Abstract
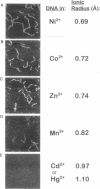
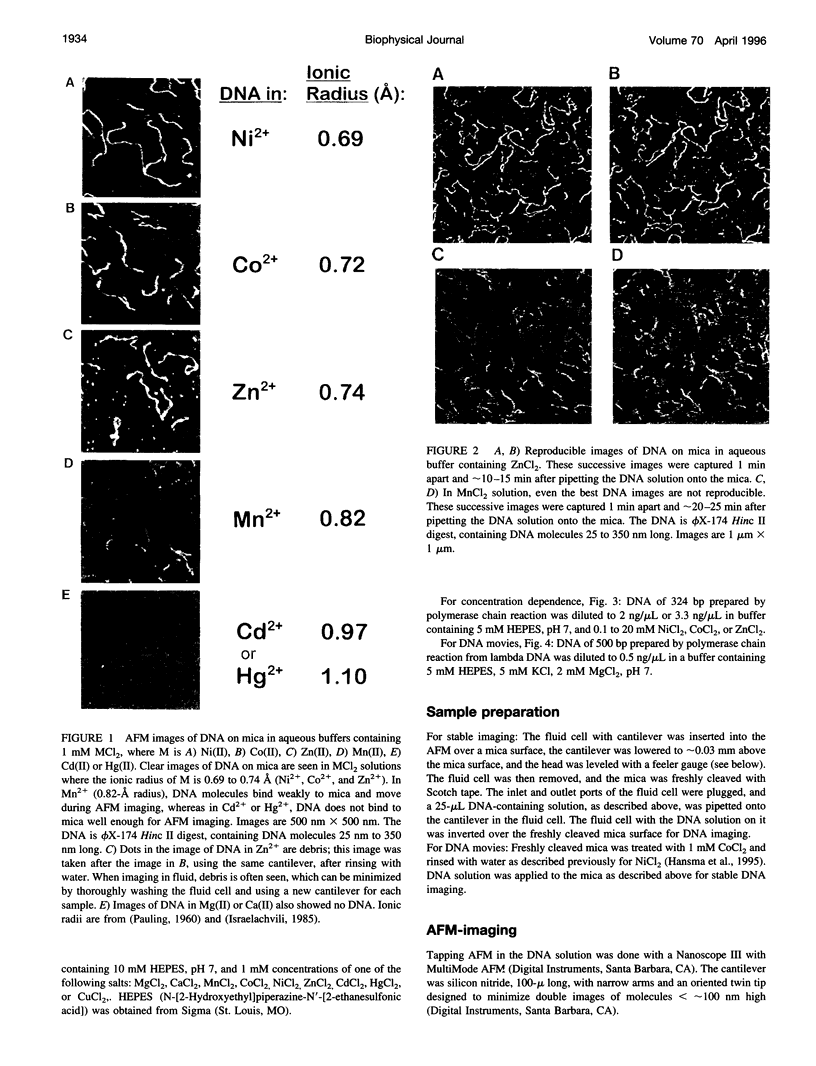
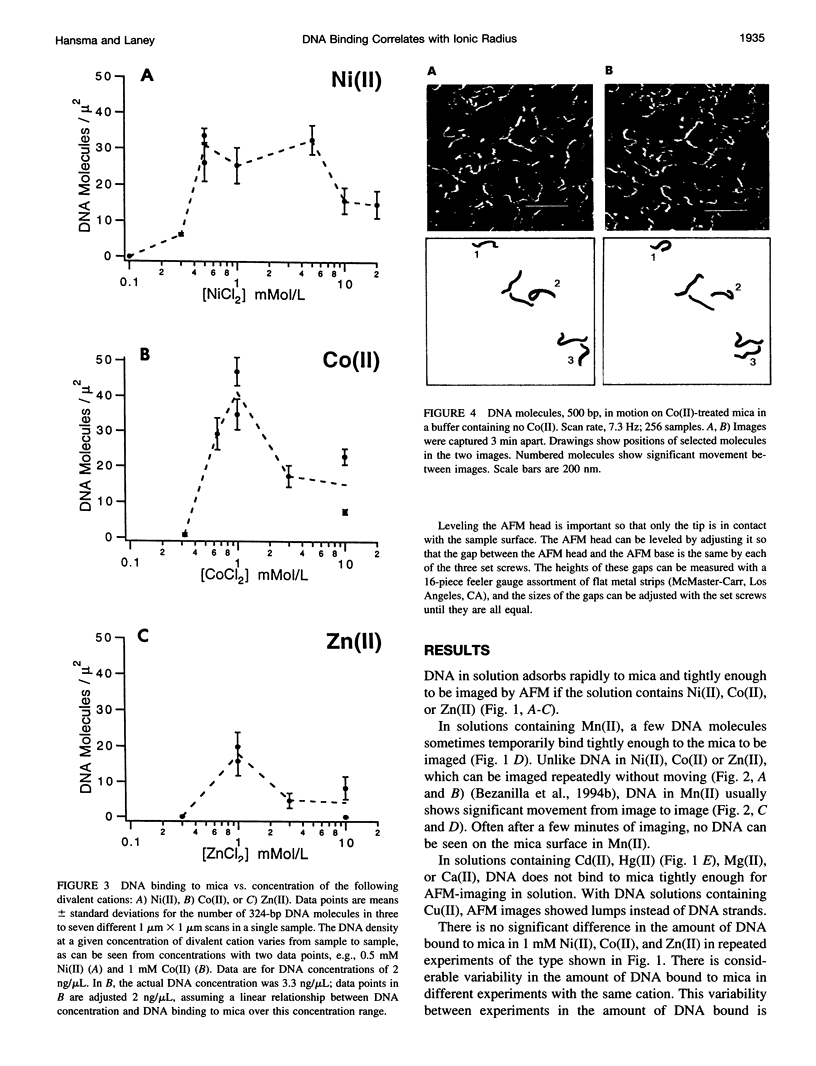
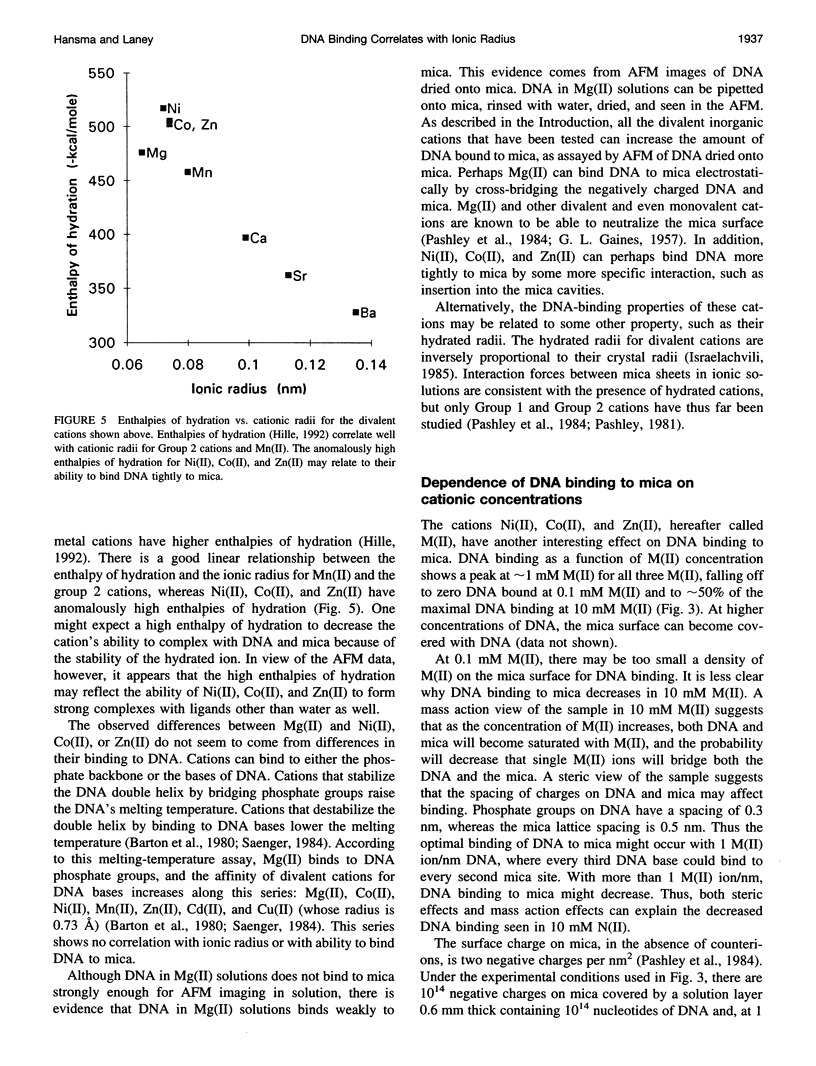
In buffers containing selected transition metal salts, DNA binds to mica tightly enough to be directly imaged in the buffer in the atomic force microscope (AFM, also known as scanning force microscope). The binding of DNA to mica, as measured by AFM-imaging, is correlated with the radius of the transition metal cation. The transition metal cations that effectively bind DNA to mica are Ni(II), Co(II), and Zn(II), which have ionic radii from 0.69 to 0.74 A. In Mn(II), ionic radius 0.82 A, DNA binds weakly to mica. In Cd(II) and Hg(II), respective ionic radii of 0.97 and 1.1 A, DNA does not bind to mica well enough to be imaged with the AFM. These results may to relate to how large a cation can fit into the cavities above the recessed hydroxyl groups in the mica lattice, although hypotheses based on hydrated ionic radii cannot be ruled out. The dependence of DNA binding on the concentrations of the cations Ni(II), Co(II), or Zn(II) shows maximal DNA binding at approximately 1-mM cation. Mg(II) does not bind DNA tightly enough to mica for AFM imaging. Mg(II) is a Group 2 cation with an ionic radius similar to that of Ni(II). Ni(II), Co(II), and Zn(II) have anomalously high enthalpies of hydration that may relate to their ability to bind DNA to mica. This AFM assay for DNA binding to mica has potential applications for assaying the binding of other polymers to mica and other flat surfaces.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bezanilla M., Drake B., Nudler E., Kashlev M., Hansma P. K., Hansma H. G. Motion and enzymatic degradation of DNA in the atomic force microscope. Biophys J. 1994 Dec;67(6):2454–2459. doi: 10.1016/S0006-3495(94)80733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys Rev Lett. 1986 Mar 3;56(9):930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- Bustamante C., Vesenka J., Tang C. L., Rees W., Guthold M., Keller R. Circular DNA molecules imaged in air by scanning force microscopy. Biochemistry. 1992 Jan 14;31(1):22–26. doi: 10.1021/bi00116a005. [DOI] [PubMed] [Google Scholar]
