Abstract
The orientation of the light-chain region of myosin heads in relaxed, rigor, and isometrically contracting fibers from rabbit psoas muscle was studied by fluorescence polarization. Cysteine 108 of chicken gizzard myosin regulatory light chain (cgRLC) was covalently modified with iodoacetamidotetramethylrhodamine (iodo-ATR). Native RLC of single glycerinated muscle fibers was exchanged for labeled cgRLC in a low [Mg2+] rigor solution at 30 degrees C. Troponin and troponin C removed in this procedure were replaced. RLC exchange had little effect on active force production. X-ray diffraction showed normal structure in rigor after RLC exchange, but loss of axial and helical order in relaxation. In isolated myofibrils labeled cgRLC was confined to the regions of the sarcomere containing myosin heads. The ATR dipoles showed a preference for orientations perpendicular to the fiber axis, combined with limited nanosecond rotational motion, in all conditions studied. The perpendicular orientation preference was more marked in rigor than in either relaxation or active contraction. Stretching relaxed fibers to sarcomere length 4 microns to eliminate overlap between actin- and myosin-containing filaments had little effect on the orientation preference. There was no change in orientation preference when fibers were put into rigor at sarcomere length 4.0 microns. Qualitatively similar results were obtained with ATR-labeled rabbit skeletal RLC.
Full text
PDF



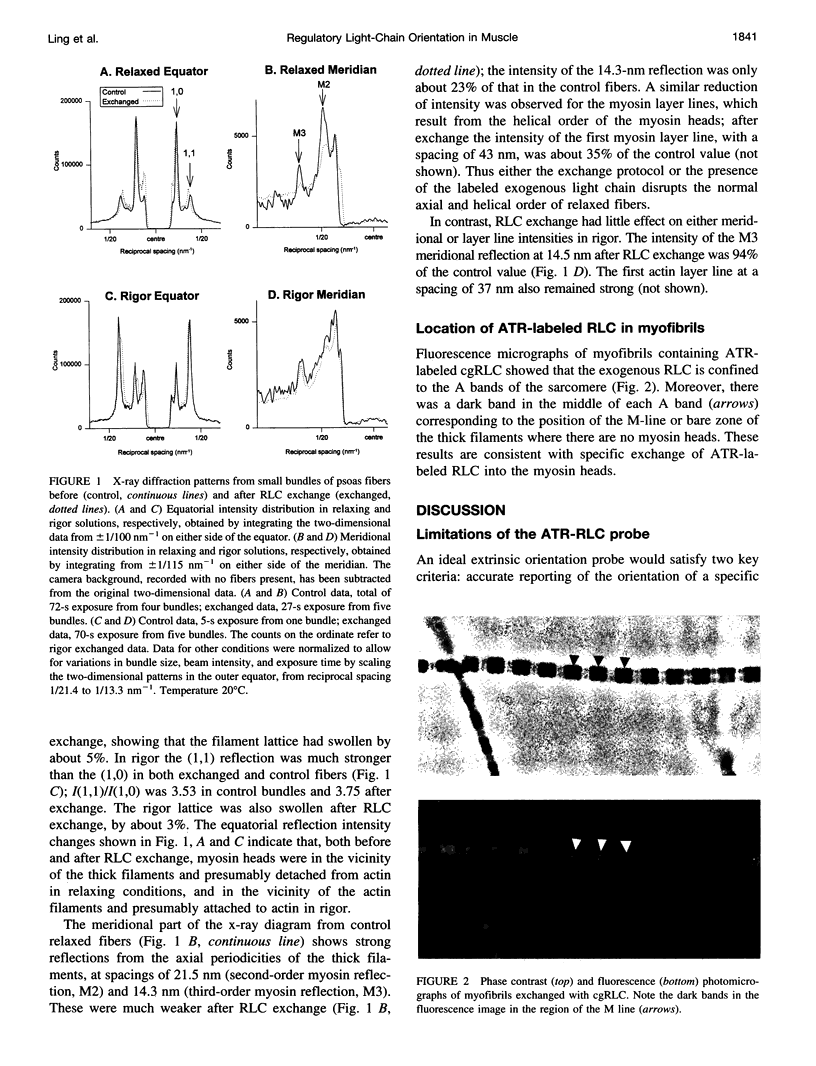

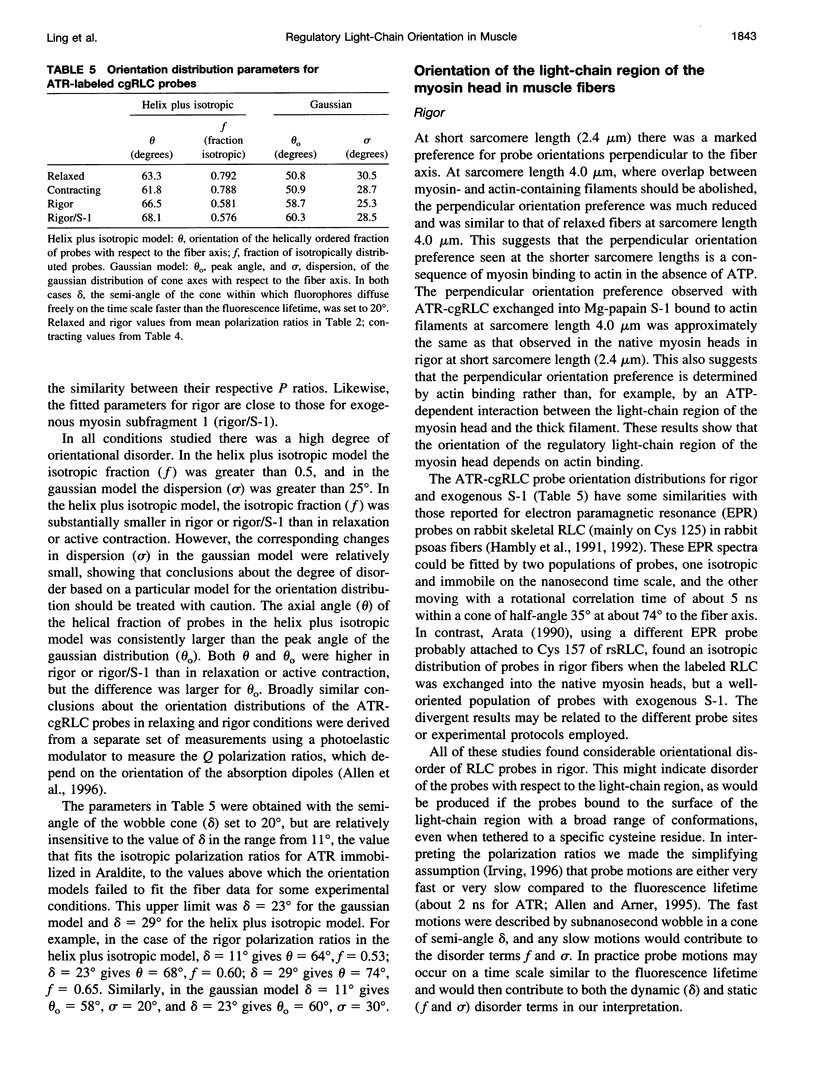



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arata T. Orientation of spin-labeled light chain 2 of myosin heads in muscle fibers. J Mol Biol. 1990 Jul 20;214(2):471–478. doi: 10.1016/0022-2836(90)90194-Q. [DOI] [PubMed] [Google Scholar]
- Borejdo J., Assulin O., Ando T., Putnam S. Cross-bridge orientation in skeletal muscle measured by linear dichroism of an extrinsic chromophore. J Mol Biol. 1982 Jul 5;158(3):391–414. doi: 10.1016/0022-2836(82)90205-4. [DOI] [PubMed] [Google Scholar]
- Borejdo J., Putnam S., Morales M. F. Fluctuations in polarized fluorescence: evidence that muscle cross bridges rotate repetitively during contraction. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6346–6350. doi: 10.1073/pnas.76.12.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt T. P., Ando T., Borejdo J. Evidence for cross-bridge order in contraction of glycerinated skeletal muscle. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7515–7519. doi: 10.1073/pnas.80.24.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN R. F., BOWMAN R. L. FLUORESCENCE POLARIZATION: MEASUREMENT WITH ULTRAVIOLET-POLARIZING FILTERS IN A SPECTROPHOTOFLUOROMETER. Science. 1965 Feb 12;147(3659):729–732. doi: 10.1126/science.147.3659.729. [DOI] [PubMed] [Google Scholar]
