1979 Sep;27(3):325–337. doi: 10.1016/S0006-3495(79)85221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral-Lilly D., Sosinsky G. E., Reed R. A., McDermott M. R., Shipley G. G. Orientation of cholera toxin bound to model membranes. Biophys J. 1994 Apr;66(4):935–941. doi: 10.1016/S0006-3495(94)80894-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cestaro B., Barenholz Y., Gatt S. Hydrolysis of di- and trisialo gangliosides in micellar and liposomal dispersion by bacterial neuraminidases. Biochemistry. 1980 Feb 19;19(4):615–619. doi: 10.1021/bi00545a002. [DOI] [PubMed] [Google Scholar]
- Curatolo W., Small D. M., Shipley G. G. Phase behavior and structural characteristics of hydrated bovine brain gangliosides. Biochim Biophys Acta. 1977 Jul 4;468(1):11–20. doi: 10.1016/0005-2736(77)90147-x. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Brady R. O. Biosynthesis and function of gangliosides. Science. 1976 Nov 26;194(4268):906–915. doi: 10.1126/science.185697. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Pacuszka T., Orlandi P. A. Gangliosides as receptors for bacterial enterotoxins. Adv Lipid Res. 1993;25:165–187. [PubMed] [Google Scholar]
- Fredman P. Glycosphingolipid tumor antigens. Adv Lipid Res. 1993;25:213–234. [PubMed] [Google Scholar]
- GAMMACK D. B. PHYSICOCHEMICAL PROPERTIES OF OX-BRAIN GANGLIOSIDES. Biochem J. 1963 Aug;88:373–383. doi: 10.1042/bj0880373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillard B. K., Heath J. P., Thurmon L. T., Marcus D. M. Association of glycosphingolipids with intermediate filaments of human umbilical vein endothelial cells. Exp Cell Res. 1991 Feb;192(2):433–444. doi: 10.1016/0014-4827(91)90062-y. [DOI] [PubMed] [Google Scholar]
- Haas N. S., Shipley G. G. Structure and properties of N-palmitoleoylgalactosylsphingosine (cerebroside). Biochim Biophys Acta. 1995 Dec 13;1240(2):133–141. doi: 10.1016/0005-2736(95)00174-3. [DOI] [PubMed] [Google Scholar]
- Hakomori S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990 Nov 5;265(31):18713–18716. [PubMed] [Google Scholar]
- Hakomori S. Glycosphingolipids in cellular interaction, differentiation, and oncogenesis. Annu Rev Biochem. 1981;50:733–764. doi: 10.1146/annurev.bi.50.070181.003505. [DOI] [PubMed] [Google Scholar]
- Hakomori S., Igarashi Y. Gangliosides and glycosphingolipids as modulators of cell growth, adhesion, and transmembrane signaling. Adv Lipid Res. 1993;25:147–162. [PubMed] [Google Scholar]
- Higashi H., Omori A., Yamagata T. Calmodulin, a ganglioside-binding protein. Binding of gangliosides to calmodulin in the presence of calcium. J Biol Chem. 1992 May 15;267(14):9831–9838. [PubMed] [Google Scholar]
- Hill M. W., Lester R. Mixtures of gangliosides and phosphatidylcholine in aqueous dispersions. Biochim Biophys Acta. 1972 Sep 1;282(1):18–30. doi: 10.1016/0005-2736(72)90307-0. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Körner O., Nicolau C. Influence of gangliosides GM1 and GD1a on structural and thermotropic properties of sonicated small 1,2-dipalmitoyl-L-alpha-phosphatidylcholine vesicles. Biochim Biophys Acta. 1981 May 20;643(3):557–571. doi: 10.1016/0005-2736(81)90352-7. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., Kok J. W. Trafficking of glycosphingolipids in eukaryotic cells; sorting and recycling of lipids. Biochim Biophys Acta. 1992 Dec 11;1113(3-4):277–294. doi: 10.1016/0304-4157(92)90002-r. [DOI] [PubMed] [Google Scholar]
- Koerner T. A., Jr, Prestegard J. H., Demou P. C., Yu R. K. High-resolution proton NMR studies of gangliosides. 1. Use of homonuclear two-dimensional spin-echo J-correlated spectroscopy for determination of residue composition and anomeric configurations. Biochemistry. 1983 May 24;22(11):2676–2687. doi: 10.1021/bi00280a014. [DOI] [PubMed] [Google Scholar]
- Koerner T. A., Jr, Prestegard J. H., Demou P. C., Yu R. K. High-resolution proton NMR studies of gangliosides. 2. Use of two-dimensional nuclear Overhauser effect spectroscopy and sialylation shifts for determination of oligosaccharide sequence and linkage sites. Biochemistry. 1983 May 24;22(11):2687–2690. doi: 10.1021/bi00280a015. [DOI] [PubMed] [Google Scholar]