- Erie D. A., Yang G., Schultz H. C., Bustamante C. DNA bending by Cro protein in specific and nonspecific complexes: implications for protein site recognition and specificity. Science. 1994 Dec 2;266(5190):1562–1566. doi: 10.1126/science.7985026. [DOI] [PubMed] [Google Scholar]
- Hansma H. G., Bezanilla M., Zenhausern F., Adrian M., Sinsheimer R. L. Atomic force microscopy of DNA in aqueous solutions. Nucleic Acids Res. 1993 Feb 11;21(3):505–512. doi: 10.1093/nar/21.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansma H. G., Browne K. A., Bezanilla M., Bruice T. C. Bending and straightening of DNA induced by the same ligand: characterization with the atomic force microscope. Biochemistry. 1994 Jul 19;33(28):8436–8441. doi: 10.1021/bi00194a007. [DOI] [PubMed] [Google Scholar]
- Hansma H. G., Laney D. E., Bezanilla M., Sinsheimer R. L., Hansma P. K. Applications for atomic force microscopy of DNA. Biophys J. 1995 May;68(5):1672–1677. doi: 10.1016/S0006-3495(95)80343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansma H. G., Vesenka J., Siegerist C., Kelderman G., Morrett H., Sinsheimer R. L., Elings V., Bustamante C., Hansma P. K. Reproducible imaging and dissection of plasmid DNA under liquid with the atomic force microscope. Science. 1992 May 22;256(5060):1180–1184. doi: 10.1126/science.256.5060.1180. [DOI] [PubMed] [Google Scholar]
- Hegner M., Wagner P., Semenza G. Immobilizing DNA on gold via thiol modification for atomic force microscopy imaging in buffer solutions. FEBS Lett. 1993 Dec 28;336(3):452–456. doi: 10.1016/0014-5793(93)80854-n. [DOI] [PubMed] [Google Scholar]
- Lyubchenko Y. L., Gall A. A., Shlyakhtenko L. S., Harrington R. E., Jacobs B. L., Oden P. I., Lindsay S. M. Atomic force microscopy imaging of double stranded DNA and RNA. J Biomol Struct Dyn. 1992 Dec;10(3):589–606. doi: 10.1080/07391102.1992.10508670. [DOI] [PubMed] [Google Scholar]
