Abstract
R-type Ca2+ channels play a critical role in coupling excitability to dendritic Ca2+ influx and neuronal secretion. Unlike other types of voltage-sensitive Ca2+ channels (L, N, P/Q, and T type), the molecular basis for the R-type Ca2+ channel is still unclear, thereby limiting further detailed analyses of R-type Ca2+ channel physiology. The prevailing hypothesis is that α1E (CaV2.3) gene encodes for R-type Ca2+ channels, but the dearth of critical evidence has rendered this hypothesis controversial. Here we generated α1E-deficient mice (α1E−/−) and examined the status of voltage-sensitive Ca2+ currents in central amygdala (CeA) neurons that exhibit abundant α1E expression and R-type Ca2+ currents. The majority of R-type currents in CeA neurons were eliminated in α1E−/− mice whereas other Ca2+ channel types were unaffected. These data clearly indicate that the expression of α1E gene underlies R-type Ca2+ channels in CeA neurons. Furthermore, the α1E−/− mice exhibited signs of enhanced fear as evidenced by their vigorous escaping behavior and aversion to open-field conditions. These latter findings imply a possible role of α1E-based R-type Ca2+ currents in amygdala physiology associated with fear.
Voltage-sensitive Ca2+ channels, classified into L, N, P/Q, R, and T types, are critical for coupling excitability to Ca2+-dependent processes within neurons and excitable cells (1). Whereas much has been learned about L, N, P/Q, and T types, the importance of R-type Ca2+ channels, originally defined as a channel “resistant” to blockers for L-, N-, and P/Q-type Ca2+ channels, has not been revealed up until recently. The R-type Ca2+ channel has been suggested to play a critical role in the release of neurotransmitters (2–5) and to be the major source of dendritic Ca2+ influx in response to action potentials (6–8). Thus, R-type Ca2+ channels appear to be a key component in controlling neural functions.
The identification of α1 subunit for each Ca2+ channel has been very critical for understanding Ca2+ channel physiology, and α1 subunits for L, N, P/Q, and T types have been successfully documented mainly because of the availability of a number of drugs and toxins that selectively target corresponding α1 subunits. Although the matching of R-type channel to α1 subunit is perhaps the main outstanding issue in the whole Ca2+ channel field, it has been difficult to characterize the corresponding α1 subunit for R-type Ca2+ channels mainly because of the lack of selective antagonists for the R-type channel currents (9).
It has been hypothesized that R-type Ca2+ currents result from the expression of the α1E gene (10–12). Antisense knockout of the α1E subunit in cerebellar granule neurons produced a partial reduction in R-type Ca2+ currents, in good agreement with this hypothesis (13, 14). However, a toxin (SNX-482) that selectively blocked the α1E–based Ca2+ currents in heterologous expression systems failed to block R-type Ca2+ currents in several instances, including cerebellar granule neurons (9, 14). Even more confusion has been introduced to this controversial subject by a recent study using α1E-deficient mice. This particular study has suggested that the majority of R-type Ca2+ currents in cerebellar granule neurons do not result from the expression of α1E (15). Thus, experiments using different methods give rise to contradictory results in cerebellar granule cells, rendering the molecular nature of R-type Ca2+ channels elusive.
In the present study, we provide clear-cut evidence that complete deletion of α1E specifically eliminates the majority of R-type components of Ca2+ currents in central amygdala (CeA) neurons, strongly supporting the hypothesis that R-type channels are encoded by α1E. Furthermore, we present behavioral data that provides insight into the function of α1E-based R-type channel currents in the CeA.
Materials and Methods
Generation of α1E−/− Mice.
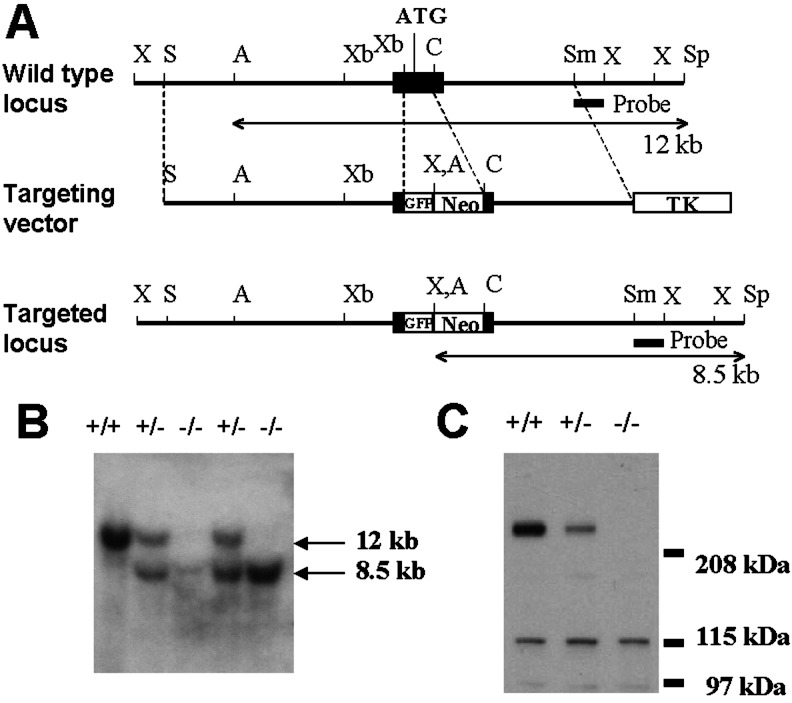