- Maggio B., Ariga T., Sturtevant J. M., Yu R. K. Thermotropic behavior of binary mixtures of dipalmitoylphosphatidylcholine and glycosphingolipids in aqueous dispersions. Biochim Biophys Acta. 1985 Aug 8;818(1):1–12. doi: 10.1016/0005-2736(85)90131-2. [DOI] [PubMed] [Google Scholar]
- Maggio B., Ariga T., Sturtevant J. M., Yu R. K. Thermotropic behavior of glycosphingolipids in aqueous dispersions. Biochemistry. 1985 Feb 26;24(5):1084–1092. doi: 10.1021/bi00326a003. [DOI] [PubMed] [Google Scholar]
- Maggio B., Cumar F. A., Caputto R. Configuration and interaction of the polar head group in gangliosides. Biochem J. 1980 Sep 1;189(3):435–440. doi: 10.1042/bj1890435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio B., Cumar F. A., Caputto R. Interactions of gangliosides with phospholipids and glycosphingolipids in mixed monolayers. Biochem J. 1978 Dec 1;175(3):1113–1118. doi: 10.1042/bj1751113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio B., Cumar F. A., Caputto R. Molecular behaviour of glycosphingolipids in interfaces. Possible participation in some properties of nerve membranes. Biochim Biophys Acta. 1981 Dec;650(2-3):69–87. doi: 10.1016/0304-4157(81)90001-0. [DOI] [PubMed] [Google Scholar]
- Maggio B., Cumar F. A., Caputto R. Surface behaviour of gangliosides and related glycosphingolipids. Biochem J. 1978 Jun 1;171(3):559–565. doi: 10.1042/bj1710559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio B., Montich G. G., Cumar F. A. Surface topography of sulfatide and gangliosides in unilamellar vesicles of dipalmitoylphosphatidylcholine. Chem Phys Lipids. 1988 Feb;46(2):137–146. doi: 10.1016/0009-3084(88)90124-7. [DOI] [PubMed] [Google Scholar]
- Masserini M., Freire E. Thermotropic characterization of phosphatidylcholine vesicles containing ganglioside GM1 with homogeneous ceramide chain length. Biochemistry. 1986 Mar 11;25(5):1043–1049. doi: 10.1021/bi00353a014. [DOI] [PubMed] [Google Scholar]
- McDaniel R. V., McIntosh T. J. X-Ray Diffraction Studies of the Cholera Toxin receptor, G(M1). Biophys J. 1986 Jan;49(1):94–96. doi: 10.1016/s0006-3495(86)83606-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Hydration force and bilayer deformation: a reevaluation. Biochemistry. 1986 Jul 15;25(14):4058–4066. doi: 10.1021/bi00362a011. [DOI] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A. Long- and short-range interactions between phospholipid/ganglioside GM1 bilayers. Biochemistry. 1994 Aug 30;33(34):10477–10486. doi: 10.1021/bi00200a032. [DOI] [PubMed] [Google Scholar]
- Mehlhorn I. E., Parraga G., Barber K. R., Grant C. W. Visualization of domains in rigid ganglioside/phosphatidylcholine bilayers: Ca2+ effects. Biochim Biophys Acta. 1986 Dec 16;863(2):139–155. doi: 10.1016/0005-2736(86)90254-3. [DOI] [PubMed] [Google Scholar]
- Myers M., Wortman C., Freire E. Modulation of neuraminidase activity by the physical state of phospholipid bilayers containing gangliosides Gd1a and Gt1b. Biochemistry. 1984 Mar 27;23(7):1442–1448. doi: 10.1021/bi00302a016. [DOI] [PubMed] [Google Scholar]
- Norton W. T., Poduslo S. E. Neuronal perikarya and astroglia of rat brain: chemical composition during myelination. J Lipid Res. 1971 Jan;12(1):84–90. [PubMed] [Google Scholar]
- Parton R. G., Simons K. Digging into caveolae. Science. 1995 Sep 8;269(5229):1398–1399. doi: 10.1126/science.7660120. [DOI] [PubMed] [Google Scholar]
- Perillo M. A., Polo A., Guidotti A., Costa E., Maggio B. Molecular parameters of semisynthetic derivatives of gangliosides and sphingosine in monolayers at the air-water interface. Chem Phys Lipids. 1993 Oct;65(3):225–238. doi: 10.1016/0009-3084(93)90020-4. [DOI] [PubMed] [Google Scholar]
- Peters M. W., Mehlhorn I. E., Barber K. R., Grant C. W. Evidence of a distribution difference between two gangliosides in bilayer membranes. Biochim Biophys Acta. 1984 Dec 19;778(3):419–428. doi: 10.1016/0005-2736(84)90389-4. [DOI] [PubMed] [Google Scholar]
- Reed R. A., Mattai J., Shipley G. G. Interaction of cholera toxin with ganglioside GM1 receptors in supported lipid monolayers. Biochemistry. 1987 Feb 10;26(3):824–832. doi: 10.1021/bi00377a025. [DOI] [PubMed] [Google Scholar]
- Reed R. A., Shipley G. G. Effect of chain unsaturation on the structure and thermotropic properties of galactocerebrosides. Biophys J. 1989 Feb;55(2):281–292. doi: 10.1016/S0006-3495(89)82803-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R. A., Shipley G. G. Structure and metastability of N-lignocerylgalactosylsphingosine (cerebroside) bilayers. Biochim Biophys Acta. 1987 Jan 26;896(2):153–164. doi: 10.1016/0005-2736(87)90175-1. [DOI] [PubMed] [Google Scholar]