- Cooke R., Crowder M. S., Thomas D. D. Orientation of spin labels attached to cross-bridges in contracting muscle fibres. Nature. 1982 Dec 23;300(5894):776–778. doi: 10.1038/300776a0. [DOI] [PubMed] [Google Scholar]
- Cooke R. The mechanism of muscle contraction. CRC Crit Rev Biochem. 1986;21(1):53–118. doi: 10.3109/10409238609113609. [DOI] [PubMed] [Google Scholar]
- Crowder M. S., Cooke R. Orientation of spin-labeled nucleotides bound to myosin in glycerinated muscle fibers. Biophys J. 1987 Feb;51(2):323–333. doi: 10.1016/S0006-3495(87)83338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaser M. L., Gergely J. Reconstitution of troponin activity from three protein components. J Biol Chem. 1971 Jul 10;246(13):4226–4233. [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Hambly B., Franks K., Cooke R. Orientation of spin-labeled light chain-2 exchanged onto myosin cross-bridges in glycerinated muscle fibers. Biophys J. 1991 Jan;59(1):127–138. doi: 10.1016/S0006-3495(91)82205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambly B., Franks K., Cooke R. Paramagnetic probes attached to a light chain on the myosin head are highly disordered in active muscle fibers. Biophys J. 1992 Nov;63(5):1306–1313. doi: 10.1016/S0006-3495(92)81717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Irving M., St Claire Allen T., Sabido-David C., Craik J. S., Brandmeier B., Kendrick-Jones J., Corrie J. E., Trentham D. R., Goldman Y. E. Tilting of the light-chain region of myosin during step length changes and active force generation in skeletal muscle. Nature. 1995 Jun 22;375(6533):688–691. doi: 10.1038/375688a0. [DOI] [PubMed] [Google Scholar]
- Jakes R., Northrop F., Kendrick-Jones J. Calcium binding regions of myosin 'regulatory' light chains. FEBS Lett. 1976 Nov;70(1):229–234. doi: 10.1016/0014-5793(76)80763-6. [DOI] [PubMed] [Google Scholar]
- Katoh T., Lowey S. Mapping myosin light chains by immunoelectron microscopy. Use of anti-fluorescyl antibodies as structural probes. J Cell Biol. 1989 Oct;109(4 Pt 1):1549–1560. doi: 10.1083/jcb.109.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishino A., Yanagida T. Force measurements by micromanipulation of a single actin filament by glass needles. Nature. 1988 Jul 7;334(6177):74–76. doi: 10.1038/334074a0. [DOI] [PubMed] [Google Scholar]