A 24.9-kb genomic DNA fragment containing the exon including the first translated region with the initiation codon of the α1E locus was isolated from a 129/svJae mouse genomic library by screening with the 476-bp (nucleotides 100–575) rat α1E cDNA probe. We designed a targeting vector to replace the 0.5-kb XbaI–ClaI fragment within the exon (from nucleotide 357 to 860 of mouse α1E cDNA) including the initiation codon and a part of the N-terminus cytosolic domain with a fragment containing the green fluorescent protein (GFP) gene and the neo cassette [phosphoglycerol kinase (PGK) promoter-neo-PGK poly(A)] to induce gene disruption and facilitate positive selection (Fig. 1A). As a result, the GFP and neo cassette is flanked by a 6.1-kb SalI–XbaI fragment and a 3.1-kb ClaI–SmaI fragment as 3′ and 5′ homologous regions, respectively. For a negative selection, the thymidine kinase cassette [PGK promoter–herpes simplex virus thymidine kinase–PGK poly(A)] was attached at the 5′ end of the 5′ homology region. Embryonic stem (ES) cell cultures and embryo manipulations were performed as described (16, 17). J1 ES cells were electroporated with the linearized targeting vector, and the positive clones were identified by Southern blotting analysis of ApaI/SpeI-digested genomic DNA. The 0.8-kb SmaI–XhoI fragment in the 5′ flanking region was used as the probe for Southern blotting analysis (Fig. 1A). ES clones harboring the desired homologous recombination were injected into C57BL/6J host blastocysts to generate chimeras. Germ-line-competent male chimeras were bred with C57BL/6J females to produce heterozygous mice (F1). Western blot analysis was used to confirm the lack of the α1E protein in the mutant by using rabbit polyclonal anti-α1E antibodies (Alomone Laboratories, Jerusalem).
Figure 1.
Generation of α1E−/− mice. (A) Structure and simplified restriction maps of the wild-type locus (Top), the targeting vector (Middle), and the disrupted locus (Bottom) of the α1E gene. The exon containing the first translated region of the α1E gene is boxed. Double-arrowhead lines under the wild type, and the targeted locus represent the expected fragments (12 kb for the wild type and 8.5 kb for the mutant) after digestion with ApaI/SpeI and hybridization with the probe (the closed box under the two loci). Restriction sites: A, ApaI; C, ClaI; S, SalI; Sm, SmaI; Sp, SpeI; X, XhoI; Xb, XbaI. GFP, green fluorescent protein; TK, thymidine kinase. (B) Southern analysis of tail DNA from wild-type, heterozygous, and homozygous mutant mice. Genomic DNA was digested with ApaI/SpeI, transferred to a nylon membrane, and hybridized with the 32P-labeled probe. +/+, wild type; +/−, heterozygote; −/−, homozygote. (C) Western blot analysis of brain membrane fractions from wild-type, heterozygous, and homozygous mutant mice. The band around 260 kDa, the predicted size of α1E protein, is specifically absent in the homozygous mutant.
Animals.
To establish parental strains, F1 α1E+/− mice were backcrossed in parallel to C57BL/6J or 129/svJae inbred mice for 3–4 generations. In all experiments, mice in mixed backgrounds obtained from these parental strains of C57BL/6J (N3–4) α1E+/− or 129/svJae (N3–4) α1E+/− mice were used. In voltage-dependence experiments and toxin mixture treatment experiments, mice obtained from crossings of C57BL/6J(N3–4) × C57BL/6J (N3–4) or C57BL/6J (N3–4) × 129/svJae (N3–4) were used. In serial toxin treatment experiments, mice obtained from crossings of C57BL/6J (N3–4) × 129/svJae (N3–4) were used. In behavioral experiments, mice obtained from crossings of C57BL/6J(N4) × C57BL/6J(N4) were used. Age-matched littermates were used as controls in all experiments. Animals were subjected to a 12-h light/dark cycle, with free access to food and water. Animal care and handling was carried out according to the guidelines of the institute.
Preparation of Acutely Dissociated CeA Neurons.
The acutely dissociated CeA neurons were prepared as described (18) from 15- to 25-day-old pups. Brains were cooled rapidly in a dissecting solution containing 120 mM NaCl, 10 mM KCl, 2 mM KH2PO4, 1 mM CaCl2, 6 mM MgSO4, 10 mM d-glucose, and 10 mM piperazine-N,N-bis-[2-ethanesulfonic acid] (pH adjusted to 7.3–7.4) through which 100% O2 was continuously bubbled at 0–5°C. The osmolarity of the dissecting solution was adjusted to 300–310 mOsM with sucrose. Transverse amygdala slices (250 or 300-μm) were sectioned by using a vibratome (Technical Products International, St. Louis) in the cold dissecting solution followed by preincubation in the oxygenated dissecting solution for 20–30 min at room temperature. The slices were then incubated for 10–12 min in the dissecting solution containing pronase E (10–15 mg, 4 units/mg, type XIV, Sigma) and trypsin (7–10 mg, 9 N-benzoyl-l-arginine ethyl ester units/mg, type XI, Sigma), oxygenated at 34.5°C. The slices were then washed twice and placed in the oxygenated dissecting solution at room temperature for another 10 min. Subsequently, the CeA was carefully removed by dissection and triturated gently to dissociate the individual neurons. Healthy-looking neurons of round, oval, or pyramidal shapes were used for patch-clamp recording.
