Abstract
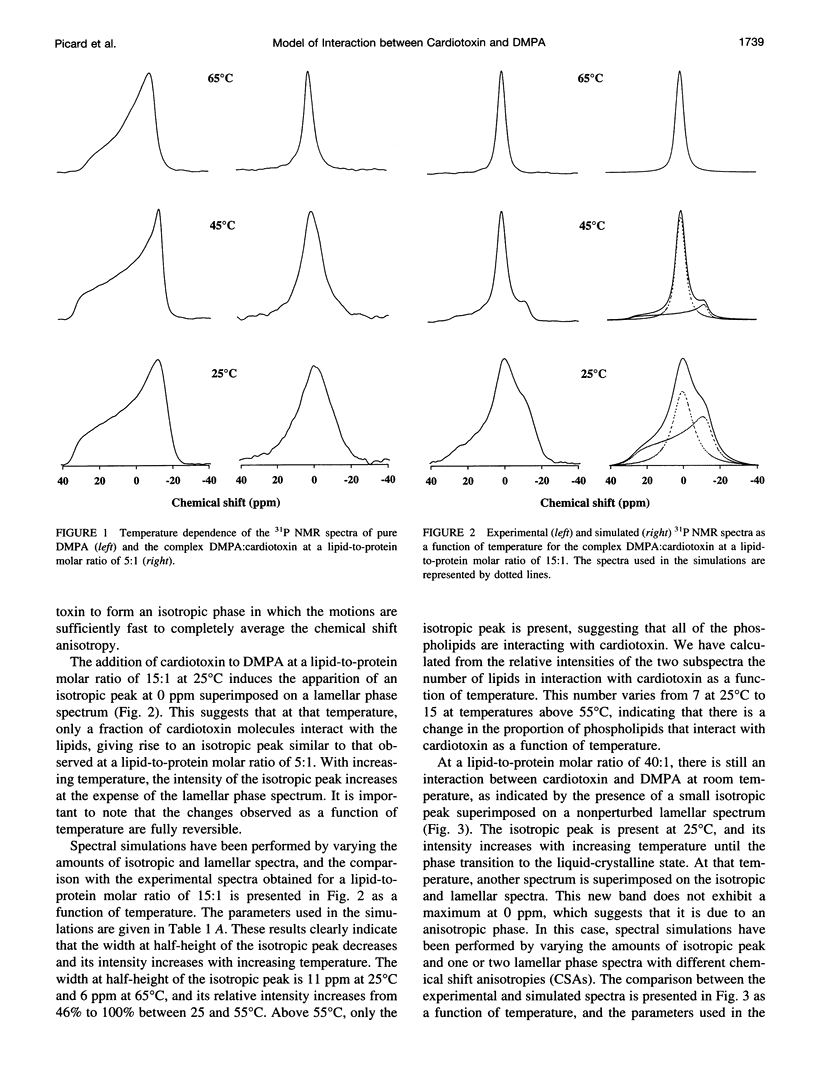
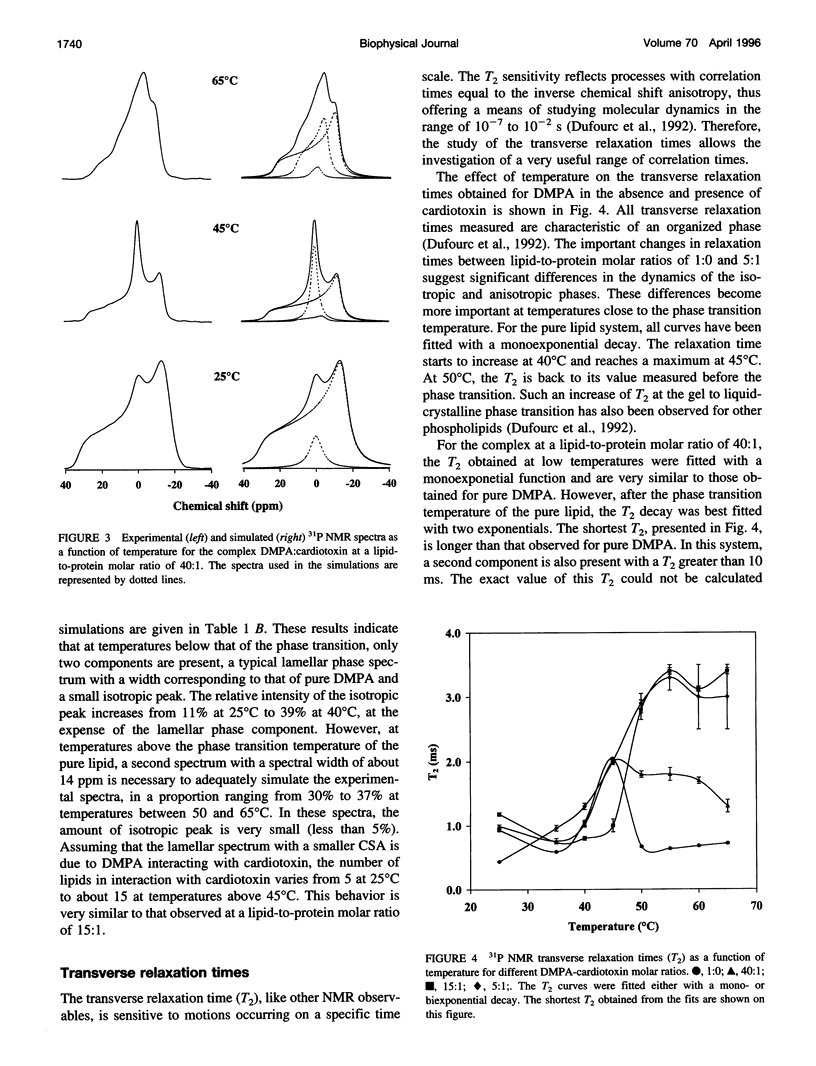
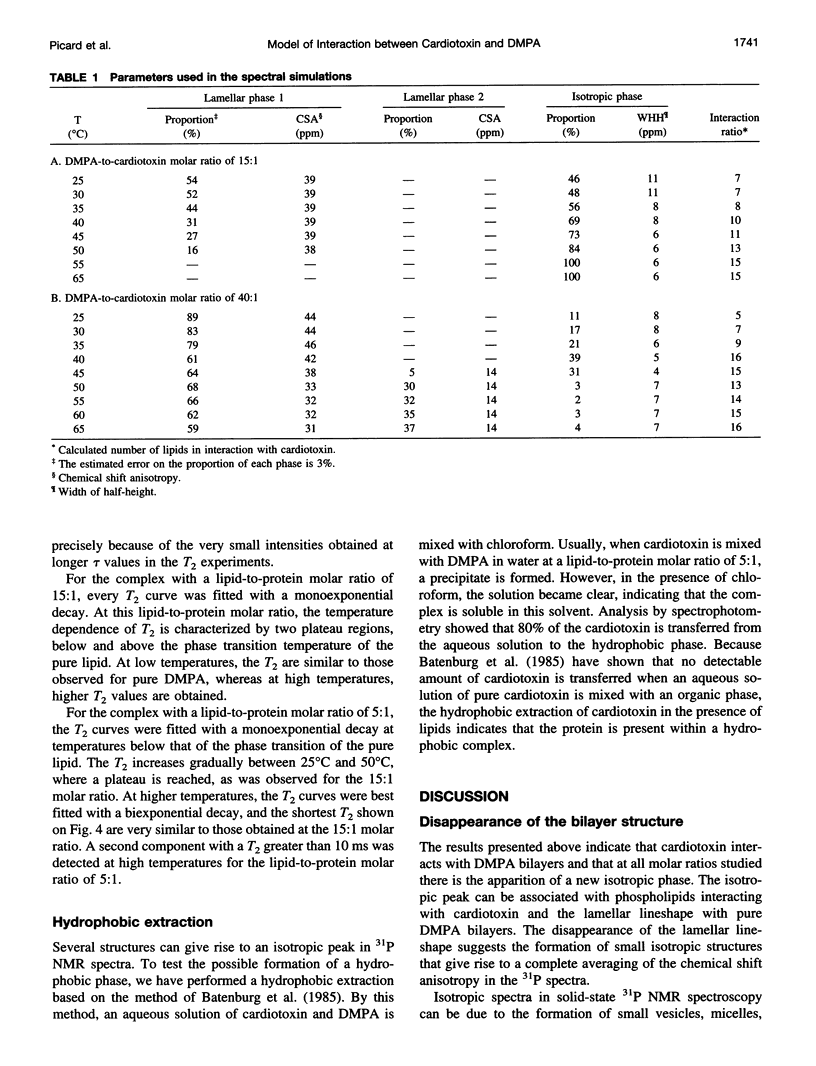
The interaction of cardiotoxin IIa, a small basic protein extracted from Naja mossambica mossambica venom, with dimyristoylphosphatidic acid (DMPA) membranes has been investigated by solid-state 31P nuclear magnetic resonance spectroscopy. Both the spectral lineshapes and transverse relaxation time values have been measured as a function of temperature for different lipid-to-protein molar ratios. The results indicate that the interaction of cardiotoxin with DMPA gives rise to the complete disappearance of the bilayer structure at a lipid-to-protein molar ratio of 5:1. However, a coexistence of the lamellar and isotropic phases is observed at higher lipid contents. In addition, the number of phospholipids interacting with cardiotoxin increases from about 5 at room temperature to approximately 15 at temperatures above the phase transition of the pure lipid. The isotropic structure appears to be a hydrophobic complex similar to an inverted micellar phase that can be extracted by a hydrophobic solvent. At a lipid-to-protein molar ratio of 40:1, the isotropic structure disappears at high temperature to give rise to a second anisotropic phase, which is most likely associated with the incorporation of the hydrophobic complex inside the bilayer.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batenburg A. M., Bougis P. E., Rochat H., Verkleij A. J., de Kruijff B. Penetration of a cardiotoxin into cardiolipin model membranes and its implications on lipid organization. Biochemistry. 1985 Dec 3;24(25):7101–7110. doi: 10.1021/bi00346a013. [DOI] [PubMed] [Google Scholar]
- Bougis P. E., Marchot P., Rochat H. Characterization of elapidae snake venom components using optimized reverse-phase high-performance liquid chromatographic conditions and screening assays for alpha-neurotoxin and phospholipase A2 activities. Biochemistry. 1986 Nov 4;25(22):7235–7243. doi: 10.1021/bi00370a070. [DOI] [PubMed] [Google Scholar]
