Abstract
It is well known that melittin, an amphipathic helical peptide, causes the micellization of phosphatidylcholine vesicles. In the present work, we conclude that the extent of micellization is dependent on the level of unsaturation of the lipid acyl chains. We report the results obtained on two systems: dipalmitoylphosphatidylcholine (DPPC), containing 10(mol)% saturated or unsaturated fatty acid (palmitic, oleic, or linoleic), and DPPC, containing 10(mol)% positively charged diacyloxy-3-(trimethylammonio)propane bearing palmitic or oleic acyl chains. For both systems, the presence of unsaturation in the lipid acyl chains inhibits melittin-induced micellization. Conversely, the addition of saturated palmitic acid to the DPPC matrix enhances the micellization. This modulation is proposed to be associated with the cohesion of the hydrophobic core. When the lipid chain packing of the gel-phase bilayer is already perturbed by the presence of unsaturation, it seems easier for the membrane to accommodate melittin at the interface, and the distribution of the peptide in the bilayer could be the origin of the inhibition of the micellization. The cohesion of the apolar core is shown to play an unquestionable role in melittin-induced micellization; however, this contribution does not appear to be as important as the electrostatic interactions between melittin and positively or negatively charged lipids.
Full text
PDF

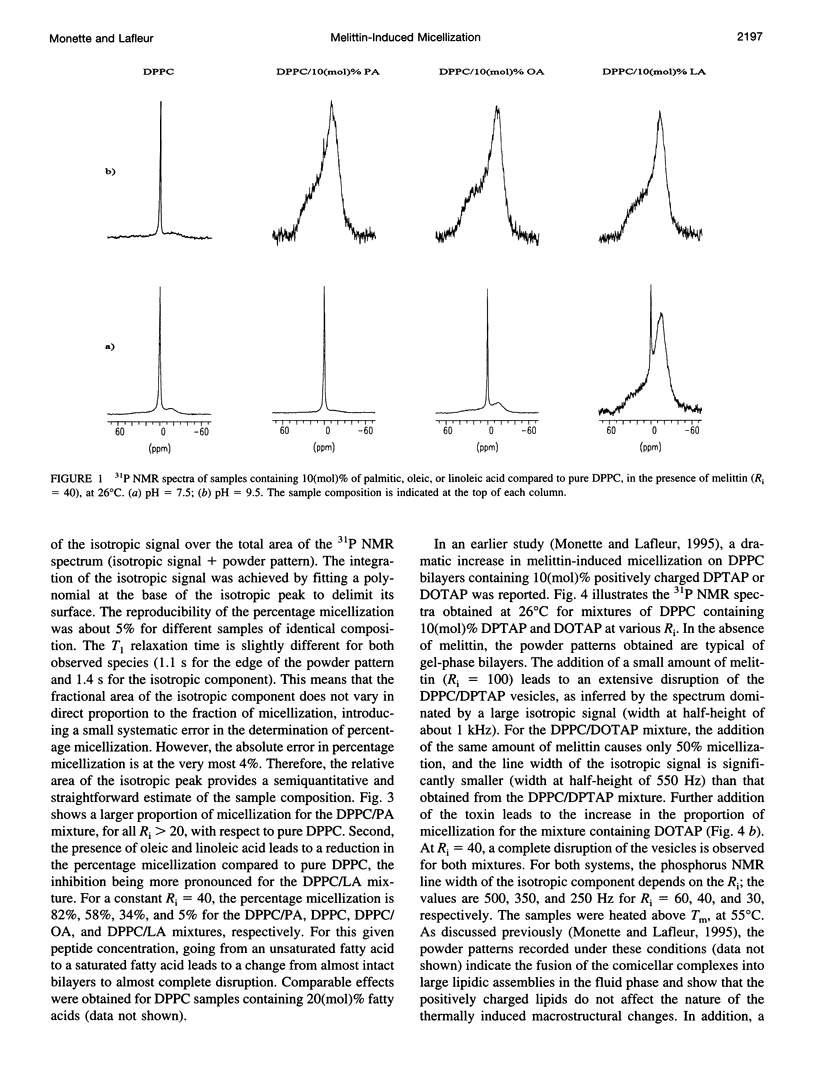
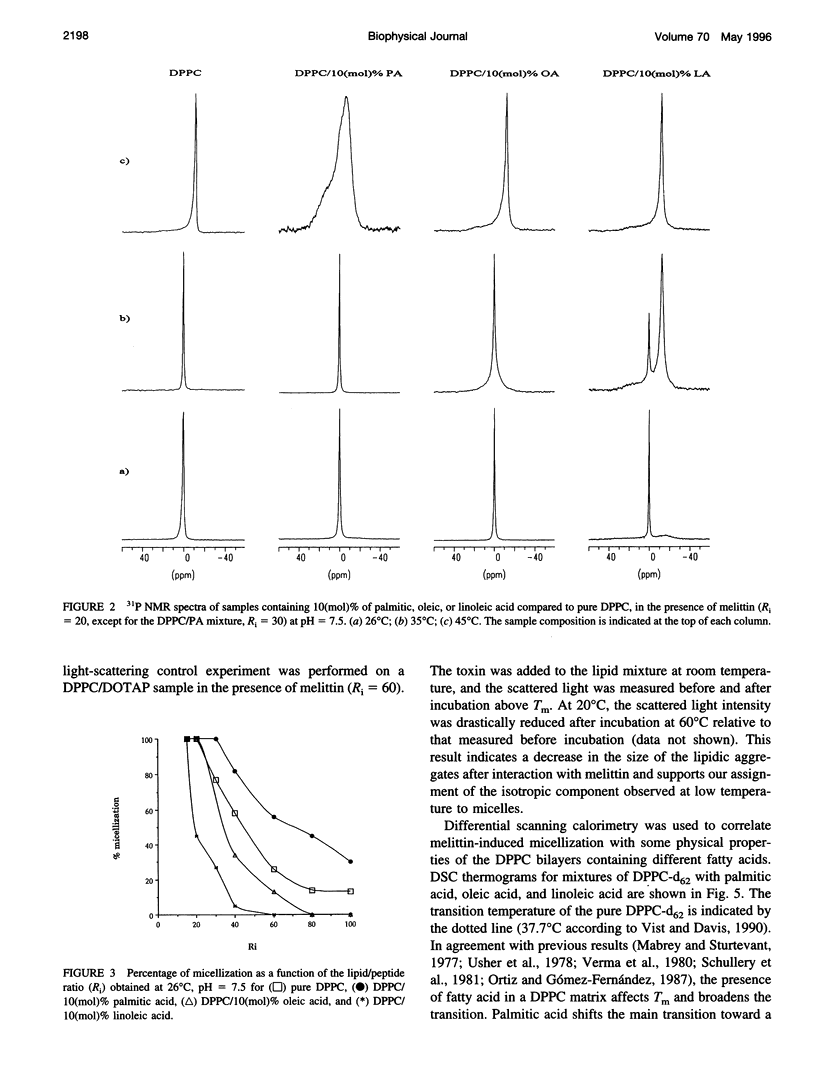
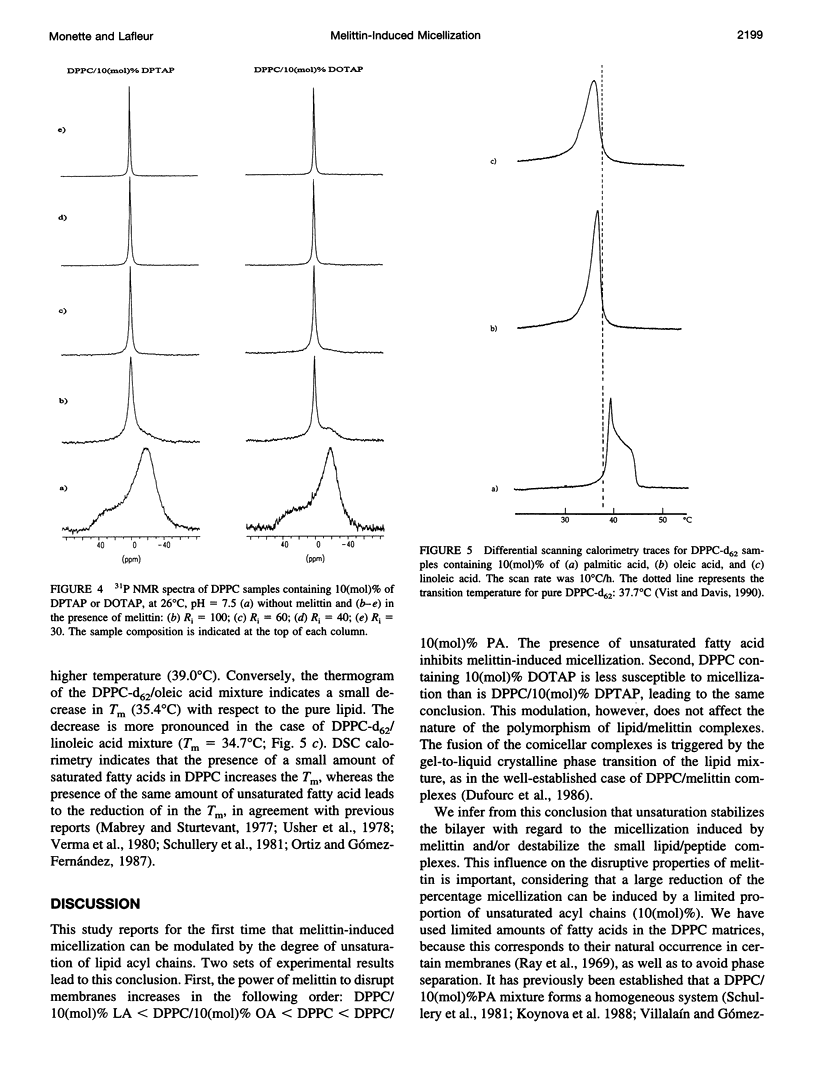



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnell E. E., Cullis P. R., de Kruijff B. Effects of tumbling and lateral diffusion on phosphatidylcholine model membrane 31P-NMR lineshapes. Biochim Biophys Acta. 1980 Dec 2;603(1):63–69. doi: 10.1016/0005-2736(80)90391-0. [DOI] [PubMed] [Google Scholar]
