Abstract
1. Synchronization of intercostal motoneurones was studied by the construction of cross-correlation histograms which related the firing times of paired groups of efferent inspiratory or expiratory discharges recorded from filaments of the external or internal nerves of anaesthetized or decerebrate cats.
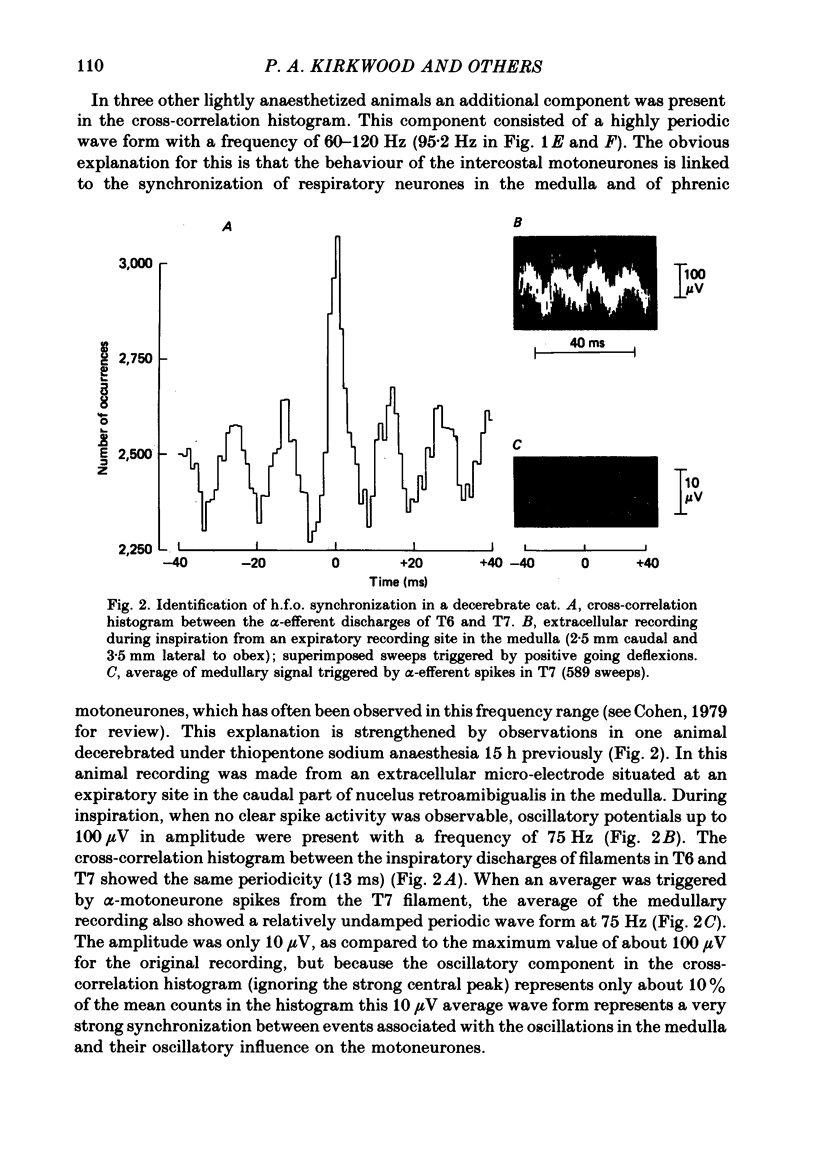
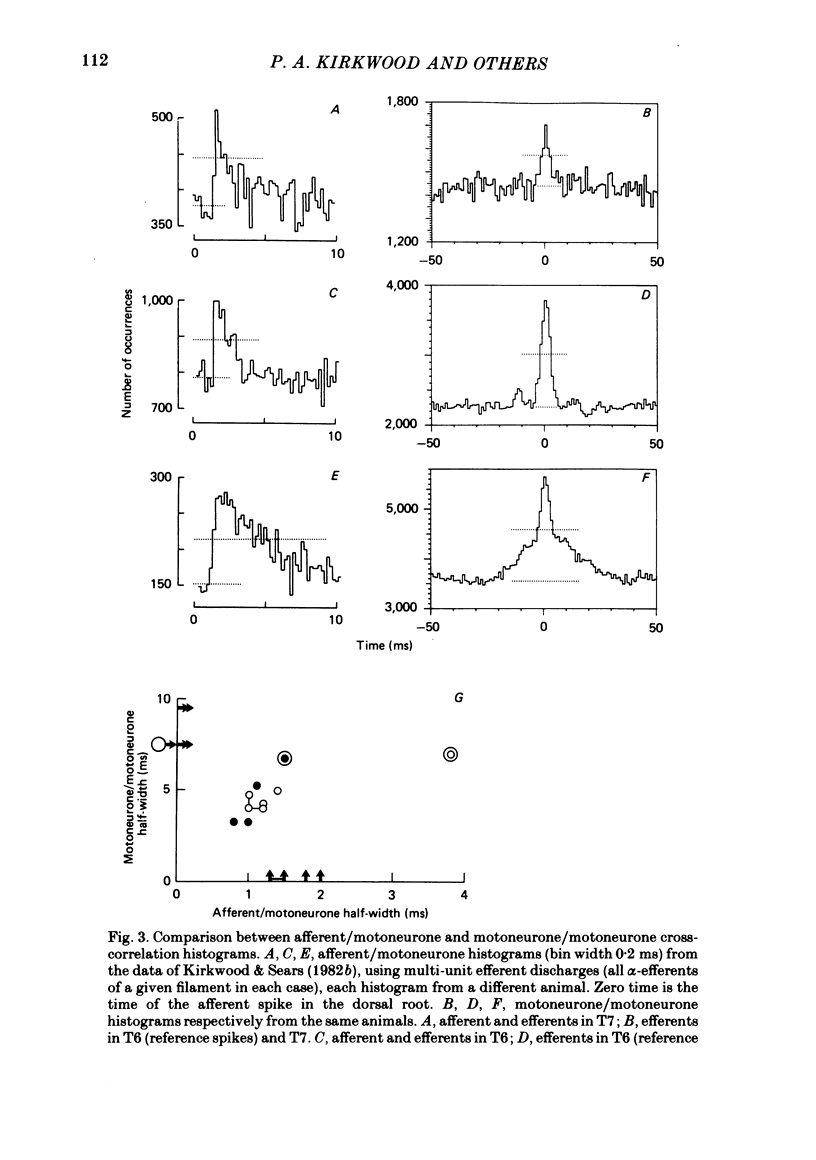
2. The principal feature of the histograms was always a central peak but the time course of the central peak showed considerable variation. Three forms of synchronization were defined on the basis of the time