- Knight P. J., Trinick J. A. Preparation of myofibrils. Methods Enzymol. 1982;85(Pt B):9–12. doi: 10.1016/0076-6879(82)85004-0. [DOI] [PubMed] [Google Scholar]
- Lowey S., Waller G. S., Trybus K. M. Skeletal muscle myosin light chains are essential for physiological speeds of shortening. Nature. 1993 Sep 30;365(6445):454–456. doi: 10.1038/365454a0. [DOI] [PubMed] [Google Scholar]
- Lowy J., Popp D., Stewart A. A. X-ray studies of order-disorder transitions in the myosin heads of skinned rabbit psoas muscles. Biophys J. 1991 Oct;60(4):812–824. doi: 10.1016/S0006-3495(91)82116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Margossian S. S., Lowey S. Preparation of myosin and its subfragments from rabbit skeletal muscle. Methods Enzymol. 1982;85(Pt B):55–71. doi: 10.1016/0076-6879(82)85009-x. [DOI] [PubMed] [Google Scholar]
- Matsuda G., Maita T., Suzuyama Y., Setoguchi M., Umegane T. Amino acid sequence of the L-2 light chain of rabbit skeletal muscle myosin. J Biochem. 1977 Mar;81(3):809–811. doi: 10.1093/oxfordjournals.jbchem.a131520. [DOI] [PubMed] [Google Scholar]
- Milligan R. A., Flicker P. F. Structural relationships of actin, myosin, and tropomyosin revealed by cryo-electron microscopy. J Cell Biol. 1987 Jul;105(1):29–39. doi: 10.1083/jcb.105.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss R. L. Ca2+ regulation of mechanical properties of striated muscle. Mechanistic studies using extraction and replacement of regulatory proteins. Circ Res. 1992 May;70(5):865–884. doi: 10.1161/01.res.70.5.865. [DOI] [PubMed] [Google Scholar]
- Moss R. L., Giulian G. G., Greaser M. L. Physiological effects accompanying the removal of myosin LC2 from skinned skeletal muscle fibers. J Biol Chem. 1982 Aug 10;257(15):8588–8591. [PubMed] [Google Scholar]
- Ostap E. M., Barnett V. A., Thomas D. D. Resolution of three structural states of spin-labeled myosin in contracting muscle. Biophys J. 1995 Jul;69(1):177–188. doi: 10.1016/S0006-3495(95)79888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham M., Irving M. Myosin crossbridge orientation in demembranated muscle fibres studied by birefringence and X-ray diffraction measurements. J Mol Biol. 1989 Nov 5;210(1):113–126. doi: 10.1016/0022-2836(89)90294-5. [DOI] [PubMed] [Google Scholar]
- Penefsky H. S. Reversible binding of Pi by beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1977 May 10;252(9):2891–2899. [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Reedy M. K., Holmes K. C., Tregear R. T. Induced changes in orientation of the cross-bridges of glycerinated insect flight muscle. Nature. 1965 Sep 18;207(5003):1276–1280. doi: 10.1038/2071276a0. [DOI] [PubMed] [Google Scholar]