- Cushley R. J., Treleaven W. D., Parmar Y. I., Chana R. S., Fenske D. B. Surface diffusion in human serum lipoproteins. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1139–1145. doi: 10.1016/0006-291x(87)90766-2. [DOI] [PubMed] [Google Scholar]
- Dempsey C. E. The actions of melittin on membranes. Biochim Biophys Acta. 1990 May 7;1031(2):143–161. doi: 10.1016/0304-4157(90)90006-x. [DOI] [PubMed] [Google Scholar]
- Dempsey C. E., Watts A. A deuterium and phosphorus-31 nuclear magnetic resonance study of the interaction of melittin with dimyristoylphosphatidylcholine bilayers and the effects of contaminating phospholipase A2. Biochemistry. 1987 Sep 8;26(18):5803–5811. doi: 10.1021/bi00392a033. [DOI] [PubMed] [Google Scholar]
- Dufourc E. J., Bonmatin J. M., Dufourcq J. Membrane structure and dynamics by 2H- and 31P-NMR. Effects of amphipatic peptidic toxins on phospholipid and biological membranes. Biochimie. 1989 Jan;71(1):117–123. doi: 10.1016/0300-9084(89)90141-7. [DOI] [PubMed] [Google Scholar]
- Dufourcq J., Faucon J. F., Fourche G., Dasseux J. L., Le Maire M., Gulik-Krzywicki T. Morphological changes of phosphatidylcholine bilayers induced by melittin: vesicularization, fusion, discoidal particles. Biochim Biophys Acta. 1986 Jul 10;859(1):33–48. doi: 10.1016/0005-2736(86)90315-9. [DOI] [PubMed] [Google Scholar]
- Faucon J. F., Bonmatin J. M., Dufourcq J., Dufourc E. J. Acyl chain length dependence in the stability of melittin-phosphatidylcholine complexes. A light scattering and 31P-NMR study. Biochim Biophys Acta. 1995 Mar 22;1234(2):235–243. doi: 10.1016/0005-2736(94)00298-4. [DOI] [PubMed] [Google Scholar]
- Habermann E. Bee and wasp venoms. Science. 1972 Jul 28;177(4046):314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- Koynova R. D., Tenchov B. G., Quinn P. J., Laggner P. Structure and phase behavior of hydrated mixtures of L-dipalmitoylphosphatidylcholine and palmitic acid. Correlations between structural rearrangements, specific volume changes and endothermic events. Chem Phys Lipids. 1988 Oct;48(3-4):205–214. doi: 10.1016/0009-3084(88)90091-6. [DOI] [PubMed] [Google Scholar]