course of the central peak: (i) short-term synchronization (Sears & Stagg, 1976), where the peak was narrow, extending over about ±3 ms but sometimes with weak shoulders to about ±5 ms; (ii) broad-peak synchronization where the peak was wider than this (often ±20 ms or more) but where there were no strong periodicities; (iii) high-frequency oscillation (h.f.o) synchronization, which was named from the related phenomena in medullary and phrenic recordings (Cohen, 1979), where there were periodic peaks on either side of the central peak with a frequency in the range 60-120 Hz. Combinations of these forms of synchronization were seen in some histograms.
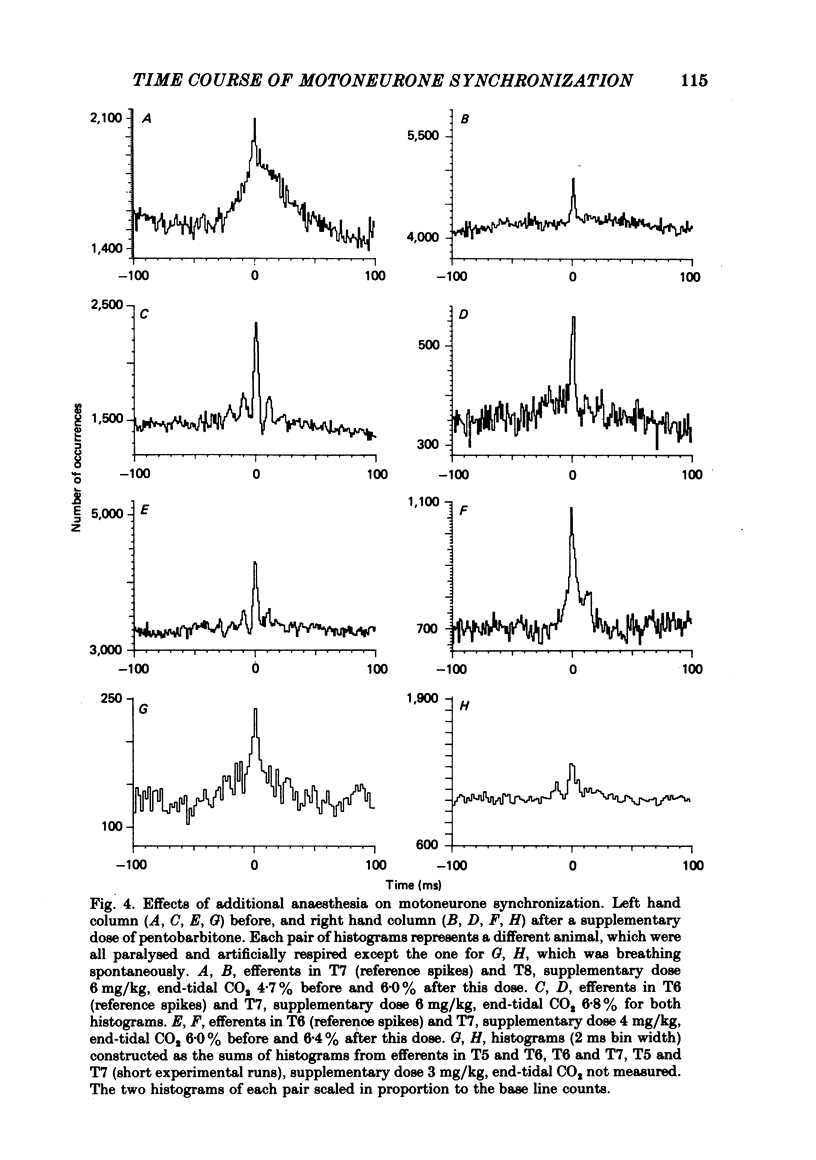
3. When different animals were compared, broad peak synchronization was seen in association with light anaesthesia and with polysynaptic excitation of the motoneurones from muscle spindle afferents.
4. In individual animals, additional anaesthesia depressed both broad peak and h.f.o. synchronization.
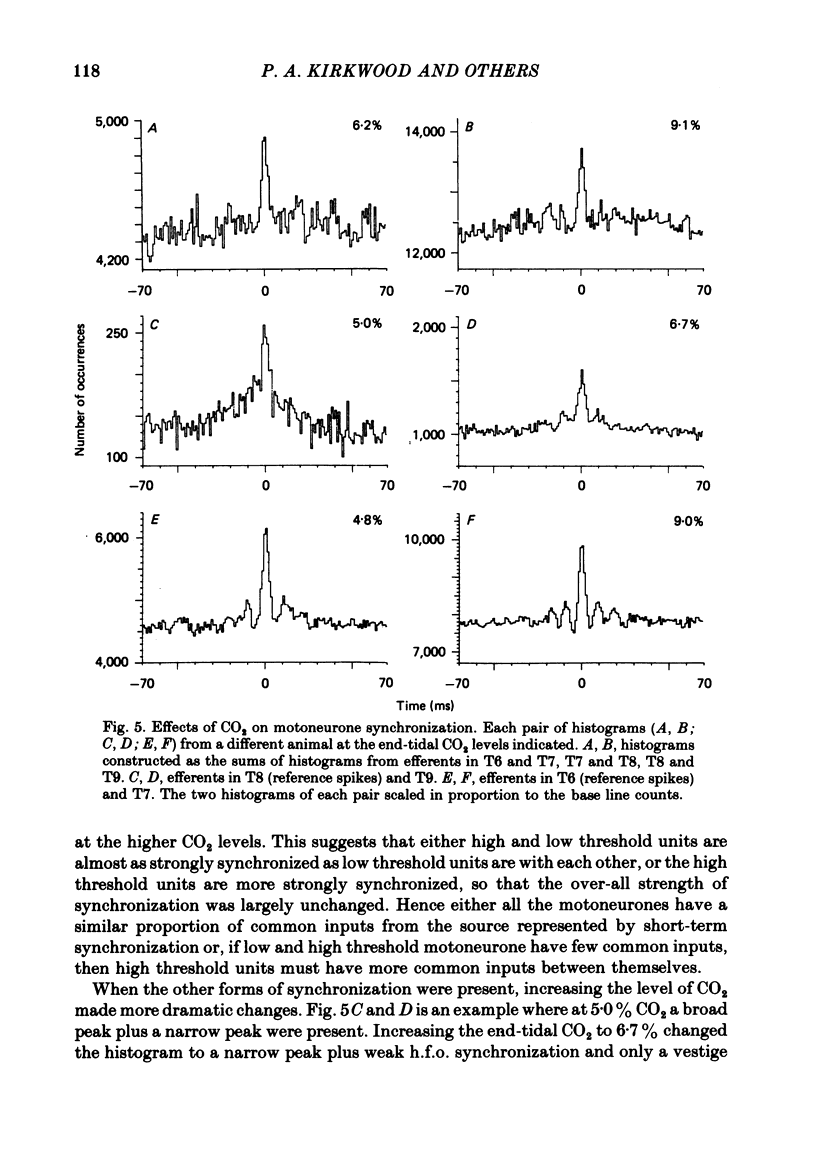
5. Raising PA, CO2, which increased the respiratory drive to the motoneurones, favoured short-term or h.f.o. synchronization at the expense of broad-peak synchronization.