- Bougis P., Rochat H., Piéroni G., Verger R. Penetration of phospholipid monolayers by cardiotoxins. Biochemistry. 1981 Aug 18;20(17):4915–4920. doi: 10.1021/bi00520a017. [DOI] [PubMed] [Google Scholar]
- Bougis P., Tessier M., Van Rietschoten J., Rochat H., Faucon J. F., Dufourcq J. Are interactions with phospholipids responsible for pharmacological activities of cardiotoxins? Mol Cell Biochem. 1983;55(1):49–64. doi: 10.1007/BF00229242. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., Hope M. J. Effects of fusogenic agent on membrane structure of erythrocyte ghosts and the mechanism of membrane fusion. Nature. 1978 Feb 16;271(5646):672–674. doi: 10.1038/271672a0. [DOI] [PubMed] [Google Scholar]
- Dufourc E. J., Mayer C., Stohrer J., Althoff G., Kothe G. Dynamics of phosphate head groups in biomembranes. Comprehensive analysis using phosphorus-31 nuclear magnetic resonance lineshape and relaxation time measurements. Biophys J. 1992 Jan;61(1):42–57. doi: 10.1016/S0006-3495(92)81814-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufourcq J., Faucon J. F., Bernard E., Pezolet M., Tessier M., van Rietschoten J., Delori P., Rochat H. Structure-function relationships for cardiotoxins interacting with phospholipids. Toxicon. 1982;20(1):165–174. doi: 10.1016/0041-0101(82)90187-8. [DOI] [PubMed] [Google Scholar]
- Dufourcq J., Faucon J. F. Specific binding of a cardiotoxin from Naja mossambica mossambica to charged phospholipids detected by intrinsic fluorescence. Biochemistry. 1978 Apr 4;17(7):1170–1176. doi: 10.1021/bi00600a006. [DOI] [PubMed] [Google Scholar]
- Désormeaux A., Laroche G., Bougis P. E., Pézolet M. Characterization by infrared spectroscopy of the interaction of a cardiotoxin with phosphatidic acid and with binary mixtures of phosphatidic acid and phosphatidylcholine. Biochemistry. 1992 Dec 8;31(48):12173–12182. doi: 10.1021/bi00163a029. [DOI] [PubMed] [Google Scholar]
- Faucon J. F., Bernard E., Dufourcq J., Pezolet M., Bougis P. Perturbations of charged phospholipid bilayers induced by melittin and cardiotoxins. A fluorescence, differential scanning calorimetry and Raman spectroscopy study. Biochimie. 1981 Nov-Dec;63(11-12):857–861. doi: 10.1016/s0300-9084(82)80273-3. [DOI] [PubMed] [Google Scholar]
