Abstract
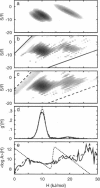
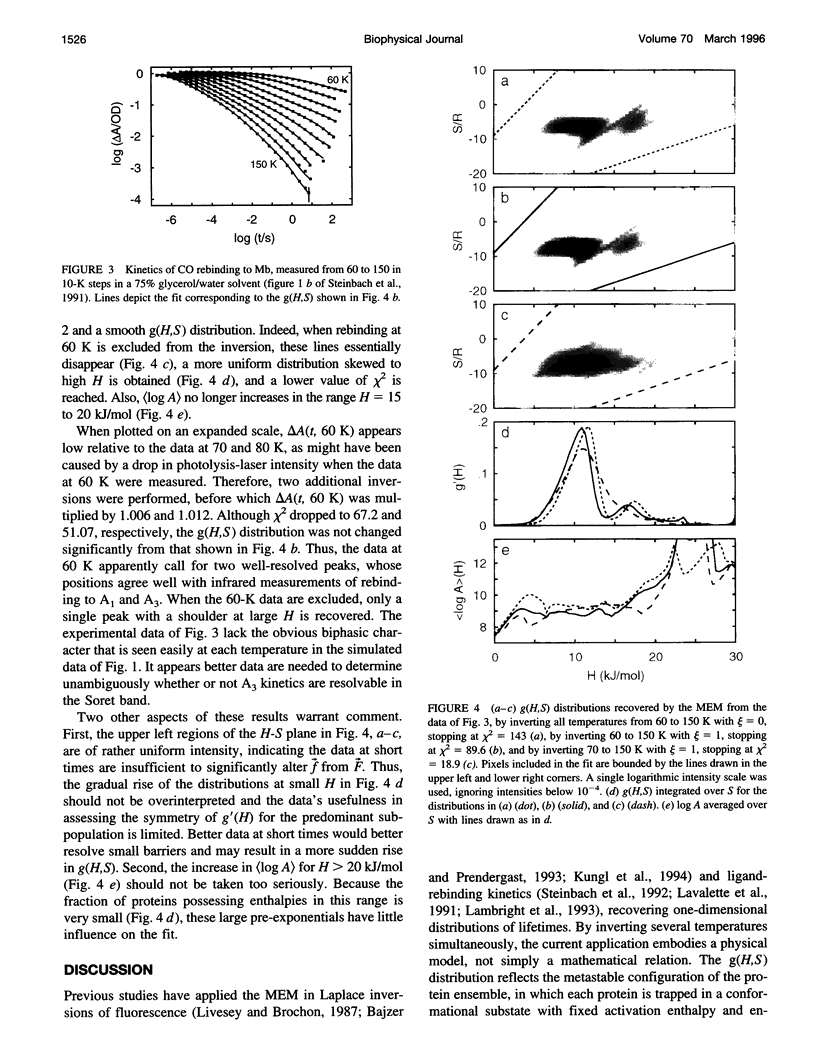
The maximum entropy method (MEM) is used to numerically invert the kinetics of ligand rebinding at low temperatures to obtain the underlying two-dimensional distribution of activation enthalpies and entropies, g(H,S). A global analysis of the rebinding of carbon monoxide (CO) to myoglobin (Mb), monitored in the Soret band at temperatures from 60 to 150 K, is performed using a Newton-Raphson optimization algorithm. The MEM approach describes the data much better than traditional least-squares analyses, reducing chi 2 by an order of magnitude. The MEM resolves two barrier distributions suggestive of rebinding to different bound conformations of MbCO, the so-called A1 and A3 substates, whose activation barriers have been independently estimated from kinetics monitored in the infrared. The distribution corresponding to A3 possesses higher activation entropies, also consistent with infrared measurements. Within an A substate, correlations of S and H are recovered qualitatively from simulated data but can be difficult to obtain from experimental data. When the rebinding measured at 60 K is excluded from the inversion, two peaks are no longer clearly resolved. Thus, data of very high quality are required to unambiguously determine the kinetic resolvability of subpopulations and the shape of the barrier distribution for a single A substate.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberding N., Chan S. S., Eisenstein L., Frauenfelder H., Good D., Gunsalus I. C., Nordlund T. M., Perutz M. F., Reynolds A. H., Sorensen L. B. Binding of carbon monoxide to isolated hemoglobin chains. Biochemistry. 1978 Jan 10;17(1):43–51. doi: 10.1021/bi00594a007. [DOI] [PubMed] [Google Scholar]
- Ansari A., Berendzen J., Bowne S. F., Frauenfelder H., Iben I. E., Sauke T. B., Shyamsunder E., Young R. D. Protein states and proteinquakes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5000–5004. doi: 10.1073/pnas.82.15.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari A., Berendzen J., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Johnson J. B., Ormos P., Sauke T. B. Rebinding and relaxation in the myoglobin pocket. Biophys Chem. 1987 May 9;26(2-3):337–355. doi: 10.1016/0301-4622(87)80034-0. [DOI] [PubMed] [Google Scholar]
- Austin R. H., Beeson K. W., Eisenstein L., Frauenfelder H., Gunsalus I. C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975 Dec 2;14(24):5355–5373. doi: 10.1021/bi00695a021. [DOI] [PubMed] [Google Scholar]