- Reedy M. K., Lucaveche C., Naber N., Cooke R. Insect crossbridges, relaxed by spin-labeled nucleotide, show well-ordered 90 degrees state by X-ray diffraction and electron microscopy, but spectra of electron paramagnetic resonance probes report disorder. J Mol Biol. 1992 Oct 5;227(3):678–697. doi: 10.1016/0022-2836(92)90217-8. [DOI] [PubMed] [Google Scholar]
- Rowe T., Kendrick-Jones J. Chimeric myosin regulatory light chains identify the subdomain responsible for regulatory function. EMBO J. 1992 Dec;11(13):4715–4722. doi: 10.1002/j.1460-2075.1992.tb05576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J. W., Thomas D. D., Goldman Y. E. Transients in orientation of a fluorescent cross-bridge probe following photolysis of caged nucleotides in skeletal muscle fibres. J Mol Biol. 1992 Jan 5;223(1):185–203. doi: 10.1016/0022-2836(92)90725-y. [DOI] [PubMed] [Google Scholar]
- Taylor K. A., Reedy M. C., Córdova L., Reedy M. K. Three-dimensional image reconstruction of insect flight muscle. II. The rigor actin layer. J Cell Biol. 1989 Sep;109(3):1103–1123. doi: 10.1083/jcb.109.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Cooke R. Orientation of spin-labeled myosin heads in glycerinated muscle fibers. Biophys J. 1980 Dec;32(3):891–906. doi: 10.1016/S0006-3495(80)85024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D. Spectroscopic probes of muscle cross-bridge rotation. Annu Rev Physiol. 1987;49:691–709. doi: 10.1146/annurev.ph.49.030187.003355. [DOI] [PubMed] [Google Scholar]
- Tregear R. T., Mendelson R. A. Polarization from a helix of fluorophores and its relation to that obtained from muscle. Biophys J. 2009 Jan 1;15(5):455–467. doi: 10.1016/S0006-3495(75)85830-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert P., Cohen C. Domains, motions and regulation in the myosin head. J Muscle Res Cell Motil. 1988 Aug;9(4):296–305. doi: 10.1007/BF01773873. [DOI] [PubMed] [Google Scholar]
- Wagner P. D. Preparation and fractionation of myosin light chains and exchange of the essential light chains. Methods Enzymol. 1982;85(Pt B):72–81. doi: 10.1016/0076-6879(82)85010-6. [DOI] [PubMed] [Google Scholar]
- Yanagida T. Angles of nucleotides bound to cross-bridges in glycerinated muscle fiber at various concentrations of epsilon-ATP, epsilon-ADP and epsilon-AMPPNP detected by polarized fluorescence. J Mol Biol. 1981 Mar 15;146(4):539–560. doi: 10.1016/0022-2836(81)90046-2. [DOI] [PubMed] [Google Scholar]