Whole-Cell Patch-Clamp Recording.
Whole-cell patch-clamp experiments were carried out as described (19–22). Patch electrodes were made from borosilicate capillary tubes (1.2 mm OD, standard wall, Warner Instruments, Hamden, CA), pulled with a micropipette puller (model P-87, Sutter Instruments, Novato, CA), and polished with a microforge (MF-9, Narishige, Tokyo). The patch electrodes had a resistance of 2.5–4 MΩ when filled with an internal solution composed of 90 mM cesium acetate, 18 mM tetraethylammonium chloride, 18 mM N-[2-hydroxyethyl]piperazine-N-[2-ethanesulfonic acid], 9 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N,N-tetraacetic acid, 9 mM d-glucose, 5 mM Mg-ATP, 0.2 mM sodium guanosine 5-triphosphate, and 0.1 mM leupeptin, pH adjusted to 7.1–7.2 with 1 M CsOH at room temperature, final osmolarity of 270–280 mOsM. The external solution consisted of 120 mM tetraethylammonium chloride, 3 mM CaCl2, 10 mM N-[2-hydroxyethyl]piperazine-N-[2-ethanesulfonic acid], 10 mM CsCl, 5 mM 4-aminopyridine, 10 mM d-glucose, and 1 μM tetrodotoxin. The pH of the external solution was titrated to ≈7.4 with 1 M HCl or 1 M CsOH, and the osmolarity was adjusted to 320 ± 5 mOsM with sucrose. Currents were recorded by using the Axopatch 200B amplifier, filtered at 1 or 2 kHz by a built-in filter, and stored in the computer. Leak corrections were performed by using a P/4 trace subtraction. The series resistance (<20 MΩ) was compensated >50%. Recordings exhibiting >30% change in series resistance were discarded whereas junction potentials were not corrected. Command pulses were delivered at 15- or 30-s intervals from −70 mV to 10 mV to elicit Ca2+ currents. To examine the voltage dependence of Ca2+ currents, a series of command pulses (−50 to 50 mV) were delivered from a holding potential of −70 mV at 10-s intervals. All of the experiments were performed at room temperature (24–28°C). Data are expressed as means ± SE, and statistical comparisons were made by using the Student's t test.
Drug solutions were delivered by gravity feed (≈1 ml/min) from a linear array of glass capillaries. Nifedipine (Sigma) was prepared as a concentrated stock solution in DMSO and protected from light. ω-Conotoxin GVIA (Alomone Labs) was prepared as a concentrated stock in the external solution and stored in aliquots at −70°C. The stocks of drugs were diluted in the external solution just before each experiment. Cytochrome c (0.1 mg/ml) was included in all external solutions to block nonspecific binding of toxins.
Behavioral Analysis.
Animals at the age of 7–12 weeks were used in behavioral studies with all animals being brought to the experimental room at least 1 h before the behavioral testing. Averaged values were compared statistically by t test (criterion for significance, P < 0.05), unless otherwise noted.
Rotarod test.
The rotarod test was performed as described (20). Briefly, mice were placed in a neutral position on a stationary 3-cm diameter cylinder of the rotarod apparatus (Letica Scientific Instruments, Barcelona). Rotation was initiated and gradually increased from 4 to 35 rpm over the course of 5 min. Latency to fall was recorded for each mouse in three trials with 20-min intervals between trials. The rotarod test was performed between 11 a.m. and 4 p.m.
Open-field test.
The open field (23) was made of white plastic plate and was 40 cm long × 40 cm wide × 37 cm high in size. The light intensity on the central platform was 40 lux. Each mouse was transferred to the periphery of the field, and the total horizontal distance moved and the time spent in the central area were measured for 5 min by using a video tracking system (HVS Image, Buckingham, U.K.). A computer program was used to overlay grid lines that divided the open field into 16 square regions. The inner four square regions were defined as the central area. The open-field test was performed between 11 a.m. and 4 p.m.
Elevated plus maze.
The elevated plus maze (24) consisted of two open arms (25 cm × 8 cm) and two closed arms with a 15-cm high wall arranged such that arms of the same type were opposite each other with arms being connected by an open central area. The light intensity on the central platform was 20 lux. All parts of the apparatus were made of black plastic plate, and the maze was placed at a height of 50 cm from the floor. Each mouse was transferred to the closed arm facing the central area and was allowed to explore freely for 5 min. The amount of time spent in the open and closed arms was measured. The elevated plus maze was performed during the early dark phase of light-dark cycles.
Results
Generation of α1E−/− Mice.
Homozygous α1E−/− mice were obtained by mating heterozygotes. Wild-type, heterozygous, and homozygous mutant offspring were produced at the predicted Mendelian ratio (1:2:1). Homozygous mice were healthy and fertile and exhibited no gross phenotypic abnormality. To confirm the null mutation of the α1E gene we performed Western blot analysis with wild-type, heterozygote, and homozygote mouse brains (Fig. 1C). Probing of membrane fractions of wild-type brain with anti-α1E antibodies resulted in an intense band at around 260 kDa, which is the predicted size of the α1E protein, together with additional weaker bands with molecular masses less than 130 kDa. The smaller bands are believed to be nonspecific, crossreactive bands. The 260-kDa band was specifically absent in membrane fractions of homozygous mutant brain as depicted in Fig. 1C. From this result, we conclude that a null mutation of α1E was successfully produced by our targeting strategy.
