Abstract
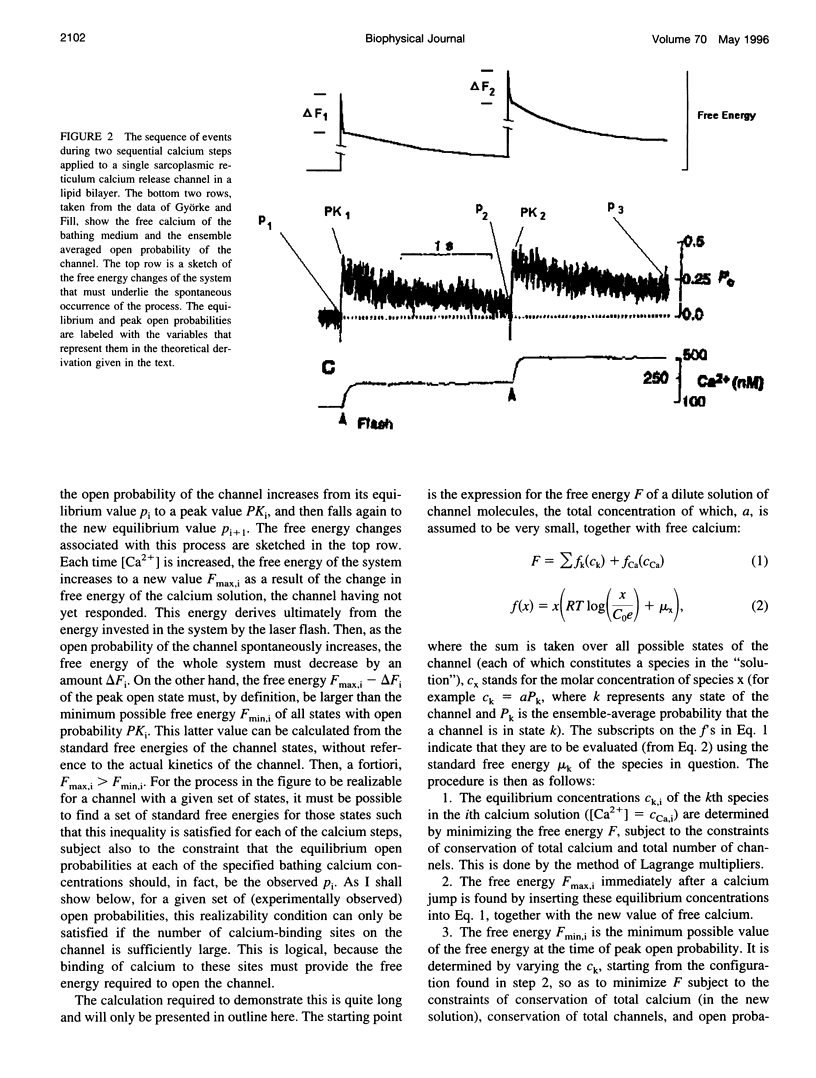
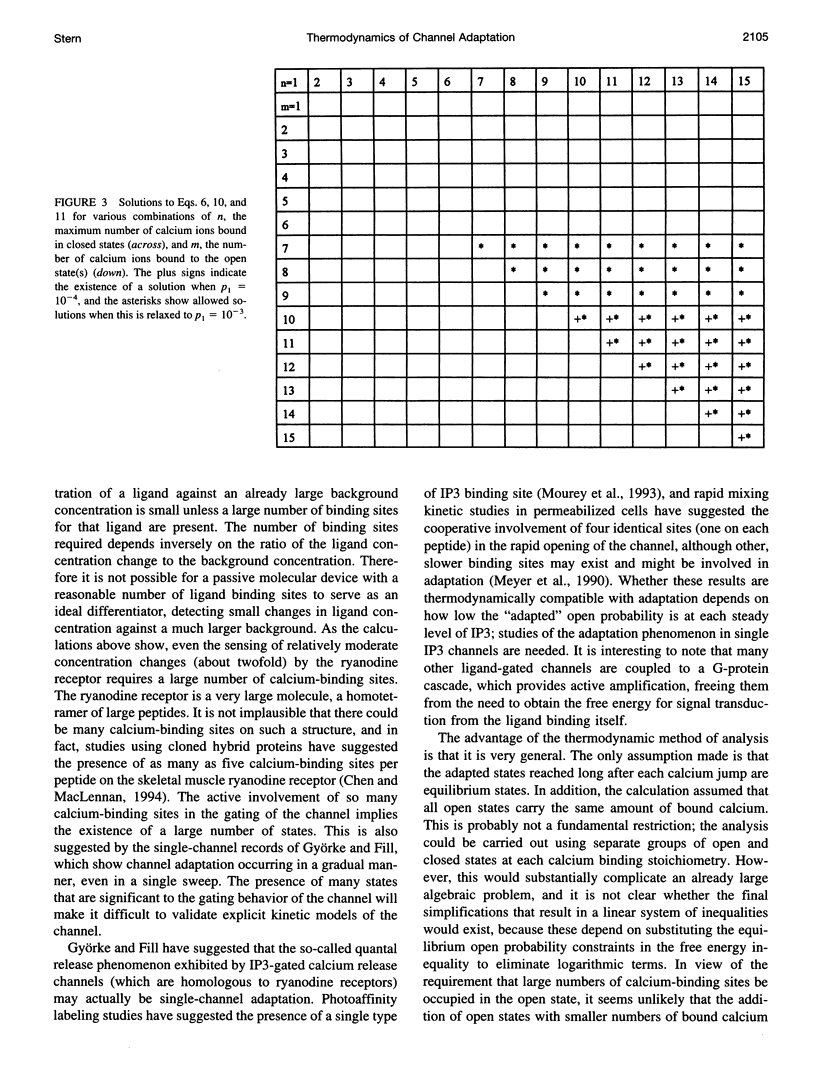
The calcium-induced calcium release channel of the cardiac sarcoplasmic reticulum has been reported to inactivate in a novel manner (termed "adaptation"), which permits reactivation by exposure to successively higher concentrations of calcium. I examined the limitations placed by thermodynamics on the possible kinetic mechanisms for such behavior. The mechanism suggested by Gyorke and Fill, in which the affinity of a calcium-binding site decreases during adaptation, is not thermodynamically feasible for a passive system, but requires an external input of free energy. Possible sources of such energy are 1) metabolic energy, which is excluded by the fact that adaptation was observed in isolated channels in the absence of ATP, or 2) coupling of ion permeation to gating, for which there is currently no evidence. I derived a general limit on the thermodynamic feasibility of a sequence of channel activations and adaptations, irrespective of channel kinetics, from the requirement that the free energy must decrease during the spontaneous evolution of the system from the state existing immediately after a step increase in [Ca2+] to the state of maximum open probability that follows. The opening of the channel must involve an increase in free energy, which must be compensated by the free energy released by the incremental binding of calcium. This requirement leads to a complicated system of inequalities, which was simplified and manipulated algebraically into the form of a linear programming problem. Numerical solution of this problem showed that the sequence of adaptations of the SR channel observed by Gyorke and Fill requires the presence of at least 10 calcium-binding sites on the channel if it is to occur in the absence of exogenous sources of free energy. This indicates either that a large number of calcium-binding sites participate in the regulation of the SR calcium release channel, or that the existing data are significantly flawed with respect to the low open probability in the resting state, the importance of "calcium spike" artifacts from flash photolysis, or both.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen S. R., MacLennan D. H. Identification of calmodulin-, Ca(2+)-, and ruthenium red-binding domains in the Ca2+ release channel (ryanodine receptor) of rabbit skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1994 Sep 9;269(36):22698–22704. [PubMed] [Google Scholar]
