Abstract
Mitochondrial ATP-sensitive K (mitoKATP) channels play a central role in protecting the heart from injury in ischemic preconditioning. In isolated mitochondria exposed to elevated extramitochondrial Ca, Pi, and anoxia to simulate ischemic conditions, the selective mitoKATP channel agonist diazoxide (25–50 μM) potently reduced mitochondrial injury by preventing both the mitochondrial permeability transition (MPT) and cytochrome c loss from the intermembrane space. Both effects were blocked completely by the selective mitoKATP antagonist 5-hydroxydecanoate. The protective effect against Ca-induced MPT was most evident under conditions in which the ability of electron transport to support membrane potential (Δψm) was decreased and inner membrane leakiness was increased moderately. Under these conditions, mitoKATP channel activity strongly regulated Δψm, and diazoxide prevented MPT by inhibiting the driving force for Ca uptake. Phorbol 12-myristate 13-acetate mimicked the protective effects of diazoxide, unless 5-hydroxydecanoate was present, indicating that protein kinase C activation also protects mitochondria by activating mitoKATP channels. Because Δψm recovery ultimately is required for heart functional recovery, these results may explain how mitoKATP channel activation mimics ischemic preconditioning by protecting mitochondria as they pass through a critical vulnerability window during ischemia/reperfusion.
In heart, the most potent method for reducing ischemia/reperfusion injury is to precondition with brief ischemic episodes before subjecting the heart to prolonged ischemia, termed ischemic preconditioning (IPC). IPC can be mimicked by pharmacologic preconditioning (PPC), in which stimulation of various protein kinase pathways or preexposure to K channel openers that activate mitochondrial ATP-sensitive K (mitoKATP) channels results in a degree of cardioprotection during prolonged ischemia comparable to IPC (for reviews, see refs. 1–3). In addition, IPC can be prevented by mitoKATP antagonists in many species, indicating that IPC and PPC are closely linked. This has led to the conclusion that mitoKATP channels are the downstream target of signaling pathways that induce IPC (1–3). However, the mechanism is poorly understood. The cardioprotective effect of IPC and PPC on mitochondrial function during cardiac ischemia/reperfusion has been well documented (4–6) and, ultimately, must protect mitochondria from undergoing the mitochondrial permeability transition (MPT). The mitoKATP channel must stay open during ischemia and reperfusion for mitochondrial protection to occur, as verified by preserved mitochondrial ATP production rate, reduced structural damage, matrix Ca increase, and improved functional recovery on reperfusion (4, 5, 7–9).
Several hypotheses have been proposed to explain how mitoKATP channel activation prevents MPT. One is that mitoKATP activation induces depolarization of the inner membrane potential (Δψm), reducing the driving force for Ca uptake by mitochondria and thereby preventing mitochondrial matrix Ca overload, the major trigger for MPT (10, 11). However, cardioprotective concentrations of diazoxide (7-chloro-3-methyl2H-1,2,4-benzothiadiazine 1,1-dioxide), which fully open mitoKATP channels (e.g., 25–50 μM), have very modest depolarizing effects on Δψm under the conditions studied (energized mitochondria with normal Δψm). Although high diazoxide concentrations significantly dissipate Δψm (10, 12), they also inhibit succinate oxidation, suggesting that its effect on Δψm is not solely related to mitoKATP channel opening (12). An alternative hypothesis is that mitoKATP channel activation promotes mild mitochondrial swelling, which may protect mitochondria by preserving structure—function of the intermembrane space (12). A third hypothesis is that diazoxide induces cardioprotection by modulating reactive oxygen species production by mitochondria (13–15).
The goal here was to examine the mechanism of mitochondrial protection by mitoKATP agonists in isolated cardiac mitochondria. We studied isolated mitochondria under conditions designed to mimic some elements of the ischemic/hypoxic environment, rather than under well energized conditions in most previous investigations.
Methods
Isolation of Mitochondria.
Mitochondria were isolated from adult rabbit hearts by enzymatic digestion, homogenization, and differential centrifugation, as described previously (16), and were resuspended in EGTA-free homogenization buffer (250 mM sucrose/10 mM Hepes, pH 7.4, with Tris) to yield 30–50 mg/ml mitochondrial protein. Mitochondria were incubated on ice and used within 5 h after isolation.
Experimental Conditions.
All measurements were carried out by using a fiber-optic spectrofluorimeter (Ocean Optics, Dunedin, FL) in a closed, continuously stirred cuvette at room temperature (22–24°C). Mitochondria (0.3–0.6 mg/ml) were added in the cuvette to standard buffer consisting of 120 mM KCl and 10 mM Hepes, pH 7.4, with KOH. Substrates, Ca, Pi, EGTA, various drugs, and fluorescent indicators were added in the concentrations indicated. For the anoxia/reoxygenation experiments, succinate-energized mitochondria were made anoxic by directing a stream of nitrogen through the hole in the cuvette cover at the buffer (2 ml), so that the stirred buffer had no contact with the air (16). Reoxygenation was accomplished by replacing nitrogen with oxygen (95% O2/5% CO2). pO2 in the buffer was recorded continuously by means of a fiber-optic oxygen sensor inserted through the same hole.
Spectrofluorimetric Techniques and Other Assays.
Mitochondrial membrane potential (Δψm).
Tetramethylrhodamine methyl ester (TMRM; 400 nM) was included in the cuvette solution, and Δψm was estimated from TMRM fluorescence at 580 nm as described previously (16). Δψm is expressed as the percentage of the TMRM fluorescence in the presence of coupled mitochondria and substrates (100%) relative to that after the addition of 0.5 μM carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) to fully depolarize mitochondria (0%). Both light-scattering and TMRM fluorescence emission were recorded simultaneously with pO2.
Mitochondrial matrix volume.