- Faucon J. F., Dufourcq J., Bernard E., Duchesneau L., Pézolet M. Abolition of the thermotropic transition of charged phospholipids induced by a cardiotoxin from Naja mossambica mossambica as detected by fluorescence polarization, differential scanning calorimetry, and Raman spectroscopy. Biochemistry. 1983 Apr 26;22(9):2179–2185. doi: 10.1021/bi00278a019. [DOI] [PubMed] [Google Scholar]
- Faucon J. F., Dufourcq J., Couraud F., Rochat H. Lipid-protein interactions. A comparative study of the binding of cardiotoxins and neurotoxins to phospholipid vesicles. Biochim Biophys Acta. 1979 Jul 5;554(2):332–339. doi: 10.1016/0005-2736(79)90374-2. [DOI] [PubMed] [Google Scholar]
- Gulik-Krzywicki T., Balerna M., Vincent J. P., Lazdunski M. Freeze-fracture study of cardiotoxin action on axonal membrane and axonal membrane lipid vesicles. Biochim Biophys Acta. 1981 Apr 22;643(1):101–114. doi: 10.1016/0005-2736(81)90222-4. [DOI] [PubMed] [Google Scholar]
- Harvey A. L., Hider R. C., Khader F. Effect of phospholipase A on actions of cobra venom cardiotoxins on erythrocytes and skeletal muscle. Biochim Biophys Acta. 1983 Feb;728(2):215–221. doi: 10.1016/0005-2736(83)90474-1. [DOI] [PubMed] [Google Scholar]
- Laroche G., Carrier D., Pézolet M. Study of the effect of poly(L-lysine) on phosphatidic acid and phosphatidylcholine/phosphatidic acid bilayers by raman spectroscopy. Biochemistry. 1988 Aug 23;27(17):6220–6228. doi: 10.1021/bi00417a005. [DOI] [PubMed] [Google Scholar]
- Laroche G., Dufourc E. J., Dufourcq J., Pézolet M. Structure and dynamics of dimyristoylphosphatidic acid/calcium complexes by 2H NMR, infrared, spectroscopies and small-angle x-ray diffraction. Biochemistry. 1991 Mar 26;30(12):3105–3114. doi: 10.1021/bi00226a018. [DOI] [PubMed] [Google Scholar]
- Lauterwein J., Wüthrich K. A possible structural basis for the different modes of action of neurotoxins and cardiotoxins from snake venoms. FEBS Lett. 1978 Sep 15;93(2):181–184. doi: 10.1016/0014-5793(78)81100-4. [DOI] [PubMed] [Google Scholar]