6. In three decerebrate animals only short-term or h.f.o. synchronization was seen.
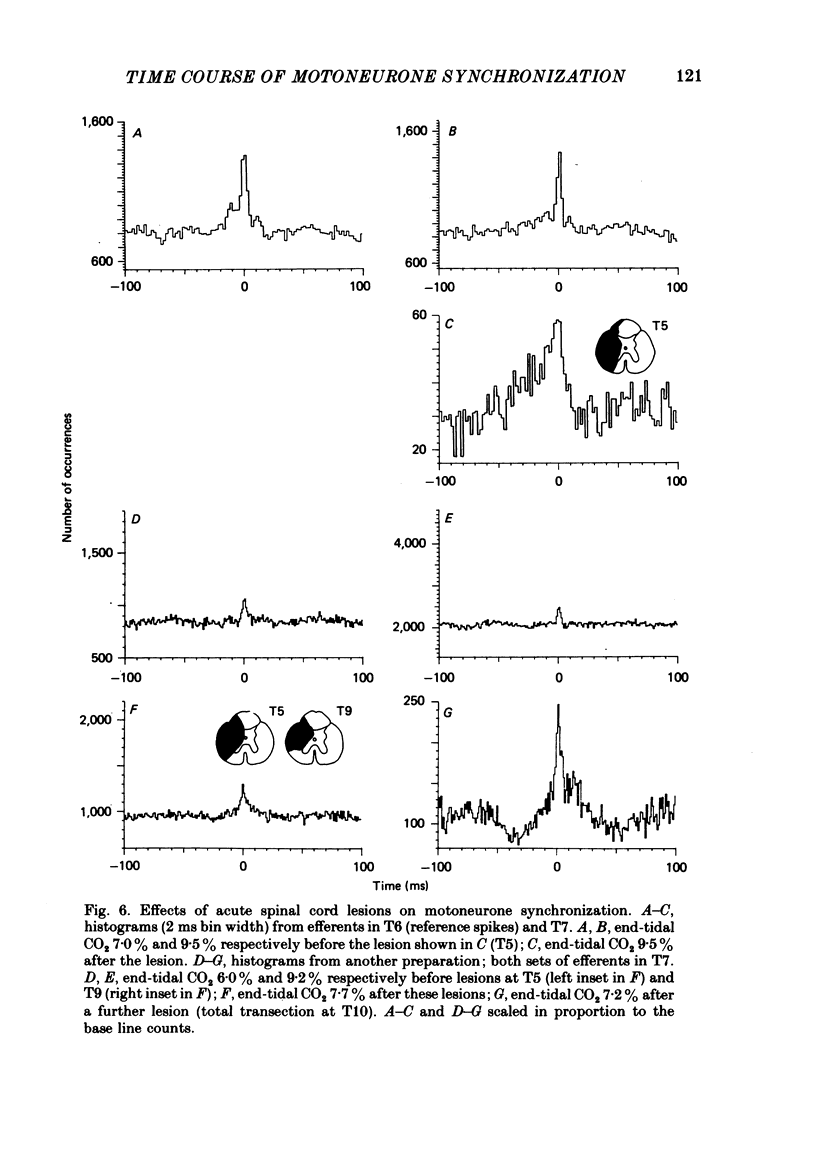
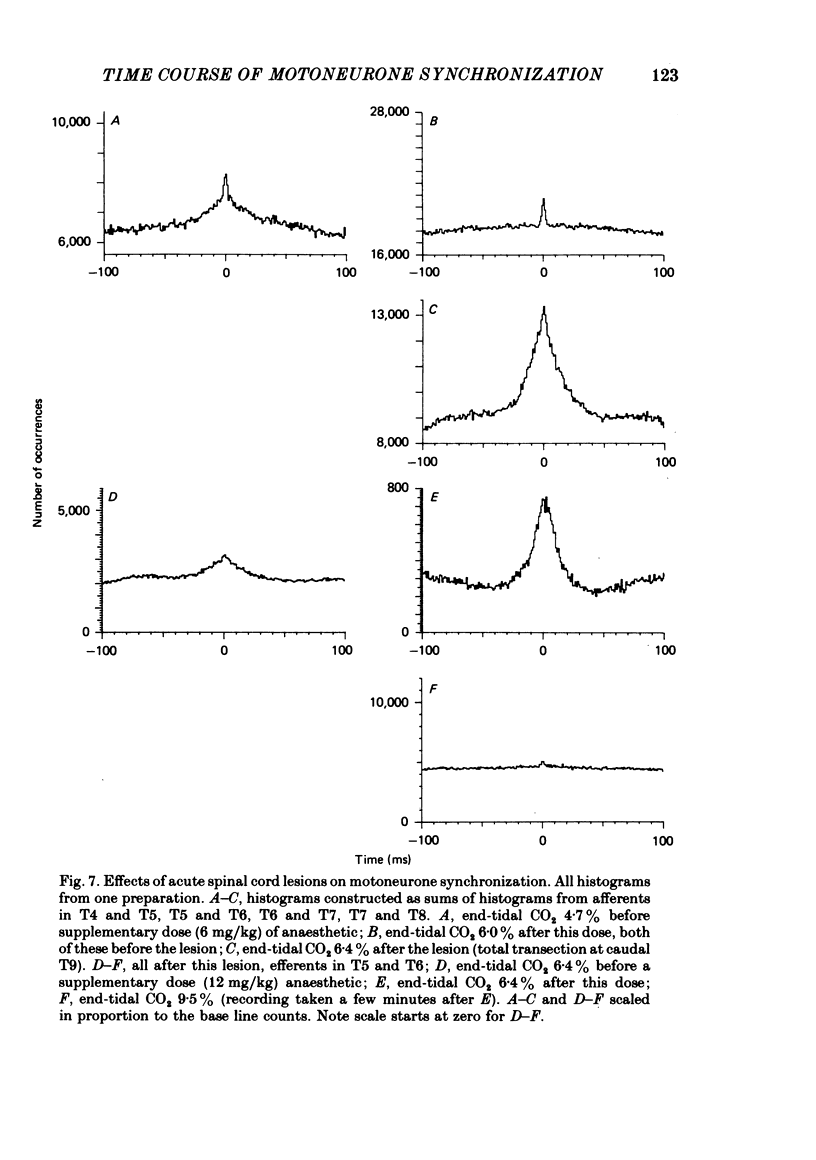
7. Spinal cord lesions above or below the segments of interest promoted broad-peak synchronization, even with high PA, CO2 or deep anaesthesia.
8. We conclude: (i) that short-term synchronization, due mainly to the branching of presynaptic axons, is generated mainly by those axons which transmit the respiratory drive, that drive providing most of the excitation of the motoneurones in moderately deep anaesthesia; (ii) that h.f.o. synchronization arises from the periodic synchronization of the discharges in these same presynaptic axons; (iii) that broad-peak synchronization is generated by the activity of other presynaptic neurones whose discharges are also synchronized, but aperiodically, these neurones most likely including spinal cord interneurones which are active in light anaesthesia or when released by spinal cord lesions.