Reduced R-Type Currents in the α1E−/− CeA Neurons.
Both mRNA and translated protein products of the α1E gene are abundantly expressed in the amygdaloid complex (25–27). R-type currents comprise the largest component of total Ca2+ currents in the CeA neurons and resemble the general characteristics of α1E-based currents defined in heterologous expression systems (18). To study the relationship between the α1E subunits and R-type currents we examined the properties of the voltage-sensitive Ca2+ currents in CeA neurons acutely dissociated from α1E−/− mice and their wild-type littermates.
Fig. 2A illustrates a typical Ca2+ current elicited by step depolarizations to a wide range of voltages. In α1E+/+ neurons, the Ca2+ currents are normally activated at around −20 mV and peaked at around 10 mV. The current voltage relationship in α1E−/− neurons was similar to that evident in α1E+/+ neurons, whereas the total current density in α1E−/− neurons was significantly reduced at all testing voltages above −10 mV (Fig. 2B). Thus, the component of Ca2+ currents absent in α1E−/− neurons appears to be the high voltage-activated type. This finding is consistent with a previous report in which α1E encoded a high voltage-activated type channel in a heterologous expression system when Ca2+ ions were used as charge carriers (26, 28).
Figure 2.
Voltage dependence of the total Ca2+ current density in acutely dissociated CeA neurons. (A) Representative superimposed current traces of CeA neurons from α1E+/+ [Left, whole-cell capacitance (WC) = 5.5 pF] and α1E−/− (Right, WC = 7 pF) mice are shown. Ca2+ currents were elicited by a series of voltage steps from −50 to +20 mV (with 10-mV increments) delivered at 10-s intervals with holding potential at −70 mV. (B) The current-voltage plot in both genotypes. Ca2+ currents were elicited by a series of voltage steps from −50 to +50 mV (with 10-mV increments) delivered at 10-s intervals with holding potential at −70 mV. ●, α1E+/+ (n = 17); ○, α1E−/− (n = 12).
We have used two different protocols to estimate the proportion of R-type Ca2+ current in the neurons. In the first protocol, the simultaneous blockade of L-, N- and P/Q-type Ca2+ channels was achieved by applying a mixture of 10 μM nifedipine, 2 μM ω-conotoxin GVIA, and 0.4 μM ω-agatoxin IVA. In the second protocol, the successive application of specific blockers was performed including 10 μM nifedipine for L-type, 1 μM ω-conotoxin GVIA for N-type, and 0.4 μM ω-agatoxin IVA for P/Q-type Ca2+ channels. In response to the mixture treatment, the current density was reduced from 69.6 ± 8.9 pA/pF before treatment (n = 8) to 28.0 ± 3.9 pA/pF after treatment (n = 8) in α1E+/+ neurons. On the other hand, in α1E−/− neurons, the current density was reduced from 45.3 ± 7.9 pA/pF before treatment (n = 6) to 5.5 ± 1.3 pA/pF after treatment (n = 6) (Fig. 3A). This result represents a decrease of the R-type current density in the mutant compared with the wild type by 81% (P < 0.001). The experiments using serial blockers gave a comparable result to that obtained with the mixture treatment (Fig. 3B): the current density was reduced from 83.7 ± 7.2 pA/pF before treatment (n = 9) to 37.1 ± 3.1 pA/pF after treatment (n = 5) in α1E+/+ neurons, whereas, in α1E−/− neurons, the current density was reduced from 46.2 ± 7.4 pA/pF before treatment (n = 15) to 4.6 ± 0.96 pA/pF after treatment (n = 10), a decrease of 88% in the R-type current density in the mutant compared with the wild type (P < 0.0001). When the data from both protocols were pooled together, total current density was decreased from 77.0 ± 5.7 pA/pF (n = 17) in α1E+/+ to 46.0 ± 5.7 pA/pF (n = 21) in α1E−/− neurons (P < 0.001), whereas the residual R-type component was reduced from 31.5 ± 2.9 pA/pF (n = 13) in α1E+/+ to 4.9 ± 0.7 pA/pF (n = 16) in α1E−/− neurons, a decrease of 84% (P < 0.0001) (Fig. 3C). Therefore, the reduction in the R-type component accounted for a majority of the reduction in the total current density, suggesting that α1E is responsible for a majority of the R-type component in the CeA neurons. However, it should be noted that some current components, albeit very small, were resistant to the mixture of blockers in α1E−/− neurons, which suggests the possibility that other Ca2+ channels may contribute to R-type currents (Fig. 3) (15).
Figure 3.