- Louw A. I., Visser L. The synergism of cardiotoxin and phospholipase A2 in hemolysis. Biochim Biophys Acta. 1978 Sep 11;512(1):163–171. doi: 10.1016/0005-2736(78)90227-4. [DOI] [PubMed] [Google Scholar]
- Menez A., Langlet G., Tamiya N., Fromageot P. Conformation of snake toxic polypeptides studied by a method of prediction and circular dichroism. Biochimie. 1978 Sep 4;60(5):505–516. doi: 10.1016/s0300-9084(78)80866-9. [DOI] [PubMed] [Google Scholar]
- Milburn M. P., Jeffrey K. R. Dynamics of the phosphate group in phospholipid bilayers. A 31P angular dependent nuclear spin relaxation time study. Biophys J. 1989 Sep;56(3):543–549. doi: 10.1016/S0006-3495(89)82701-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn M. P., Jeffrey K. R. Dynamics of the phosphate group in phospholipid bilayers. A 31P nuclear relaxation time study. Biophys J. 1987 Nov;52(5):791–799. doi: 10.1016/S0006-3495(87)83273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn M. P., Jeffrey K. R. Dynamics of the phosphate group in phospholipid bilayers. A 31P-1H transient Overhauser effect study. Biophys J. 1990 Jul;58(1):187–194. doi: 10.1016/S0006-3495(90)82364-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménez A., Gatineau E., Roumestand C., Harvey A. L., Mouawad L., Gilquin B., Toma F. Do cardiotoxins possess a functional site? Structural and chemical modification studies reveal the functional site of the cardiotoxin from Naja nigricollis. Biochimie. 1990 Aug;72(8):575–588. doi: 10.1016/0300-9084(90)90121-v. [DOI] [PubMed] [Google Scholar]
- O'Connell J. F., Bougis P. E., Wüthrich K. Determination of the nuclear-magnetic-resonance solution structure of cardiotoxin CTX IIb from Naja mossambica mossambica. Eur J Biochem. 1993 May 1;213(3):891–900. doi: 10.1111/j.1432-1033.1993.tb17833.x. [DOI] [PubMed] [Google Scholar]
- Orädd G., Lindblom G., Fontell K., Ljusberg-Wahren H. Phase diagram of soybean phosphatidylcholine-diacylglycerol-water studied by x-ray diffraction and 31P- and pulsed field gradient 1H-NMR: evidence for reversed micelles in the cubic phase. Biophys J. 1995 May;68(5):1856–1863. doi: 10.1016/S0006-3495(95)80362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pott T., Maillet J. C., Dufourc E. J. Effects