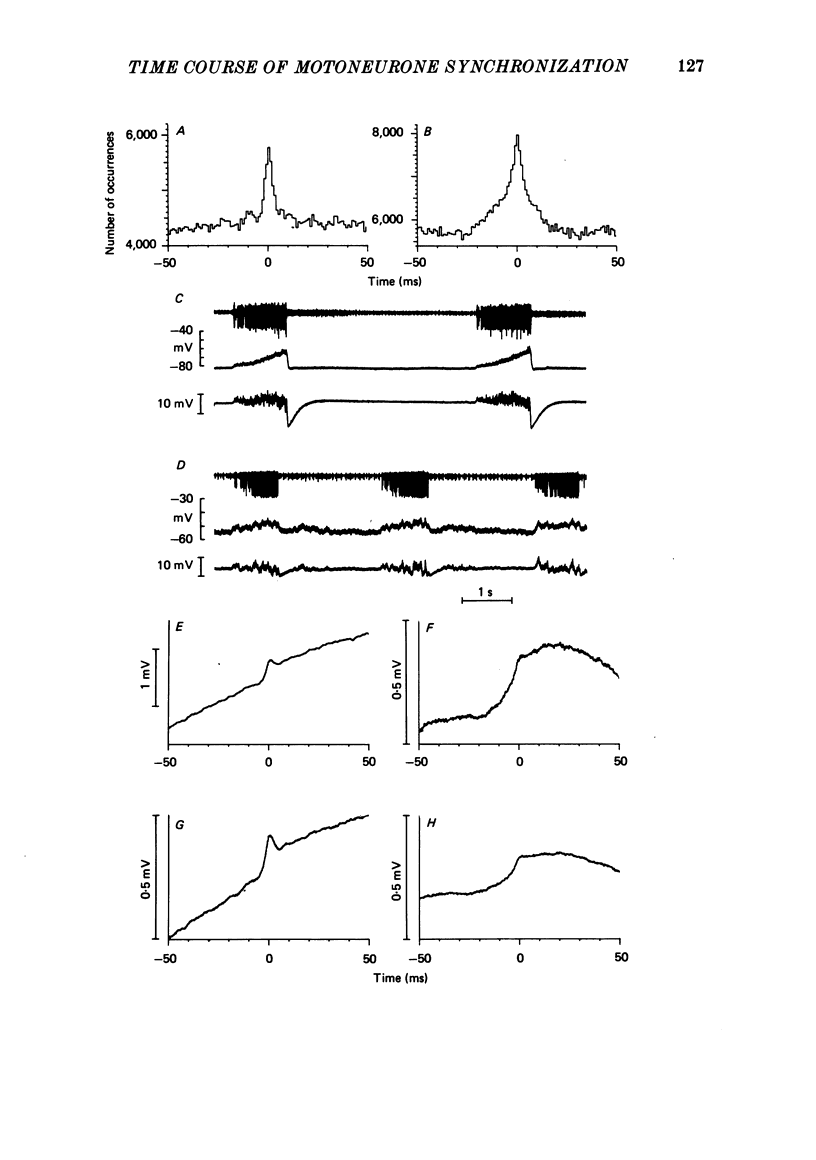
9. These conclusions are supported by comparisons between intracellular recordings from inspiratory motoneurones in animals showing different forms of motoneurone synchronization, the comparison including the measurements of `average common excitation' (a.c.e.) potentials (Kirkwood & Sears, 1978).
Full text
PDF























Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aminoff M. J., Sears T. A. Spinal integration of segmental, cortical and breathing inputs to thoracic respiratory motoneurones. J Physiol. 1971 Jun;215(2):557–575. doi: 10.1113/jphysiol.1971.sp009485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett D. W. Correlation analysis of units recorded in the cat dorsal lateral geniculate nucleus. Exp Brain Res. 1975 Dec 22;24(2):111–130. doi: 10.1007/BF00234058. [DOI] [PubMed] [Google Scholar]
- Arnett D., Spraker T. E. Cross-correlation analysis of the maintained discharge of rabbit retinal ganglion cells. J Physiol. 1981 Aug;317:29–47. doi: 10.1113/jphysiol.1981.sp013812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton C. R., Kirkwood P. A., Sears T. A. On the transmission of the stimulating effects of carbon dioxide to the muscles of respiration. J Physiol. 1978 Jul;280:249–272. doi: 10.1113/jphysiol.1978.sp012383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainton C. R., Kirkwood P. A. The effect of carbon dioxide on the tonic and the rhythmic discharges of expiratory bulbospinal neurones. J Physiol. 1979 Nov;296:291–314. doi: 10.1113/jphysiol.1979.sp013006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRITCHLOW V., VON EULER INTERCOSTAL MUSCLE SPINDLE ACTIVITY AND ITS GAMMA MOTOR CONTROL. J Physiol. 1963 Oct;168:820–847. doi: 10.1113/jphysiol.1963.sp007225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. I. Neurogenesis of respiratory rhythm in the mammal. Physiol Rev. 1979 Oct;59(4):1105–1173. doi: 10.1152/physrev.1979.59.4.1105. [DOI] [PubMed] [Google Scholar]
- Cohen M. I., Piercey M. F., Gootman P. M., Wolotsky P. Synaptic connections between medullary inspiratory neurons and phrenic motoneurons as revealed by cross-correlation. Brain Res. 1974 Dec 6;81(2):319–324. doi: 10.1016/0006-8993(74)90946-9. [DOI] [PubMed] [Google Scholar]
- Cohen M. I. Synchronization of discharge, spontaneous and evoked, between inspiratory neurons. Acta Neurobiol Exp (Wars) 1973;33(1):189–218. [PubMed] [Google Scholar]
- DOWNMAN C. B., HUSSAIN A. Spinal tracts and supraspinal centres influencing visceromotor and allied reflexes in cats. J Physiol. 1958 May 28;141(3):489–499. doi: 10.1113/jphysiol.1958.sp005990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOWNMAN C. B. Skeletal muscle reflexes of splanchnic and intercostal nerve origin in acute spinal and decerebrate cats. J Neurophysiol. 1955 May;18(3):217–235. doi: 10.1152/jn.1955.18.3.217. [DOI] [PubMed] [Google Scholar]
- Dietz V., Bischofberger E., Wita C., Freund H. J. Correlation between the dischanges of two simultaneously recorded motor units and physiological tremor. Electroencephalogr Clin Neurophysiol. 1976 Jan;40(1):97–105. doi: 10.1016/0013-4694(76)90183-8. [DOI] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Supraspinal control of interneurones mediating spinal reflexes. J Physiol. 1959 Oct;147:565–584. doi: 10.1113/jphysiol.1959.sp006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J. L., Sommer D., Cohen M. I. Short time scale correlations between discharges of medullary respiratory neurons. J Neurophysiol. 1980 May;43(5):1284–1295. doi: 10.1152/jn.1980.43.5.1284. [DOI] [PubMed] [Google Scholar]