Decreased total and R-type Ca2+ current densities in CeA neurons in α1E−/− mice. (A) Analysis of currents using a mixture of blockers. Peak ICa, activated by 80 or 60 msec depolarization from −70 mV to 10 mV every 30 s, is plotted against time for an α1E+/+ neuron (WC = 17 pF) and an α1E−/− neuron (WC = 9 pF). R-type Ca2+ current was isolated by application of a mixture consisting of 10 μM nifedipine, 2 μM ω-conotoxin GVIA, and 0.4 μM ω-agatoxin-IVA, and the Ca2+ current was identified by a final application of 200 μM Cd2+. (Right) Examples of typical tracings are shown. (Upper) α1E+/+. (Lower) α1E−/−. CeA neurons of α1E−/− exhibited a large reduction in the amount of R-type current density. (B) L-, N-, P/Q-, and R-type Ca2+ current of CeA neurons in α1E+/+ and α1E−/− CeA neurons. Peak ICa activated by 60-msec depolarization from −70 mV to 10 mV every 15 s is plotted against time for an α1E+/+ neuron (WC = 8 pF) and an α1E−/− neuron (WC = 18 pF). L-, N-, P/Q-, and R-type currents were blocked by serial applications of 10 μM nifedipine, 1 μM ω-conotoxin-GVIA, 0.4 μM ω-agatoxin-IVA, and 200 μM Cd2+. (Right) Examples of typical tracings are shown. (Upper) α1E+/+. (Lower) α1E−/−. (C) Histogram of total and individual channel type current densities in α1E+/+ (filled bars), and α1E−/− (empty bars) CeA neurons. Total current: +/+, n = 15; −/−, n =22; P < 0.001. L type: +/+, n = 8; −/−, n = 14; P > 0.2. N type: +/+, n = 8; −/−, n = 14; P > 0.05. P/Q type: +/+, n = 7; −/−, n = 12; P > 0.9. R type: +/+, n = 13; −/−, n = 16, P < 0.0001. It was noted that the proportion of R-type Ca2+ currents in α1E+/+ neurons exhibited some variation (around 50% of total Ca2+ current in 6/13 cells; around 30% of total Ca2+ current in 7/13 cells.). Data from the two groups of neurons were pooled to calculate R-type currents of the α1E+/+ neurons. Because the total and the R-type current density values were not significantly different between the mixture and the serial treatment protocols, results from both protocols were combined in this histogram.
In an effort to characterize the nature of α1E-based R-type currents, we compared the inactivation kinetics of Ca2+ currents between α1E+/+ and α1E−/− neurons. The inactivation kinetics of total Ca2+ currents in α1E+/+ neurons was more rapid than that evident in α1E−/− neurons (+/+: τ = 29.9 ± 1.2, n = 17; −/−: τ = 46.8 ± 4.0, n = 21; P < 0.001), indicating that the component absent in α1E−/− neurons, supposedly R-type currents, has rapid inactivation kinetics. In fact, after a treatment with the blocker mixture the R-type currents in α1E+/+ neurons exhibited relatively rapid inactivation kinetics (τ = 24.1 ± 1.1, n = 13). These observations are consistent with the previous finding that α1E-based Ca2+ channels exhibited relatively rapid inactivation kinetics in heterologous expression systems (25, 26, 29). This result reinforces the idea that the expression of α1E underlies the majority of R-type currents.
The currents blocked by nifedipine or ω-agatoxin IVA were not significantly altered by deletion of α1E (L type: +/+, 13.4 ± 0.8 pA/pF, n = 8, −/−, 15.2 ± 1.0 pA/pF, n = 14, P > 0.2; P/Q type: +/+, 21.1 ± 2.2 pA/pF, n = 7, −/−, 20.3 ± 7.5 pA/pF, n = 12, P > 0.9) (Fig. 3 B and C). Interestingly, the current component inhibited by ω-conotoxin GVIA appeared to be reduced from 14.9 ± 4.1 pA/pF (n = 8) in α1E+/+ to 7.8 ± 1.3 pA/pF (n = 14) in α1E−/− neurons, but this difference did not reach statistical significance (P > 0.05) (Fig. 3 B and C).
In summary, the majority of R-type currents in CeA neurons were eliminated without a significant alteration of other high voltage-activated Ca2+ channels in α1E-deficient mice.
Emotional Abnormality of α1E−/− Mice.
To examine the behavioral effect of impairment of R-type currents in CeA neurons, we explored the anxiety-related behavior of α1E−/− mice, because the CeA is involved in the regulation of fear or stress-related behavior (30–32). α1E−/− mice appeared normal with no significant motor defects evident in the rotarod test (Fig. 4A). However, α1E−/− mice did exhibit some signs of fear or anxiety. They often showed vigorous escaping behavior during experimental handling. This escaping behavior of α1E−/− mice was characterized by fast explosive runs from corner to corner of a cage and was not observed in wild-type littermates. Mutant mice would occasionally jump out of the cage when the hand of an experimenter approached them. In the open-field test, the α1E−/− mice spent significantly less time in the central area (P < 0.01) but there was no reduction of total locomotor activity, suggesting that the mutant had an increased anxiety level (Fig. 4 B and C). However, no significant differences were noted between wild-type and the α1E−/− mice in the elevated plus maze assay (Fig. 4 D and E). The behavioral phenotypes of the α1E−/− mice are generally consistent with those of a previous study (33) and are suggestive of a fear-related emotional abnormality in α1E−/− mice.
Figure 4.