of pH and cholesterol on DMPA membranes: a solid state 2H- and 31P-NMR study. Biophys J. 1995 Nov;69(5):1897–1908. doi: 10.1016/S0006-3495(95)80060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees B., Samama J. P., Thierry J. C., Gilibert M., Fischer J., Schweitz H., Lazdunski M., Moras D. Crystal structure of a snake venom cardiotoxin. Proc Natl Acad Sci U S A. 1987 May;84(10):3132–3136. doi: 10.1073/pnas.84.10.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas E. A., Le Maire M., Gulik-Krzywicki T. Isolation of rhodopsin by the combined action of cardiotoxin and phospholipase A2 on rod outer segment membranes. Biochim Biophys Acta. 1981 Jun 9;644(1):127–133. doi: 10.1016/0005-2736(81)90067-5. [DOI] [PubMed] [Google Scholar]
- Seddon J. M. An inverse face-centered cubic phase formed by diacylglycerol-phosphatidylcholine mixtures. Biochemistry. 1990 Aug 28;29(34):7997–8002. doi: 10.1021/bi00486a031. [DOI] [PubMed] [Google Scholar]
- Seddon J. M. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim Biophys Acta. 1990 Feb 28;1031(1):1–69. doi: 10.1016/0304-4157(90)90002-t. [DOI] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Siegel D. P. Inverted micellar intermediates and the transitions between lamellar, cubic, and inverted hexagonal lipid phases. I. Mechanism of the L alpha----HII phase transitions. Biophys J. 1986 Jun;49(6):1155–1170. doi: 10.1016/S0006-3495(86)83744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D. P. Inverted micellar intermediates and the transitions between lamellar, cubic, and inverted hexagonal lipid phases. II. Implications for membrane-membrane interactions and membrane fusion. Biophys J. 1986 Jun;49(6):1171–1183. doi: 10.1016/S0006-3495(86)83745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D. P. Inverted micellar structures in bilayer membranes. Formation rates and half-lives. Biophys J. 1984 Feb;45(2):399–420. doi: 10.1016/S0006-3495(84)84164-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz W. E., Bougis P. E., Rochat H., Redwine O. D., Braun W., Wüthrich K. 1H nuclear-magnetic-resonance studies of the three-dimensional structure of the cardiotoxin CTXIIb from Naja mossambica mossambica in aqueous solution and comparison with the crystal structures of homologous toxins. Eur J Biochem. 1988 Feb 15;172(1):101–116. doi: 10.1111/j.1432-1033.1988.tb13861.x. [DOI] [PubMed] [Google Scholar]