Changes in matrix volume were estimated by measuring 90° light scattering with excitation and emission wavelengths set at 520 nm (16). Changes in matrix volume are reported as a percentage of maximum (100%) swelling induced by adding 10 μg of alamethicin at the end of the experiment.
Mitochondrial Ca2+ uptake and efflux.
Changes in extramitochondrial [Ca2+] were followed by measuring Calcium Green-5N (1 μM, salt form) fluorescence at excitation/emission wavelengths of 475/515 nm. [Ca2+] was calibrated by adding known amounts of Ca2+ to the buffer in the presence of mitochondria and FCCP to block Ca2+ uptake (16).
Mitochondrial calcein efflux.
Mitochondrial calcein efflux indicating MPT was measured by loading mitochondria with calcein-AM (10 μM, 2-h incubation on ice; AM = acetoxymethyl ester) and recording fluorescence at excitation/emission wavelengths of 475/515 nm after washing out extramitochondrial calcein-AM from the buffer. Calcein efflux results in fluorescence increase as a result of dequenching, and 100% efflux was promoted at the end of the experiment by adding alamethicin.
Mitochondrial protein.
Protein content was determined by the Lowry method.
Chemicals and Data Analysis.
Cyclosporin A (CsA) was a generous gift of CIBA–Geigy. Fluorescent dyes were purchased from Molecular Probes, and all other chemicals were purchased from Sigma. Mitochondrial substrates were added as free acids by using Tris to buffer pH.
Because during prolonged incubation of mitochondria on ice mitochondrial sensitivity to Ca-induced Δψm dissipation and MPT increased, interventions were always recorded in parallel with control experiments (within 15 min). Results are presented as original tracings, with summary data as mean ± SD. Student's t test was used to assess statistical significance, using the Bonferroni correction for more than two groups.
Results
Effects of Diazoxide and 5-HD on Δψm Regulation and MPT in Nonenergized Mitochondria.
Addition of a sufficiently large Ca load to isolated mitochondria, energized with succinate in the presence of elevated extramitochondrial [Pi] and without adenine nucleotides present, dissipated Δψm and caused matrix swelling. Recovery of Δψm required both Ca chelation by EGTA and CsA, indicating that MPT had occurred. Thus, under control conditions, Ca loading induced MPT in the absence of either diazoxide or 5-HD. In addition, pretreatment of mitochondria with diazoxide (up to 200 μM) or 5-HD (up to 300 μM) under otherwise identical conditions had no major effects on Δψm and did not protect against or accelerate PTP opening.
To simulate conditions more representative of ischemia/reperfusion, we examined the effects of removing substrates and further elevating the concentration of extramitochondrial Pi [which accumulates rapidly during ischemia (17) and is a known MTP inducer (18)] in freshly isolated mitochondria (Fig. 1A). Fig. 1A illustrates that nonenergized mitochondria developed modest Δψm that increased upon adding 5 mM Pi in the absence of extramitochondrial Ca. Generation of the proton gradient from oxidation of endogenous substrates was closely balanced by its dissipation as a result of inner membrane leakiness, which began to prevail over time as demonstrated by a slow decrease in Δψm. Subsequent addition of 50 μM diazoxide markedly accelerated Δψm dissipation because of a summation of the effects of inner membrane leakiness and mitoKATP activation, as seen when a low concentration of BSA (0.05 mg/ml), which is known to reduce proton leak, probably by chelating free fatty acids (19), was added to allow some Δψm recovery. Addition of 40 μM 5-HD then led to nearly full Δψm recovery. Under these conditions, diazoxide's known inhibition of succinate dehydrogenase cannot have caused dissipation of Δψm, because Δψm generation mainly depended on endogenous site I substrates as shown by Δψm dissipation with rotenone. Diazoxide, even at 100 μM, has no effect on site I substrate oxidation either in state 3 or 4 respiration (20). Thus, despite the absence of extramitochondrial ATP or Mg under these conditions, diazoxide was capable of increasing further the open probability of mitoKATP channels, and 5-HD was effective at blocking this increase. Our observations also show that the ability of mitoKATP channels to significantly affect Δψm depends largely on two factors: electron transport capability and the status of inner membrane leakiness to protons, the latter of which can be reversed by BSA. In energized mitochondria, the abundance of exogenous substrates allows electron transport activity to overpower Δψm dissipation because of inner membrane leakiness or mitoKATP channel opening, so that diazoxide has little effect on Δψm. In nonenergized mitochondria, neither diazoxide nor 5-HD significantly affected Δψm when relatively high concentrations (>0.2 mg/ml) of BSA were applied first, because total inner membrane leakiness to protons and K remained within the range for which residual electron transport could compensate.
Figure 1.
Effect of diazoxide and 5-HD on Δψm, Ca uptake, and MPT in nonenergized, isolated mitochondria. (A and B) Mitochondria (0.5 mg) were added to KCl buffer containing TMRM. For A, additions at arrows are: 100 μM EGTA, 5 mM Pi, 50 μM diazoxide, 0.05 mg/ml BSA, 40 μM 5-HD, and 5 μM rotenone. For B (buffer contained 100 μM EGTA), additions are: 5 mM Pi, 40 μM 5-HD, 0.05 mg/ml BSA, 50 μM diazoxide, and 5 μM rotenone. (C and D) Mitochondria (0.6 mg) were added to KCl buffer containing 1 μM Calcium Green-5N. For C, additions are: 5 mM Pi, 50 μM diazoxide, 20 μM Ca, 0.1 mg/ml BSA, and site I substrates. For D, additions are: 5 mM Pi, 100 μM 5-HD, 20 μM Ca, 0.1 mg/ml BSA, 5 mM each of pyruvate, malate, and glutamate, 5 mM succinate, and 1.5 μM CsA. Inset shows the same experiment in the presence of 1.5 μM CsA. Mitochondria rapidly accumulated Ca after adding site I substrates. (E and F) Same protocols as in C and D, using calcein-AM-loaded mitochondria to validate MPT. (E) In diazoxide-treated mitochondria, Ca-induced calcein release was reduced significantly. In F, 5-HD-treated mitochondria released calcein in response to Ca load, indicating MPT. Calcein release resulting from pore opening was confirmed in an experiment with CsA that completely prevented Ca-induced calcein release (Inset). (G) Summary of average substrate-induced Ca uptake in the presence of diazoxide or 5-HD for three different preparations. (H) Summary of Ca-induced calcein release, expressed as a percentage from maximal release induced with alamethicin (100%). Values are mean ± SE for the number of determinations in three different preparations.