Rotarod, open-field, and elevated plus maze tests. (A) Rotarod test. No significant difference was observed in the retention time on the rotating cylinder between α1E+/+ and α1E−/− mice (+/+, n = 13; −/−, n = 9). (B and C) Open-field test. The total moving distance (B) and the percentage of time spent in the central area (C) were measured during the first 5 min after placing each mouse in the apparatus (filled bars, +/+, n = 13; empty bars, −/−, n = 9). The mutant mice spent significantly less time in the center area than the wild type. *, P < 0.01. (D and E) Elevated plus maze. The percentage of time spent in the open arms (D) and the total crossing number (E) were counted (filled bar: +/+, n = 13; empty bar: −/−, n = 9). No significant difference was found between α1E+/+ and α1E−/− mice either in the open arms time or in the crossing number.
Discussion
Our findings provide strong evidence that the α1E subunit underlies R-type, toxin-resistant Ca2+ channel currents in CeA neurons and support the hypothesis that R-type Ca2+ channel currents result from the expression of the α1E gene (10–12).
There has been much debate concerning the molecular nature of R-type Ca2+ channels (9, 15, 28). Experiments using antisense oligonucleotides to suppress α1E expression generally support the idea that the α1E gene encodes the Ca2+ channel subunit underlying R-type currents in cerebellar granule neurons (13, 14). In contrast, studies using either the SNX-482 toxin or genetic deletion of α1E suggest that the majority of R-type Ca2+ channel currents did not result from the expression of α1E in cerebellar granule neurons (9, 15). In particular, deletion of α1E in cerebellar granule neurons did not significantly alter the total Ca2+ current density or the R-type current component, implying either the presence of heterogeneity in molecular components for R-type channel currents or some functional compensation by other Ca2+ channels in response to the α1E deletion (15). By contrast, in the present study, genetic deletion of α1E in CeA neurons produced a significant reduction of the total current density that was largely accounted for by a reduction of the R-type current density. These data provide an unequivocal example where complete deletion of α1E specifically eliminates the majority of R-type, toxin-resistant components of Ca2+ currents.
The discrepancy between our results and the results by Wilson et al. (15) could be explained by the fact that the two labs used different concentrations of dihydropyridines to block L-type Ca2+ channels. Wilson et al. (15) used 500 nM nimodipine, whereas we used 10 μM nifedipine, a saturating concentration. Apparently, 500 nM nimodipine has been shown to be a subsaturating concentration to block L-type Ca2+ channel currents including α1D-based currents (34). If subsaturating concentrations of the blocker are used, the R-type current will be contaminated with other Ca2+ currents and lead to an overestimation of the actual R-type component. Not surprisingly, in cerebellar granule neurons, the proportion of L-type Ca2+ current reported by Wilson et al. (15) is substantially smaller than that reported in the previous study using a saturating concentration of 10 μM nimodipine (19). Therefore, the use of a subsaturating concentration of nimodipine in the study of Wilson et al. (15) may cause incomplete blockade of L-type calcium channels, which could contribute to the presence of substantial, toxin-resistant components in α1E−/− neurons.
It should be noted that in α1E−/− CeA neurons some currents, albeit very small, were still resistant to the mixture of blockers for L, N, and P/Q channels in our experimental condition. As discussed in the previous section, such a resistant current component might have resulted from incomplete blockade of L-type calcium channel currents, especially α1D-based currents, by dihydropyridines. α1D-based currents in heterologous expression systems exhibited a very poor sensitivity to dihydropyridine (34, 35). In that study, only ≈50% of α1D-based Ca2+ current was inhibited by 3 μM nimodipine, a saturating concentration (34). Consistent with this idea is a recent report that some of the R-type components in dorsal root ganglion neurons were blocked by very high concentrations of dihydropyridine, thereby implying that α1D-based currents contribute to the R-type currents.** An alternative possibility is that the residual, toxin-resistant current in α1E−/− neurons is caused by incomplete blockade of Q-type currents by the concentration of ω-agatoxin IVA used in our study (400 nM). This concentration of ω-agatoxin IVA has been shown to block around 90% of ω-agatoxin IVA-sensitive currents in cerebellar granule cells (11). Thus, the remaining, small fraction of the Q-type currents resistant to 400 nM ω-agatoxin IVA might contribute to the residual, toxin-resistant currents in α1E−/− neurons.
Deletion of α1E did not appear to alter the expression of other types of Ca2+ channels in CeA neurons, with the possible exception of N-type Ca2+ channels, which exhibited a slight, although not statistically significant, decrease in expression in α1E-deficient mice. Recently the expression of N-type Ca2+ channels has been shown to change in proportion to total Ca2+ current density in superior cervical ganglion neurons (36), which may underlie the slightly diminished expression of N-type Ca2+ channels in α1E-deficient neurons.
What is the functional relevance of α1E-based, R-type Ca2+ channel currents in CeA neurons? In light of their prominent somatic and dendritic location (27), it is likely that R-type channel currents in CeA neurons are involved in the regulation of neuronal excitability. In fact, the blockade of Ca2+ channels dramatically increased neuronal excitability of CeA neurons and reduced afterhyperpolarization, suggesting that voltage-dependent Ca2+ channels regulate somatic and dendritic excitability through Ca2+-activated K+ channels (37). This notion also is supported by a recent study in which dendritic R-type Ca2+ channels were demonstrated to play a major role in mediating Ca2+ influx in response to action potentials (6–8). Because some CeA neurons (≈40% of α1E+/+ CeA neurons) exhibit an extremely large amount of α1E-based, R-type currents (see Fig. 3A and ref. 18), the modulation of R-type currents by other G protein-coupled neurotransmitter receptors (2, 38, 39) would be sufficient to regulate neuronal excitability in the CeA. The α1E−/− mouse therefore will be a very useful tool to examine this question in the future.