- Lafleur M., Dasseux J. L., Pigeon M., Dufourcq J., Pézolet M. Study of the effect of melittin on the thermotropism of dipalmitoylphosphatidylcholine by Raman spectroscopy. Biochemistry. 1987 Feb 24;26(4):1173–1179. doi: 10.1021/bi00378a027. [DOI] [PubMed] [Google Scholar]
- Lafleur M., Samson I., Pézolet M. Investigation of the interaction between melittin and dipalmitoylphosphatidylglycerol bilayers by vibrational spectroscopy. Chem Phys Lipids. 1991 Oct;59(3):233–244. doi: 10.1016/0009-3084(91)90023-5. [DOI] [PubMed] [Google Scholar]
- Lauterwein J., Bösch C., Brown L. R., Wüthrich K. Physicochemical studies of the protein-lipid interactions in melittin-containing micelles. Biochim Biophys Acta. 1979 Sep 21;556(2):244–264. doi: 10.1016/0005-2736(79)90046-4. [DOI] [PubMed] [Google Scholar]
- Mabrey S., Sturtevant J. M. Incorporation of saturated fatty acids into phosphatidylcholine bilayers. Biochim Biophys Acta. 1977 Mar 25;486(3):444–450. doi: 10.1016/0005-2760(77)90094-7. [DOI] [PubMed] [Google Scholar]
- Monette M., Lafleur M. Modulation of melittin-induced lysis by surface charge density of membranes. Biophys J. 1995 Jan;68(1):187–195. doi: 10.1016/S0006-3495(95)80174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monette M., Van Calsteren M. R., Lafleur M. Effect of cholesterol on the polymorphism of dipalmitoylphosphatidylcholine/melittin complexes: an NMR study. Biochim Biophys Acta. 1993 Jul 4;1149(2):319–328. doi: 10.1016/0005-2736(93)90217-n. [DOI] [PubMed] [Google Scholar]
- Ortiz A., Gómez-Fernández J. C. A differential scanning calorimetry study of the interaction of free fatty acids with phospholipid membranes. Chem Phys Lipids. 1987 Oct;45(1):75–91. doi: 10.1016/0009-3084(87)90041-7. [DOI] [PubMed] [Google Scholar]