The relative importance of reducing inner membrane leakiness with BSA vs. closing mitoKATP channels with 5-HD in the regulation of Δψm in nonenergized mitochondria is illustrated further in Fig. 1B, in which the order of application of diazoxide and 5-HD was reversed. After Pi, Δψm increased and began to dissipate slowly because of increased inner membrane leak. Addition of 5-HD stabilized Δψm, indicating that, with mitoKATP channels closed, residual electron transport could compensate. Addition of BSA led to further Δψm hyperpolarization, after which addition of diazoxide had no significant effect.
Fig. 1 C and D shows that in nonenergized mitochondria, diazoxide and 5-HD had significant effects on Ca uptake and Ca-induced MPT. In both cases, a 20 μM Ca load was administered at the same time point at which BSA had been applied in Fig. 1 A and B. This Ca load was accumulated easily by energized mitochondria with intact membranes without inducing MPT (not shown). In nonenergized mitochondria, preexposure to diazoxide completely prevented matrix Ca uptake, and subsequent addition of site I substrates allowed regeneration of Δψm and Ca uptake without triggering MPT (Fig. 1C). In nonenergized mitochondria pretreated with 5-HD, however, the same Ca load led to initial Ca uptake followed by rapid Ca release (Fig. 1D). This was due to MPT, because adding BSA and energizing mitochondria with site I as well as site II substrates stimulated relatively little Ca uptake, whereas closing PTP with CsA resulted in a large, sustained Ca uptake. Fig. 1D Inset shows that when the experiment was performed in the presence of CsA to prevent PTP opening, complete Ca uptake occurred after adding substrates, as in the diazoxide-treated mitochondria.
Fig. 1 E and F shows the same protocols performed in calcein-AM-loaded mitochondria. Upon addition of the Ca load, markedly less calcein efflux occurred in diazoxide-treated mitochondria (Fig. 1E) compared with 5-HD-treated mitochondria (Fig. 1F). Pretreatment with CsA prevented calcein efflux in the latter case (Fig. 1F Inset).
Effects of mitoKATP Channel Activation/Inhibition During Anoxia/Reoxygenation.
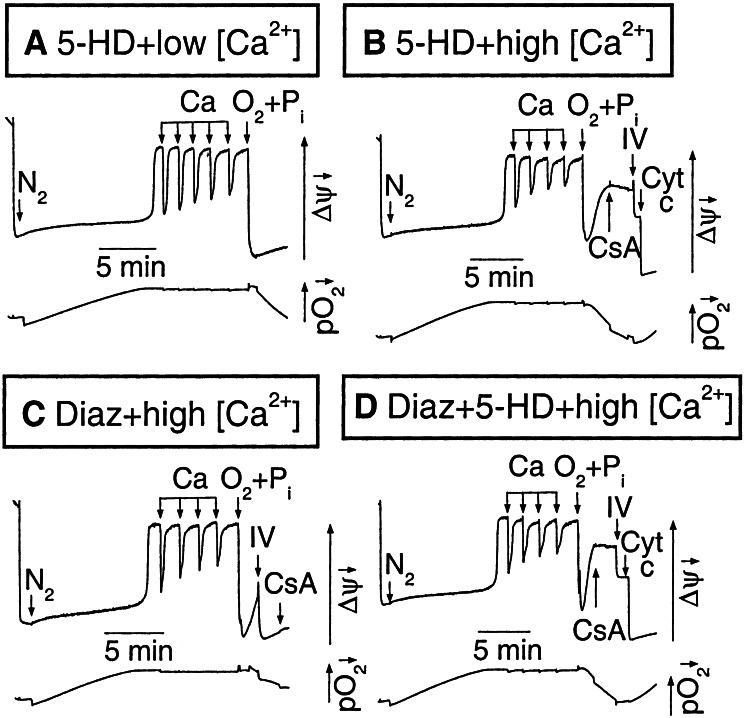
We next investigated the effects of diazoxide and 5-HD in isolated mitochondria subjected to anoxia/reoxygenation in the presence of Pi at an increased extramitochondrial concentration. In Fig. 2A, a freshly isolated, succinate-energized mitochondrial suspension was made anoxic until Δψm fully dissipated (16) and then subjected to a Ca load of five 5 μM Ca pulses with 300 μM 5-HD present to block mitoKATP channels. With each Ca pulse, a small amount of O2 also was injected (still below the threshold of the oxygen electrode), producing a rapid transient Δψm increase. The suspension then was reoxygenated while 5 mM Pi was added simultaneously. Although Δψm recovered under these conditions, with a larger Ca load of four 10 μM pulses (Fig. 2B), reoxygenation + Pi resulted in only transient Δψm recovery, which then dissipated again as MPT was triggered. CsA and complex IV substrates partially restored Δψm at this point, but full recovery was achieved only after addition of exogenous cytochrome c (2.5 μM). This observation indicates that significant cytochrome c loss from the intermembrane space had occurred and thereby compromised the ability of complex IV substrates to regenerate Δψm.
Figure 2.