- Ruocco M. J., Atkinson D., Small D. M., Skarjune R. P., Oldfield E., Shipley G. G. X-ray diffraction and calorimetric study of anhydrous and hydrated N-palmitoylgalactosylsphingosine (cerebroside). Biochemistry. 1981 Oct 13;20(21):5957–5966. doi: 10.1021/bi00524a006. [DOI] [PubMed] [Google Scholar]
- Ruocco M. J., Shipley G. G. Interaction of cholesterol with galactocerebroside and galactocerebroside-phosphatidylcholine bilayer membranes. Biophys J. 1984 Dec;46(6):695–707. doi: 10.1016/S0006-3495(84)84068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruocco M. J., Shipley G. G., Oldfield E. Galactocerebroside-phospholipid interactions in bilayer membranes. Biophys J. 1983 Jul;43(1):91–101. doi: 10.1016/S0006-3495(83)84327-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruocco M. J., Shipley G. G. Thermal and structural behavior of natural cerebroside 3-sulfate in bilayer membranes. Biochim Biophys Acta. 1986 Jul 24;859(2):246–256. doi: 10.1016/0005-2736(86)90220-8. [DOI] [PubMed] [Google Scholar]
- Scarsdale J. N., Prestegard J. H., Yu R. K. NMR and computational studies of interactions between remote residues in gangliosides. Biochemistry. 1990 Oct 23;29(42):9843–9855. doi: 10.1021/bi00494a014. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. E., McIntosh D. P., Dvorak A. M., Liu J., Oh P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science. 1995 Sep 8;269(5229):1435–1439. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
- Siebert H. C., Reuter G., Schauer R., von der Lieth C. W., Dabrowski J. Solution conformations of GM3 gangliosides containing different sialic acid residues as revealed by NOE-based distance mapping, molecular mechanics, and molecular dynamics calculations. Biochemistry. 1992 Aug 4;31(30):6962–6971. doi: 10.1021/bi00145a014. [DOI] [PubMed] [Google Scholar]
- Sillerud L. O., Schafer D. E., Yu R. K., Konigsberg W. H. Calorimetric properties of mixtures of ganglioside GM1 and dipalmitoylphosphatidylcholine. J Biol Chem. 1979 Nov 10;254(21):10876–10880. [PubMed] [Google Scholar]
- Singh D., Jarrell H. C., Barber K. R., Grant C. W. Glycosphingolipids: 2H NMR study of the influence of ceramide fatty acid characteristics on the carbohydrate headgroup in phospholipid bilayers. Biochemistry. 1992 Mar 17;31(10):2662–2669. doi: 10.1021/bi00125a005. [DOI] [PubMed] [Google Scholar]
- Svennerholm L., Fredman P. A procedure for the quantitative isolation of brain gangliosides. Biochim Biophys Acta. 1980 Jan 18;617(1):97–109. doi: 10.1016/0005-2760(80)90227-1. [DOI] [PubMed] [Google Scholar]
- Tettamanti G., Riboni L. Gangliosides and modulation of the function of neural cells. Adv Lipid Res. 1993;25:235–267. [PubMed] [Google Scholar]
- Thompson T. E., Allietta M., Brown R. E., Johnson M. L., Tillack T. W. Organization of ganglioside GM1 in phosphatidylcholine bilayers. Biochim Biophys Acta. 1985 Jul 25;817(2):229–237. doi: 10.1016/0005-2736(85)90024-0. [DOI] [PubMed] [Google Scholar]
- Torbet J., Wilkins M. H. X-ray diffraction studies of lecithin bilayers. J Theor Biol. 1976 Oct 21;62(2):447–458. doi: 10.1016/0022-5193(76)90129-6. [DOI] [PubMed] [Google Scholar]
- Worthington C. R., Blaurock A. E. A structural analysis of nerve myelin. Biophys J. 1969 Jul;9(7):970–990. doi: 10.1016/S0006-3495(69)86431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller C. B., Marchase R. B. Gangliosides as modulators of cell function. Am J Physiol. 1992 Jun;262(6 Pt 1):C1341–C1355. doi: 10.1152/ajpcell.1992.262.6.C1341. [DOI] [PubMed] [Google Scholar]
- Zhang R. G., Scott D. L., Westbrook M. L., Nance S., Spangler B. D., Shipley G. G., Westbrook E. M. The three-dimensional crystal structure of cholera toxin. J Mol Biol. 1995 Aug 25;251(4):563–573. doi: 10.1006/jmbi.1995.0456. [DOI] [PubMed] [Google Scholar]
- Zhang R. G., Westbrook M. L., Westbrook E. M., Scott D. L., Otwinowski Z., Maulik P. R., Reed R. A., Shipley G. G. The 2.4 A crystal structure of cholera toxin B subunit pentamer: choleragenoid. J Mol Biol. 1995 Aug 25;251(4):550–562. doi: 10.1006/jmbi.1995.0455. [DOI] [PubMed] [Google Scholar]