- Lyubchenko Y. L., Oden P. I., Lampner D., Lindsay S. M., Dunker K. A. Atomic force microscopy of DNA and bacteriophage in air, water and propanol: the role of adhesion forces. Nucleic Acids Res. 1993 Mar 11;21(5):1117–1123. doi: 10.1093/nar/21.5.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubchenko Y., Shlyakhtenko L., Harrington R., Oden P., Lindsay S. Atomic force microscopy of long DNA: imaging in air and under water. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2137–2140. doi: 10.1073/pnas.90.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees W. A., Keller R. W., Vesenka J. P., Yang G., Bustamante C. Evidence of DNA bending in transcription complexes imaged by scanning force microscopy. Science. 1993 Jun 11;260(5114):1646–1649. doi: 10.1126/science.8503010. [DOI] [PubMed] [Google Scholar]
- Schaper A., Pietrasanta L. I., Jovin T. M. Scanning force microscopy of circular and linear plasmid DNA spread on mica with a quaternary ammonium salt. Nucleic Acids Res. 1993 Dec 25;21(25):6004–6009. doi: 10.1093/nar/21.25.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thundat T., Allison D. P., Warmack R. J., Brown G. M., Jacobson K. B., Schrick J. J., Ferrell T. L. Atomic force microscopy of DNA on mica and chemically modified mica. Scanning Microsc. 1992 Dec;6(4):911–918. [PubMed] [Google Scholar]
- Vesenka J., Guthold M., Tang C. L., Keller D., Delaine E., Bustamante C. Substrate preparation for reliable imaging of DNA molecules with the scanning force microscope. Ultramicroscopy. 1992 Jul;42-44(Pt B):1243–1249. doi: 10.1016/0304-3991(92)90430-r. [DOI] [PubMed] [Google Scholar]
- Wyman C., Grotkopp E., Bustamante C., Nelson H. C. Determination of heat-shock transcription factor 2 stoichiometry at looped DNA complexes using scanning force microscopy. EMBO J. 1995 Jan 3;14(1):117–123. doi: 10.1002/j.1460-2075.1995.tb06981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]