Effects of mitoKATP activation on Ca-induced MPT during anoxia/reoxygenation. In each image, the upper trace shows TMRM fluorescence and the lower trace shows pO2. Mitochondria (0.5 mg/ml, <3 h on ice) were added to KCl buffer containing 5 mM succinate and exposed to a N2 stream until Δψm depolarized abruptly at a critically low pO2. A Ca load then was administered followed by reoxygenation plus the addition of 5 mM Pi (O2+Pi). (A) After a small Ca load (five 5 μM pulses) with 300 μM 5-HD present, Δψm recovered fully with O2+Pi. (B) After a larger Ca load (four 10 μM pulses), Δψm recovery after O2+Pi was transient, requiring CsA + complex IV substrates (IV) as well as 5 μM cytochrome c for full recovery. (C) With 50 μM diazoxide in place of 5-HD, Δψm recovery after O2+Pi was complete without CsA or cytochrome c. (D) Coadministration of 300 μM 5-HD blocked protection by diazoxide.
When the same experiment was performed with 50 μM diazoxide in place of 5-HD, however, full Δψm recovery after reoxygenation was achieved solely after complex IV substrates (Fig. 2C). CsA had no additional effect, and cytochrome c was not necessary for full recovery. The protective effects of diazoxide were completely blocked by 5-HD (Fig. 2D). Similar findings were obtained with three different preparations.
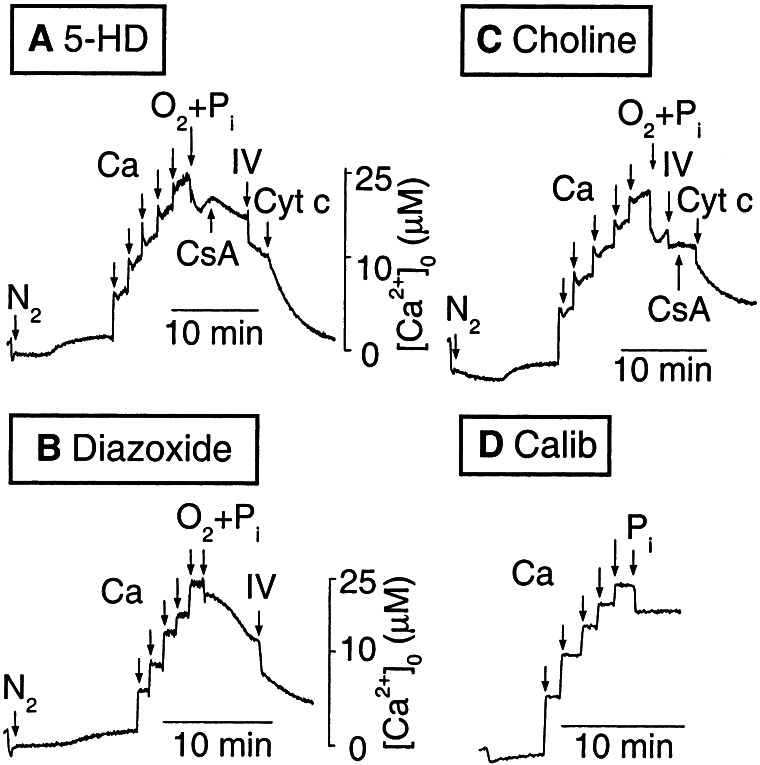
In Fig. 2, the rapid Δψm dissipation associated with critical O2 depletion as well as the transient increases in Δψm associated with the Ca additions were similar in diazoxide- and 5-HD-treated mitochondria. Thus, the mechanism of diazoxide-induced protection against cytochrome c depletion appears to be independent of its effects on Δψm. Fig. 3 shows the corresponding effects on Ca uptake and release by mitochondria during the same anoxia/reperfusion protocol in Fig. 2. After a duration of anoxia sufficient to dissipate Δψm with 5-HD present [as indicated by the small, rapid increase in extramitochondrial Ca (16)], Ca pulses were added (Fig. 3A). Each pulse resulted in a transient, small Ca uptake generated by the transient Δψm increase (see Fig. 2A) from O2 in the pulse. Upon reoxygenation with 5 mM Pi, transient Ca uptake followed by Ca efflux occurred. The latter was due to MPT, because it was reversed by CsA. Addition of complex IV substrates stimulated further Ca uptake, but full recovery required cytochrome c, as in Fig. 2B. In contrast, with diazoxide in place of 5-HD (Fig. 3B), the small, rapid Ca release corresponding to anoxic Δψm dissipation was blunted. After the Ca pulses, full recovery of Ca reuptake after reoxygenation + Pi was achieved with complex IV substrates alone, without any requirement for CsA or cytochrome c. Fig. 3C shows that replacing KCl with choline chloride prevented the protective effects of diazoxide, consistent with diazoxide's effects being mediated via mitoKATP channels.
Figure 3.
Effects of mitoKATP activation on mitochondrial Ca uptake/release during anoxia/reoxygenation. Extramitochondrial Ca was recorded by using 1 μM Calcium Green-5N during the same protocol as in Fig. 2. (A) Mitochondria (0.3 mg/ml, <3 h on ice) were added to KCl buffer containing succinate and 300 μM 5-HD, made anoxic and challenged with five 5 μM Ca pulses. Reoxygenation with 5 mM Pi (O2+Pi) led to transient Ca uptake followed by re-release requiring CsA + complex IV substrates (IV) + cytochrome c addition to reverse fully. (B) With 50 μM diazoxide replacing 5-HD, Ca reuptake after O2 +Pi was complete with ascorbic acid + TMPD without requiring CsA or cytochrome c. (C) With 50 μM diazoxide but choline chloride replacing KCl, Ca reuptake after O2+Pi again required both CsA and cytochrome c. (D) Method of calibrating extramitochondrial Ca, in which Ca pulses (5 μM) were added to a mitochondrial suspension, with FCCP present to prevent mitochondrial Ca uptake (see Methods). The addition of 5 mM Pi mildly quenched Calcium Green-5N fluorescence.