- Cheng H., Fill M., Valdivia H., Lederer W. J. Models of Ca2+ release channel adaptation. Science. 1995 Mar 31;267(5206):2009–2010. doi: 10.1126/science.7701326. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke S., Fill M. Ryanodine receptor adaptation: control mechanism of Ca(2+)-induced Ca2+ release in heart. Science. 1993 May 7;260(5109):807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- Lamb G. D., Fryer M. W., Stephenson D. G. Ca(2+)-induced Ca2+ release in response to flash photolysis. Science. 1994 Feb 18;263(5149):986–988. doi: 10.1126/science.8310298. [DOI] [PubMed] [Google Scholar]
- Meyer T., Wensel T., Stryer L. Kinetics of calcium channel opening by inositol 1,4,5-trisphosphate. Biochemistry. 1990 Jan 9;29(1):32–37. doi: 10.1021/bi00453a004. [DOI] [PubMed] [Google Scholar]
- Mourey R. J., Estevez V. A., Marecek J. F., Barrow R. K., Prestwich G. D., Snyder S. H. Inositol 1,4,5-trisphosphate receptors: labeling the inositol 1,4,5-trisphosphate binding site with photoaffinity ligands. Biochemistry. 1993 Feb 23;32(7):1719–1726. doi: 10.1021/bi00058a004. [DOI] [PubMed] [Google Scholar]
- Richard E. A., Miller C. Steady-state coupling of ion-channel conformations to a transmembrane ion gradient. Science. 1990 Mar 9;247(4947):1208–1210. doi: 10.1126/science.2156338. [DOI] [PubMed] [Google Scholar]
- Sachs F., Qin F., Palade P. Models of Ca2+ release channel adaptation. Science. 1995 Mar 31;267(5206):2010–2011. doi: 10.1126/science.7701327. [DOI] [PubMed] [Google Scholar]
- Stern M. D. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992 Aug;63(2):497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Othmer H. G. A model of calcium dynamics in cardiac myocytes based on the kinetics of ryanodine-sensitive calcium channels. Biophys J. 1994 Dec;67(6):2223–2235. doi: 10.1016/S0006-3495(94)80707-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