- Guilbaud G., Benelli G., Besson J. M. Responses of thoracic dorsal horn interneurons to cutaneous stimulation and to the administration of algogenic substances into the mesenteric artery in the spinal cat. Brain Res. 1977 Apr 1;124(3):437–448. doi: 10.1016/0006-8993(77)90945-3. [DOI] [PubMed] [Google Scholar]
- Hilaire G., Monteau R. Connexions entre les neurones inspiratoires bulbaires et les motoneurones phréniques et intercostaux. J Physiol (Paris) 1976;72(8):987–1000. [PubMed] [Google Scholar]
- Holmes O., Houchin J. Units in the cerebral cortex of the anaesthetized rat and the correlations between their discharges. J Physiol. 1966 Dec;187(3):651–671. doi: 10.1113/jphysiol.1966.sp008116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNO M., PERL E. R. Alteration of spinal reflexes by interaction with suprasegmental and dorsal root activity. J Physiol. 1960 Apr;151:103–122. [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Excitatory post-synaptic potentials from single muscle spindle afferents in external intercostal motoneurones of the cat. J Physiol. 1982 Jan;322:287–314. doi: 10.1113/jphysiol.1982.sp014038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. Proceedings: Monosynaptic excitation of thoracic expiratory motoneurones from lateral respiratory neurones in the medulla of the cat. J Physiol. 1973 Oct;234(2):87P–89P. [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Stagg D. Proceedings: Synchronized firing of respiratory motoneurones during spontaneous breathing in the anaesthetized cat. J Physiol. 1974 May;239(1):11P–13P. [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Stagg D., Westgaard R. H. The spatial distribution of synchronization of intercostal motoneurones in the cat. J Physiol. 1982 Jun;327:137–155. doi: 10.1113/jphysiol.1982.sp014224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The effects of single afferent impulses on the probability of firing of external intercostal motoneurones in the cat. J Physiol. 1982 Jan;322:315–336. doi: 10.1113/jphysiol.1982.sp014039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A. The synaptic connexions to intercostal motoneurones as revealed by the average common excitation potential. J Physiol. 1978 Feb;275:103–134. doi: 10.1113/jphysiol.1978.sp012180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood P. A., Sears T. A., Westgaard R. H. Recurrent inhibition of intercostal motoneurones in the cat. J Physiol. 1981;319:111–130. doi: 10.1113/jphysiol.1981.sp013895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell L. M., Henneman E. Terminals of single Ia fibers: distribution within a pool of 300 homonymous motor neurons. Science. 1968 Apr 5;160(3823):96–98. doi: 10.1126/science.160.3823.96. [DOI] [PubMed] [Google Scholar]
- Milner-Brown H. S., Stein R. B., Lee R. G. Synchronization of human motor units: possible roles of exercise and supraspinal reflexes. Electroencephalogr Clin Neurophysiol. 1975 Mar;38(3):245–254. doi: 10.1016/0013-4694(75)90245-x. [DOI] [PubMed] [Google Scholar]