Protein Kinase C (PKC) Activation and Mitochondrial Protection Against MPT.
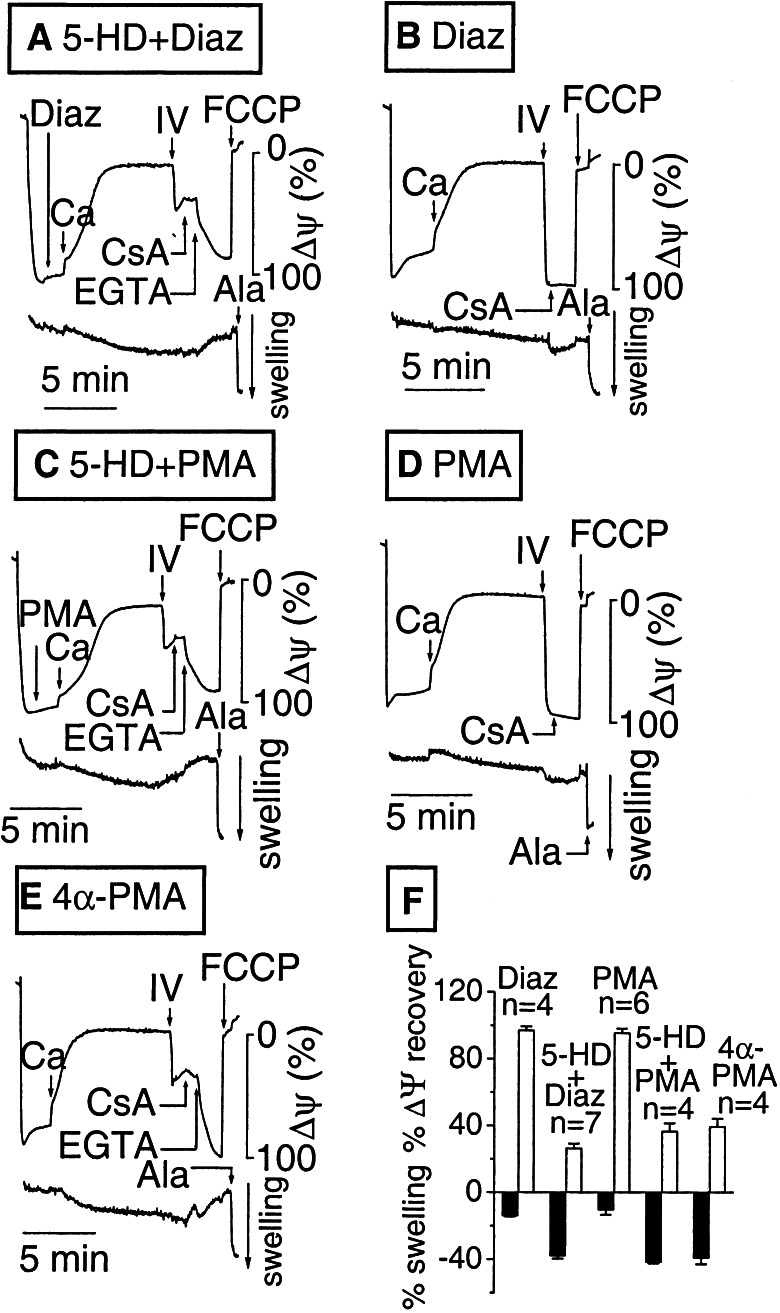
PKC activation is strongly implicated in cardioprotection by IPC and has been shown to potentiate diazoxide's effects on mitoKATP channels (21). In Fig. 4A, freshly isolated mitochondria were added to substrate-free buffer containing 5 mM Pi and 300 μM 5-HD. Mitochondria developed near normal Δψm, but Δψm dissipated slowly because of membrane leakiness that was not fully compensated by endogenous substrate oxidation (Fig. 1). Upon addition of 50 μM diazoxide and a small Ca load (10 μM), Δψm gradually depolarized and the matrix became progressively more swollen. When energized with site IV substrates, Δψm failed to recover completely and swelling did not reverse, even after CsA was added, until Ca was chelated with EGTA. These findings indicate that MPT had occurred. Similar results were obtained in the absence of both diazoxide and 5-HD (not shown). However, when this experiment was repeated with the same preparation of mitochondria with diazoxide present but no 5-HD (Fig. 4B), Δψm dissipation after the Ca load was faster, matrix swelling was much smaller, and Δψm recovered fully upon energization with complex IV substrates, with no additional recovery by CsA, similar to the results in Fig. 1.
Figure 4.
Comparison of the effects of diazoxide and phorbol esters on Ca-induced MPT in nonenergized mitochondria. In each image, the upper trace shows TMRM fluorescence and the lower trace shows matrix swelling. Fresh, isolated, nonenergized mitochondria (1–3 h on ice) were subjected to a small Ca load (10 μM) sufficient to dissipate Δψm and cause matrix swelling. Ala, alamethicin. (A) With 300 μM 5-HD added to diazoxide, full Δψm recovery required arachidonic acid + TMPD, EGTA, and CsA, indicating MPT had occurred. (B) With 50 μM diazoxide present, energization with complex IV substrates (IV) led to full Δψm recovery. (C–E) Treatment with 400 nM PMA (D), but not the inactive phorbol ester 4α-PMA (E), reproduced the effects of diazoxide. The effects of PMA were blocked by 300 μM 5-HD (C). (F) Data summary showing the degree of matrix swelling after Ca loading (relative to alamethicin, solid bars) and the extent of Δψm recovery after the addition of complex IV substrates (TMPD) for the various interventions in A–E.