The CeA is the major output structure of the amygdaloid complex and projects to brainstem and hypothalamic nuclei that are critically involved in fear responses (30–32). Electrical stimulation of the CeA induced fear-like responses (40–42), whereas lesions of the CeA reduced or completely blocked many autonomic, neuroendocrine, and behavioral responses evoked by unconditioned or conditioned fear-arousing stimuli (43–45). In this respect, the α1E-deficient mice provided us with an opportunity to examine the relationship between R-type channel currents and amygdala-dependent behaviors. The α1E−/− mice exhibited fear-related emotional abnormalities presumably as a result of enhanced neuronal activity in the CeA. As mentioned previously, loss of somatic and dendritic Ca2+ current density in α1E-deficient mice could result in an increased excitability of the CeA (37), which in turn enhances the neuronal output of the whole amygdaloid complex. Thus, a deletion of α1E would enhance fear-related behaviors through heightened outputs from the amygdaloid complex. Further physiological and behavioral analyses of the α1E-deficient mice are merited in the future.
Acknowledgments
We thank K. W. Lee for blastocyst injection, K. Jun for primary screening, J. L. Kim for help in embryonic stem cell culture, S. S. Choi for help in vector construction, Y. Namkung for help in patch-clamp recording, M. P. Kong for animal care, and S. W. Hong for help in genotyping. This work was supported by a grant from the National Creative Research Initiative Program, the Ministry of Science and Technology, Korea (to H.-S.S.), and Korea Ministry of Science and Technology Grant M1–0108-00–0051 under the neurobiology research program to S.C.
Abbreviations
- CeA
central amygdala
- WC
whole-cell capacitance
Footnotes
Oh, S. B., Monteil, A., Bhattacharyya, B., Ren, D. & Miller, R. J. (2001) Soc. Neurosci. Abstr. 31, no. 381.24.
References
- 1.Tsien R W, Wheeler D B. In: Calcium as a Cellular Regulator. Carafoli E, Klee C B, editors. New York: Oxford Univ. Press; 1999. pp. 171–199. [Google Scholar]
- 2.Wu L G, Borst J G, Sakmann B. Proc Natl Acad Sci USA. 1998;95:4720–4725. doi: 10.1073/pnas.95.8.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu L G, Westenbroek R E, Borst J G, Catterall W A, Sakmann B. J Neurosci. 1999;19:726–736. doi: 10.1523/JNEUROSCI.19-02-00726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang G, Dayanithi G, Newcomb R, Lemos J R. J Neurosci. 1999;19:9235–9241. doi: 10.1523/JNEUROSCI.19-21-09235.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albillos A, Neher E, Moser T. J Neurosci. 2000;20:8323–8330. doi: 10.1523/JNEUROSCI.20-22-08323.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Magee J C, Johnston D. J Physiol (London) 1995;487:67–90. doi: 10.1113/jphysiol.1995.sp020862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kavalali E T, Zhuo M, Bito H, Tsien R W. Neuron. 1997;18:651–653. doi: 10.1016/s0896-6273(00)80305-0. [DOI] [PubMed] [Google Scholar]
- 8.Sabatini B L, Svoboda K. Nature (London) 2000;408:589–593. doi: 10.1038/35046076. [DOI] [PubMed] [Google Scholar]
- 9.Newcomb R, Szoke B, Palma A, Wang G, Chen X, Hopkins W, Cong R, Miller J, Urge L, Tarczy-Hornoch K, et al. Biochemistry. 1998;37:15353–15362. doi: 10.1021/bi981255g. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J F, Randall A D, Ellinor P T, Horne W A, Sather W A, Tanabe T, Schwarz T L, Tsien R W. Neuropharmacology. 1993;32:1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]
- 11.Randall A, Tsien R W. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Randall A D, Tsien R W. Neuropharmacology. 1997;36:879–893. doi: 10.1016/s0028-3908(97)00086-5. [DOI] [PubMed] [Google Scholar]
- 13.Piedras-Renteria E S, Tsien R W. Proc Natl Acad Sci USA. 1998;95:7760–7765. doi: 10.1073/pnas.95.13.7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tottene A, Volsen S, Pietrobon D. J Neurosci. 2000;20:171–178. doi: 10.1523/JNEUROSCI.20-01-00171.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson S M, Toth P T, Oh S B, Gillard S E, Volsen S, Ren D, Philipson L H, Lee E C, Fletcher C F, Tessarollo L, et al. J Neurosci. 2000;20:8566–8571. doi: 10.1523/JNEUROSCI.20-23-08566.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim D, Jun K S, Lee S B, Kang N G, Min D S, Kim Y H, Ryu S H, Suh P G, Shin H S. Nature (London) 1997;389:290–293. doi: 10.1038/38508. [DOI] [PubMed] [Google Scholar]