- Mitchell R. A., Herbert D. A. Synchronized high frequency synaptic potentials in medullary respiratory neurons. Brain Res. 1974 Jul 26;75(2):350–355. doi: 10.1016/0006-8993(74)90760-4. [DOI] [PubMed] [Google Scholar]
- Moore G. P., Segundo J. P., Perkel D. H., Levitan H. Statistical signs of synaptic interaction in neurons. Biophys J. 1970 Sep;10(9):876–900. doi: 10.1016/S0006-3495(70)86341-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda H., Adey W. R. Firing of neuron pairs in cat association cortex during sleep and wakefulness. J Neurophysiol. 1970 Sep;33(5):672–684. doi: 10.1152/jn.1970.33.5.672. [DOI] [PubMed] [Google Scholar]
- Noda H., Manohar S., Adey W. R. Spontaneous activity of cat hippocampal neurons in sleep and wakefulness. Exp Neurol. 1969 Jun;24(2):217–231. doi: 10.1016/0014-4886(69)90016-8. [DOI] [PubMed] [Google Scholar]
- Pullen A. H., Sears T. A. Modification of "C" synapses following partial central deafferentation of thoracic motoneurones. Brain Res. 1978 Apr 21;145(1):141–146. doi: 10.1016/0006-8993(78)90802-8. [DOI] [PubMed] [Google Scholar]
- Rudomin P., Burke R. E., Núez R., Madrid J., Dutton H. Control by Preynaptic Correlation: a mechanism affecting information transmission from Ia fibers to motoneurons. J Neurophysiol. 1975 Mar;38(2):267–284. doi: 10.1152/jn.1975.38.2.267. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. Activity of fusimotor fibres innervating muscle spindles in the intercostal muscles of the cat. Nature. 1963 Mar 9;197:1013–1014. doi: 10.1038/1971013a0. [DOI] [PubMed] [Google Scholar]
- SEARS T. A. EFFERENT DISCHARGES IN ALPHA AND FUSIMOTOR FIBRES OF INTERCOSTAL NERVES OF THE CAT. J Physiol. 1964 Nov;174:295–315. doi: 10.1113/jphysiol.1964.sp007488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEARS T. A. INVESTIGATIONS ON RESPIRATORY MOTONEURONES OF THE THORACIC SPINAL CORD. Prog Brain Res. 1964;12:259–273. doi: 10.1016/s0079-6123(08)60627-5. [DOI] [PubMed] [Google Scholar]
- SUMI T. Organization of spinal respiratory neurons. Ann N Y Acad Sci. 1963 Jun 24;109:561–570. doi: 10.1111/j.1749-6632.1963.tb13487.x. [DOI] [PubMed] [Google Scholar]
- SUMI T. The segmental reflex relations of cutaneous afferent inflow to thoracic respiratory motoneurons. J Neurophysiol. 1963 May;26:478–493. doi: 10.1152/jn.1963.26.3.478. [DOI] [PubMed] [Google Scholar]
- Sears T. A., Stagg D. Short-term synchronization of intercostal motoneurone activity. J Physiol. 1976 Dec;263(3):357–381. doi: 10.1113/jphysiol.1976.sp011635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears T. A. The respiratory motoneuron and apneusis. Fed Proc. 1977 Sep;36(10):2412–2420. [PubMed] [Google Scholar]
- Sherrington C. S., Sowton S. C. Observations on reflex responses to single break-shocks. J Physiol. 1915 Jul 5;49(5):331–348. doi: 10.1113/jphysiol.1915.sp001713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomori Z., Widdicombe J. G. Muscular, bronchomotor and cardiovascular reflexes elicited by mechanical stimulation of the respiratory tract. J Physiol. 1969 Jan;200(1):25–49. doi: 10.1113/jphysiol.1969.sp008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon B. R., Duffin J. Cross-correlation of medullary respiratory neurons in the cat. Exp Neurol. 1978 Aug;61(1):15–30. doi: 10.1016/0014-4886(78)90178-4. [DOI] [PubMed] [Google Scholar]