Fig. 4C shows that pretreatment of the mitochondrial suspension with phorbol 12-myristate 13-acetate (PMA; 400 nM) in the presence of 300 μM 5-HD also led to MPT, but, in the absence of 5-HD, PMA protected against MPT similar to diazoxide (Fig. 4D). The inactive phorbol ester 4α-PMA did not prevent MPT (Fig. 4E). These observations indicate that mitoKATP channels are activated by PKC, resulting in equivalent protection against MPT as direct activation of mitoKATP channel with diazoxide.
Discussion
When widespread irreversible MPT occurs throughout a cell's mitochondria, necrotic cell death is inevitable. Cardioprotection by IPC or PPC ultimately must involve prevention of irreversible MPT (22), the key issue being whether they protect at an upstream event far removed from MPT or at a very proximate level. Considerable evidence implicating mitoKATP channels in PPC and IPC support the latter possibility. However, the mechanism has been elusive. The findings of the present study shed light on this issue by demonstrating two major, protective effects of mitoKATP channel activation on mitochondrial function under conditions generally relevant to ischemia and hypoxia: reduction in mitochondrial Ca loading in the presence of elevated extramitochondrial Pi and moderately increased inner membrane leakiness, and prevention of cytochrome c loss from the intermembrane space. Our findings stress that both mechanisms, operating in a coordinated way, are essential for recovery of mitochondrial function. Indeed, Δψm recovery is possible only in mitochondria with closed PTP and sufficient electron transport power to increase proton pumping.
Prevention of Mitochondrial Ca Overload by mitoKATP Activation.
By increasing inner membrane permeability to K, mitoKATP activation has two main effects: Δψm dissipation and mild matrix swelling. The initial idea that Δψm dissipation protects against MPT by reducing the driving force for Ca entry into the matrix via the Ca uniporter, however, has been criticized because, in energized mitochondria, mitoKATP agonists such as diazoxide induced minimal Δψm dissipation at fully cardioprotective doses (10, 12). Furthermore, although mitoKATP activation increased the ability of energized mitochondria to tolerate Ca loading, mitoKATP activation in already Ca-loaded mitochondria promoted, rather than inhibited, MPT (10).
Because the above experiments typically were performed with energized mitochondria, we reasoned that mimicking some major elements of the ischemic/anoxic environment might better clarify the role of Δψm dissipation by mitoKATP activation. Changes in the intracellular milieu during ischemia/reperfusion or anoxia/reoxygenation are complex. Some factors such as acidosis protect against MPT, but many, such as elevated cytoplasmic Ca and Pi, free fatty acid accumulation, oxidative stress, and cytokine release, tend to promote MPT. Cytoplasmic [Pi] increases markedly during ischemia or anoxia because of the rapid depletion of creatine phosphate and can reach 15 mM within 30–40 min (17). Mitochondrial Pi uptake decreases matrix pH that results in a compensatory increase in Δψm (23), enhancing matrix Ca uptake and PTP open probability (18). In addition to elevated Pi, mitochondria are subjected to other factors including anoxic electron transport inhibition and free fatty acid accumulation, which may increase inner membrane leakiness (19). Our findings show that, for a critical combination of these factors, activation of mitoKATP channels by diazoxide has a potent effect at preventing the Pi-induced Δψm hyperpolarization that promotes matrix Ca uptake, triggering MPT. Supporting this hypothesis, recent experimental studies have demonstrated that IPC and mitoKATP channel activation significantly inhibited matrix Ca accumulation and improved functional recovery of mitochondria in situ during ischemia/reperfusion, effects that were abolished partially or completely by 5-HD (5). Also, mitochondria isolated from preconditioned hearts after ischemia/reperfusion demonstrated greater ATP production than non-pre-conditioned hearts unless pretreated with 5-HD (4).
It has been argued that, for isolated mitochondria suspended in buffer lacking exogenous ATP or Mg, mitoKATP channels are already at their maximum open probability, so that diazoxide cannot protect against Ca-loading by further depolarizing Δψm (12). Experimental support for this view (24) was based on an indirect measure of mitoKATP channel activity, namely, matrix swelling, which may be less sensitive than changes in Δψm. Also, the latter study did not consider the state of inner membrane leakiness, which, we show here, has a critical influence on the ability of mitoKATP channels to modulate Δψm. In deenergized mitochondria suspended in an ATP- and Mg-free buffer, we found that diazoxide enhanced Δψm dissipation in a 5-HD-sensitive manner (e.g., Fig. 1). In this setting, only endogenous site I substrates were available to support electron transport at a reduced level, excluding the possibility that inhibition of succinate oxidation by diazoxide was responsible for enhancing Δψm dissipation. Neither diazoxide nor 5-HD has been reported to have any effects on site I substrate oxidation to our knowledge. On the other hand, in the anoxia–reoxygenation experiments (Figs. 2 and 3), mitochondria were energized with succinate, raising the issue that inhibition of succinate oxidation by diazoxide may have been a factor protecting against cytochrome c loss and MPT. However, this is unlikely, because (i) during reoxygenation, mitochondria were reenergized with site IV substrates whose oxidation is not inhibited by diazoxide (12); (ii) including 5-HD abolished diazoxide's protective effect (Fig. 2D), so that KATP inhibition is the only explanation unless 5-HD acted as a substrate for electron transport; and (iii) replacing KCl with choline chloride abolished protection by diazoxide (Fig. 3C). If mitoKATP channels behave similarly to sarcolemmal KATP channels, whose maximal open probability in the absence of ATP is ≈0.6 and can fall to much lower values with rundown (25, 26), then there is potentially considerable room for K channel openers or PKC-mediated phosphorylation to increase mitoKATP channel open probability even in the absence of extramitochondrial ATP.