- Surewicz W. K., Stepanik T. M., Szabo A. G., Mantsch H. H. Lipid-induced changes in the secondary structure of snake venom cardiotoxins. J Biol Chem. 1988 Jan 15;263(2):786–790. [PubMed] [Google Scholar]
- Verkleij A. J. Lipidic intramembranous particles. Biochim Biophys Acta. 1984 Jan 27;779(1):43–63. doi: 10.1016/0304-4157(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Vincent J. P., Balerna M., Lazdunski M. Properties of association of cardiotoxin with lipid vesicles and natural membranes. A fluorescence study. FEBS Lett. 1978 Jan 1;85(1):103–108. doi: 10.1016/0014-5793(78)81258-7. [DOI] [PubMed] [Google Scholar]
- Vincent J. P., Schweitz H., Chicheportiche R., Fosset M., Balerna M., Lenoir M. C., Lazdunski M. Molecular mechanism of cardiotoxin action on axonal membranes. Biochemistry. 1976 Jul 27;15(15):3171–3175. doi: 10.1021/bi00660a002. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Cullis P. R. The influence of poly(L-lysine) on phospholipid polymorphism. Evidence that electrostatic polypeptide-phospholipid interactions can modulate bilayer/non-bilayer transitions. Biochim Biophys Acta. 1980 Sep 2;601(1):235–240. doi: 10.1016/0005-2736(80)90528-3. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Verkley A. J., van Echteld C. J., Gerritsen W. J., Mombers C., Noordam P. C., de Gier J. The occurrence of lipidic particles in lipid bilayers as seen by 31P NMR and freeze-fracture electron-microscopy. Biochim Biophys Acta. 1979 Aug 7;555(2):200–209. doi: 10.1016/0005-2736(79)90160-3. [DOI] [PubMed] [Google Scholar]
- van Dijck P. W., de Kruijff B., Verkleij A. J., van Deenen L. L., de Gier J. Comparative studies on the effects of pH and Ca2+ on bilayers of various negatively charged phospholipids and their mixtures with phosphatidylcholine. Biochim Biophys Acta. 1978 Sep 11;512(1):84–96. doi: 10.1016/0005-2736(78)90219-5. [DOI] [PubMed] [Google Scholar]