- Bajzer Z., Prendergast F. G. A model for multiexponential tryptophan fluorescence intensity decay in proteins. Biophys J. 1993 Dec;65(6):2313–2323. doi: 10.1016/S0006-3495(93)81325-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendzen J., Braunstein D. Temperature-derivative spectroscopy: a tool for protein dynamics. Proc Natl Acad Sci U S A. 1990 Jan;87(1):1–5. doi: 10.1073/pnas.87.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlott D. D., Frauenfelder H., Langer P., Roder H., DiIorio E. E. Nanosecond flash photolysis study of carbon monoxide binding to the beta chain of hemoglobin Zürich [beta 63(E7)His leads to Arg]. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6239–6243. doi: 10.1073/pnas.80.20.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenstein D., Nienhaus G. U. Conformational substates in azurin. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9681–9685. doi: 10.1073/pnas.89.20.9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frauenfelder H., Parak F., Young R. D. Conformational substates in proteins. Annu Rev Biophys Biophys Chem. 1988;17:451–479. doi: 10.1146/annurev.bb.17.060188.002315. [DOI] [PubMed] [Google Scholar]
- Hagen S. J., Hofrichter J., Eaton W. A. Protein reaction kinetics in a room-temperature glass. Science. 1995 Aug 18;269(5226):959–962. doi: 10.1126/science.7638618. [DOI] [PubMed] [Google Scholar]
- Kungl A. J., Visser A. J., Kauffmann H. F., Breitenbach M. Time-resolved fluorescence studies of dityrosine in the outer layer of intact yeast ascospores. Biophys J. 1994 Jul;67(1):309–317. doi: 10.1016/S0006-3495(94)80482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambright D. G., Balasubramanian S., Boxer S. G. Dynamics of protein relaxation in site-specific mutants of human myoglobin. Biochemistry. 1993 Sep 28;32(38):10116–10124. doi: 10.1021/bi00089a030. [DOI] [PubMed] [Google Scholar]
- Lavalette D., Tetreau C., Brochon J. C., Livesey A. Conformational fluctuations and protein reactivity. Determination of the rate-constant spectrum and consequences in elementary biochemical processes. Eur J Biochem. 1991 Mar 28;196(3):591–598. doi: 10.1111/j.1432-1033.1991.tb15854.x. [DOI] [PubMed] [Google Scholar]
- Livesey A. K., Brochon J. C. Analyzing the distribution of decay constants in pulse-fluorimetry using the maximum entropy method. Biophys J. 1987 Nov;52(5):693–706. doi: 10.1016/S0006-3495(87)83264-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. L. A model of protein conformational substates. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3670–3672. doi: 10.1073/pnas.82.11.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
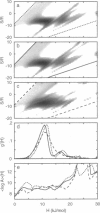
- Steinbach P. J., Ansari A., Berendzen J., Braunstein D., Chu K., Cowen B. R., Ehrenstein D., Frauenfelder H., Johnson J. B., Lamb D. C. Ligand binding to heme proteins: connection between dynamics and function. Biochemistry. 1991 Apr 23;30(16):3988–4001. doi: 10.1021/bi00230a026. [DOI] [PubMed] [Google Scholar]
- Steinbach P. J., Chu K., Frauenfelder H., Johnson J. B., Lamb D. C., Nienhaus G. U., Sauke T. B., Young R. D. Determination of rate distributions from kinetic experiments. Biophys J. 1992 Jan;61(1):235–245. doi: 10.1016/S0006-3495(92)81830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]