Several other limitations in this study should be recognized. To study MPT, it was necessary to exclude ATP and Mg from the buffer, because they are potent MPT inhibitors. In contrast, these compounds, as well as ADP (an even more potent MPT inhibitor), are present in millimolar concentrations in intact myocytes during ischemia/reperfusion. Presumably, other pro-MPT factors that become active during ischemia (free fatty acid and Pi accumulation) or reperfusion (reactive oxygen species and pH increase) counteract the anti-MPT effects of adenine nucleotides and Mg, because the occurrence of MPT during reperfusion is well documented (22). Nevertheless, this limitation highlights the general shortcoming that studies of isolated mitochondria cannot exactly replicate the ischemia/reperfusion milieu in vivo.
Prevention of Cytochrome c Loss from the Intermembrane Space.
A second hypothesis proposed to explain cardioprotection by mitoKATP channel activation involves matrix swelling, postulated to preserve intermembrane space architecture, thereby preventing increased adenine nucleotide permeability of outer membrane protein porin (12). Consistent with Ozcan et al. (13), we found that diazoxide protected against cytochrome c loss, and it is intriguing to speculate that the processes controlling porin's permeability to adenine nucleotides also might regulate permeability to cytochrome c. Cytochrome c loss from mitochondria does not necessarily require outer membrane rupture because of MPT (27). Ischemia is known to cause early alterations in outer membrane permeability (28), and IPC has been found to inhibit respiratory stimulation by exogenous cytochrome c (6), suggesting that IPC inhibits cytochrome c loss. In our experiments, when cytochrome c was added after reoxygenation in the presence of CsA, this ≈13-kDa molecule still was able to permeate the outer membrane to reach the intermembrane space, where it stimulated electron transport to restore Δψm and Ca uptake. Thus, both PTP closure and recovery of electron transport (proton-pumping capacity) were required for full recovery of mitochondrial function.
PCK Signaling and mitoKATP Channel Activation.
In addition to direct mitoKATP channel activation with diazoxide and other agonists, IPC also can be mimicked by stimulating PKC-linked plasma-membrane receptors. Our findings suggest that cardioprotection by PKC is mediated, in part, through mitoKATP channel activation, because the phorbol ester PMA protected against MPT under the same conditions in which diazoxide was protective, and protection was abolished by 5-HD. These observations suggest that adequate PKC was present in isolated mitochondria to be translocated into inner membrane in response to PMA. Because inactive PKC is a soluble protein, one possibility is that PKC conferring protection is located in the intermembrane space or matrix. Our findings are consistent with previous studies showing that PKC activation sensitizes mitoKATP channels to activation by diazoxide (21).
Summary and Conclusions.
In isolated mitochondria exposed to elevated Ca and Pi and anoxia/reoxygenation or substrate-free conditions simulating key components of ischemia/reperfusion, direct activation of mitoKATP channels with diazoxide had two major, protective effects that preserved mitochondrial function: prevention of MPT and prevention of cytochrome c loss. Under conditions in which electron transport capability was reduced and the inner membrane had become leaky to a moderate extent, diazoxide prevented Δψm from increasing in response to elevated Pi, thereby reducing matrix Ca accumulation and averting MPT. The second effect may be related to osmotic consequences of mitoKATP channel activation, which protected the outer mitochondrial membrane: diazoxide prevented cytochrome c loss from the intermembrane space, preserving electron transport capability so that Δψm could be regenerated after reoxygenation. The coordination of these two effects is essential for allowing recovery of Δψm, which ultimately is required for functional cardiac recovery. PKC stimulation with phorbol esters mimicked the protective effects of diazoxide under these conditions, indicating that cardioprotection in IPC was mediated, at least in part, through PCK-induced activation of mitoKATP channels.
These observations further support the idea that the interplay between Δψm and matrix Ca with respect to MPT is critically important (16). Δψm dissipation inhibits MPT by reducing matrix Ca uptake, but, at comparable matrix [Ca], Δψm dissipation increases PTP open probability and thereby directly promotes MPT. Thus, if mitochondria are Ca-loaded at normal Δψm and then depolarized, such as with FCCP or diazoxide, MPT will be induced (10, 29, 30). However, if mitochondria are depolarized before exposure to elevated extramitochondrial Ca, MPT will not occur unless Ca concentration remains elevated when Δψm is regenerated (16). Diazoxide has minimal effects on Δψm under physiological energized conditions but accelerates Δψm dissipation when mitochondria become vulnerable to injury by means of MPT, because of elevated Ca and Pi, reduced electron transport capability, and inner membrane leakiness. This favorable profile maximizes diaxozide's therapeutic efficacy while minimizing toxicity and may be an important mechanism of protection of mitochondria as they pass through a critical window of vulnerability during ischemia/reperfusion.
Acknowledgments
This work was supported by National Institutes of Health Specialized Center of Research in Sudden Cardiac Death Grant P50 HL52319 and by the Laubisch Fund and Kawata Endowments.