- 17.Cho C H, Kim S S, Jeong M J, Lee C O, Shin H S. Mol Cells. 2000;10:712–722. doi: 10.1007/s10059-000-0712-2. [DOI] [PubMed] [Google Scholar]
- 18.Yu B, Shinnick-Gallagher P. J Neurophysiol. 1997;77:690–701. doi: 10.1152/jn.1997.77.2.690. [DOI] [PubMed] [Google Scholar]
- 19.Jun K, Piedras-Renteria E S, Smith S M, Wheeler D B, Lee S B, Lee T G, Chin H, Adams M E, Scheller R H, Tsien R W, Shin H S. Proc Natl Acad Sci USA. 1999;96:15245–15250. doi: 10.1073/pnas.96.26.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim C, Jun K, Lee T, Kim S S, McEnery M W, Chin H, Kim H L, Park J M, Kim D K, Jung S J, et al. Mol Cell Neurosci. 2001;18:235–245. doi: 10.1006/mcne.2001.1013. [DOI] [PubMed] [Google Scholar]
- 21.Namkung Y, Skrypnyk N, Jeong M J, Lee T, Lee M S, Kim H L, Chin H, Suh P G, Kim S S, Shin H S. J Clin Invest. 2001;108:1015–1022. doi: 10.1172/JCI13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D, Song I, Keum S, Lee T, Jeong M J, Kim S S, McEnery M W, Shin H S. Neuron. 2001;31:35–45. doi: 10.1016/s0896-6273(01)00343-9. [DOI] [PubMed] [Google Scholar]
- 23.Dulawa S C, Grandy D K, Low M J, Paulus M P, Geyer M A. J Neurosci. 1999;19:9550–9556. doi: 10.1523/JNEUROSCI.19-21-09550.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pellow S, File S E. Pharmacol Biochem Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 25.Soong T W, Stea A, Hodson C D, Dubel S J, Vincent S R, Snutch T P. Science. 1993;260:1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- 26.Williams M E, Marubio L M, Deal C R, Hans M, Brust P F, Philipson L H, Miller R J, Johnson E C, Harpold M M, Ellis S B. J Biol Chem. 1994;269:22347–22357. [PubMed] [Google Scholar]
- 27.Yokoyama C T, Westenbroek R E, Hell J W, Soong T W, Snutch T P, Catterall W A. J Neurosci. 1995;15:6419–6432. doi: 10.1523/JNEUROSCI.15-10-06419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bourinet E, Zamponi G W, Stea A, Soong T W, Lewis B A, Jones L P, Yue D T, Snutch T P. J Neurosci. 1996;16:4983–4993. doi: 10.1523/JNEUROSCI.16-16-04983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Page K M, Stephens G J, Berrow N S, Dolphin A C. J Neurosci. 1997;17:1330–1338. doi: 10.1523/JNEUROSCI.17-04-01330.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LeDoux J E, Iwata J, Cicchetti P, Reis D J. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis M. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 32.Applegate C D, Kapp B S, Underwood M D, McNall C L. Physiol Behav. 1983;31:353–360. doi: 10.1016/0031-9384(83)90201-9. [DOI] [PubMed] [Google Scholar]
- 33.Saegusa H, Kurihara T, Zong S, Minowa O, Kazuno A, Han W, Matsuda Y, Yamanaka H, Osanai M, Noda T, Tanabe T. Proc Natl Acad Sci USA. 2000;97:6132–6137. doi: 10.1073/pnas.100124197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu W, Lipscombe D. J Neurosci. 2001;21:5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koschak A, Reimer D, Huber I, Grabner M, Glossmann H, Engel J, Striessnig J. J Biol Chem. 2001;276:22100–22106. doi: 10.1074/jbc.M101469200. [DOI] [PubMed] [Google Scholar]
- 36.Schorge S, Gupta S, Lin Z, McEnery M W, Lipscombe D. Nat Neurosci. 1999;2:785–790. doi: 10.1038/12153. [DOI] [PubMed] [Google Scholar]
- 37.Schiess M C, Asprodini E K, Rainnie D G, Shinnick-Gallagher P. Brain Res. 1993;604:283–297. doi: 10.1016/0006-8993(93)90380-6. [DOI] [PubMed] [Google Scholar]
- 38.Melliti K, Meza U, Adams B. J Neurosci. 2000;20:7167–7173. doi: 10.1523/JNEUROSCI.20-19-07167.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu B, Shinnick-Gallagher P. J Pharmacol Exp Ther. 1998;284:170–179. [PubMed] [Google Scholar]
- 40.Gelsema A J, McKitrick D J, Calaresu F R. Am J Physiol. 1987;253:R712–R718. doi: 10.1152/ajpregu.1987.253.5.R712. [DOI] [PubMed] [Google Scholar]
- 41.Iwata J, Chida K, LeDoux J E. Brain Res. 1987;418:183–188. doi: 10.1016/0006-8993(87)90978-4. [DOI] [PubMed] [Google Scholar]
- 42.Kapp B S, Gallagher M, Underwood M D, McNall C L, Whitehorn D. Brain Res. 1982;234:251–262. doi: 10.1016/0006-8993(82)90866-6. [DOI] [PubMed] [Google Scholar]
- 43.Davis M, Hitchcock J, Rosen J B. In: The Psychology of Learning and Motivation: Advances in Research and Theory. Bower G, editor. Academic New York; 1987. pp. 263–305. [Google Scholar]
- 44.Hitchcock J, Davis M. Behav Neurosci. 1986;100:11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- 45.LeDoux J E. Curr Opin Neurobiol. 1992;2:191–197. doi: 10.1016/0959-4388(92)90011-9. [DOI] [PubMed] [Google Scholar]