- Pott T., Dufourc E. J. Action of melittin on the DPPC-cholesterol liquid-ordered phase: a solid state 2H-and 31P-NMR study. Biophys J. 1995 Mar;68(3):965–977. doi: 10.1016/S0006-3495(95)80272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. K., Skipski V. P., Barclay M., Essner E., Archibald F. M. Lipid composition of rat liver plasma membranes. J Biol Chem. 1969 Oct 25;244(20):5528–5536. [PubMed] [Google Scholar]
- Schullery S. E., Seder T. A., Weinstein D. A., Bryant D. A. Differential thermal analysis of dipalmitoylphosphatidylcholine--fatty acid mixtures. Biochemistry. 1981 Nov 24;20(24):6818–6824. doi: 10.1021/bi00527a012. [DOI] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Stamatatos L., Leventis R., Zuckermann M. J., Silvius J. R. Interactions of cationic lipid vesicles with negatively charged phospholipid vesicles and biological membranes. Biochemistry. 1988 May 31;27(11):3917–3925. doi: 10.1021/bi00411a005. [DOI] [PubMed] [Google Scholar]
- Subbarao N. K., MacDonald R. C. Lipid unsaturation influences melittin-induced leakage of vesicles. Biochim Biophys Acta. 1994 Jan 3;1189(1):101–107. doi: 10.1016/0005-2736(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Usher J. R., Epand R. M., Papahadjopoulos D. The effect of free fatty acids on the thermotropic phase transition of dimyristoyl glycerophosphocholine. Chem Phys Lipids. 1978 Oct;22(3):245–253. doi: 10.1016/0009-3084(78)90031-2. [DOI] [PubMed] [Google Scholar]
- Verma S. P., Wallach D. F., Sakura J. D. Raman analysis of the thermotropic behavior of lecithin-fatty acid systems and of their interaction with proteolipid apoprotein. Biochemistry. 1980 Feb 5;19(3):574–579. doi: 10.1021/bi00544a028. [DOI] [PubMed] [Google Scholar]
- Vist M. R., Davis J. H. Phase equilibria of cholesterol/dipalmitoylphosphatidylcholine mixtures: 2H nuclear magnetic resonance and differential scanning calorimetry. Biochemistry. 1990 Jan 16;29(2):451–464. doi: 10.1021/bi00454a021. [DOI] [PubMed] [Google Scholar]
- Vogel H., Jähnig F. The structure of melittin in membranes. Biophys J. 1986 Oct;50(4):573–582. doi: 10.1016/S0006-3495(86)83497-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