Abbreviations
- mitoKATP channel
mitochondrial ATP-sensitive K channel
- MPT
mitochondrial permeability transition
- 5-HD
5-hydroxydecanoate
- IPC
ischemic preconditioning
- PMA
phorbol 12-myristate 13-acetate
- CsA
cyclosporin A
- FCCP
carbonyl cyanide p-trifluoromethoxyphenylhydrazone
- PKC
protein kinase C
- PPC
pharmacologic preconditioning
- Δψm
inner membrane potential
- TMRM
tetramethylrhodamine methyl ester
References
- 1.Szewczyk A, Marbán E. Trends Pharmacol Sci. 1999;20:157–161. doi: 10.1016/s0165-6147(99)01301-2. [DOI] [PubMed] [Google Scholar]
- 2.Grover G J, Garlid K D. J Mol Cell Cardiol. 2000;32:677–695. doi: 10.1006/jmcc.2000.1111. [DOI] [PubMed] [Google Scholar]
- 3.Gross G J, Fryer R M. Circ Res. 1999;84:973–979. doi: 10.1161/01.res.84.9.973. [DOI] [PubMed] [Google Scholar]
- 4.Fryer R M, Eells J T, Hsu A K, Henry M M, Gross G J. Am J Physiol. 2000;278:H305–H312. doi: 10.1152/ajpheart.2000.278.1.H305. [DOI] [PubMed] [Google Scholar]
- 5.Wang L, Cherednichenko G, Hernandez L, Halow J, Camacho S A, Figueredo V, Schaefer S. Am J Physiol. 2001;280:H2321–H2328. doi: 10.1152/ajpheart.2001.280.5.H2321. [DOI] [PubMed] [Google Scholar]
- 6.Laclau M N, Boudina S, Thambo J B, Tariosse L, Gouverneur G, Bonoron-Adele S, Saks V A, Garlid K D, Dos Santos P. J Mol Cell Cardiol. 2001;33:947–956. doi: 10.1006/jmcc.2001.1357. [DOI] [PubMed] [Google Scholar]
- 7.Fryer R M, Hsu A K, Gross G J. J Mol Cell Cardiol. 2001;33:831–834. doi: 10.1006/jmcc.2001.1350. [DOI] [PubMed] [Google Scholar]
- 8.Murata M, Akao M, O'Rourke B, Marban E. Circ Res. 2001;89:891–898. doi: 10.1161/hh2201.100205. [DOI] [PubMed] [Google Scholar]
- 9.Ozcan C, Holmuhamedov E L, Jahangir A, Terzic A. J Thorac Cardiovasc Surg. 2001;121:298–306. doi: 10.1067/mtc.2001.111421. [DOI] [PubMed] [Google Scholar]
- 10.Holmuhamedov E L, Wang L, Terzic A. J Physiol. 1999;519:347–360. doi: 10.1111/j.1469-7793.1999.0347m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Sato T, Seharaseyon J, Szewczyk A, O'Rourke B, Marbán E. Ann N Y Acad Sci. 1999;874:27–37. doi: 10.1111/j.1749-6632.1999.tb09222.x. [DOI] [PubMed] [Google Scholar]
- 12.Kowaltowski A J, Seetharaman S, Paucek P, Garlid K D. Am J Physiol. 2001;280:H649–H657. doi: 10.1152/ajpheart.2001.280.2.H649. [DOI] [PubMed] [Google Scholar]
- 13.Ozcan C, Bienengraeber M, Dzeja P, Terzic A. Am J Physiol. 2002;282:H531–H539. doi: 10.1152/ajpheart.00552.2001. [DOI] [PubMed] [Google Scholar]
- 14.Pain T, Yang X M, Critz S D, Yue Y, Nakano A, Liu G S, Heusch G, Cohen M V, Downey J M. Circ Res. 2000;87:460–466. doi: 10.1161/01.res.87.6.460. [DOI] [PubMed] [Google Scholar]
- 15.Forbes R A, Steenbergen C, Murphy E. Circ Res. 2001;88:802–809. doi: 10.1161/hh0801.089342. [DOI] [PubMed] [Google Scholar]
- 16.Korge P, Honda H M, Weiss J N. Am J Physiol. 2001;280:C517–C526. doi: 10.1152/ajpcell.2001.280.3.C517. [DOI] [PubMed] [Google Scholar]
- 17.Koretsune Y, Marban E. Am J Physiol. 1990;258:H9–H16. doi: 10.1152/ajpheart.1990.258.1.H9. [DOI] [PubMed] [Google Scholar]
- 18.Zoratti M, Szabo I. Biochim Biophys Acta. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 19.Borutaite V, Morkuniene R, Budriunaite A, Krasauskaite D, Ryselis S, Toleikis A, Brown G C. J Mol Cell Cardiol. 1996;28:2195–2201. doi: 10.1006/jmcc.1996.0211. [DOI] [PubMed] [Google Scholar]
- 20.Ovide-Bordeaux S, Ventura-Clapier R, Veksler V. J Biol Chem. 2000;275:37291–37325. doi: 10.1074/jbc.M005772200. [DOI] [PubMed] [Google Scholar]
- 21.Sato T, O'Rourke B, Marbán E. Circ Res. 1998;83:110–114. doi: 10.1161/01.res.83.1.110. [DOI] [PubMed] [Google Scholar]
- 22.Halestrap A P, Kerr P M, Javadov S, Woodfield K Y. Biochim Biophys Acta. 1998;1366:79–94. doi: 10.1016/s0005-2728(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 23.Jung D W, Panzeter E, Baysal K, Brierley G P. Biochim Biophys Acta. 1997;1320:310–320. doi: 10.1016/s0005-2728(97)00036-4. [DOI] [PubMed] [Google Scholar]
- 24.Jaburek M, Yarov-Yarovoy V, Paucek P, Garlid K D. J Biol Chem. 1998;273:13578–13582. [PubMed] [Google Scholar]
- 25.Ribalet B, John S A, Weiss J N. J Gen Physiol. 2000;116:391–410. doi: 10.1085/jgp.116.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Light P E, Bladen C, Winkfein R J, Walsh M P, French R J. Proc Natl Acad Sci USA. 2000;97:9058–9063. doi: 10.1073/pnas.160068997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doran E, Halestrap A P. Biochem J. 2000;348:343–350. [PMC free article] [PubMed] [Google Scholar]
- 28.Rossi A, Kay L, Saks V. Mol Cell Biochem. 1998;184:401–408. [PubMed] [Google Scholar]
- 29.Sztark F, Ichas F, Ouhabi R, Dabadie P, Mazat J P. FEBS Lett. 1995;368:101–104. doi: 10.1016/0014-5793(95)00610-l. [DOI] [PubMed] [Google Scholar]
- 30.Petronilli V, Cola C, Massari S, Colonna R, Bernardi P. J Biol Chem. 1993;268:21939–21945. [PubMed] [Google Scholar]