Abstract
The damaging effects of synchrotron-derived x rays on aqueous phospholipid dispersions have been evaluated. The effect of degree of lipid hydration, phospholipid chemical structure, mesophase identity, aqueous medium composition, and incident flux on the severity and progress of damage was quantified using time-resolved x-ray diffraction and chromatographic analysis of damage products. Electron spin resonance measurements of spin-trapped intermediates generated during irradiation suggest a free radical-mediated process. Surprisingly, radiation damage effects revealed by x-ray diffraction were imperceptible when the lamellar phases were prepared under water-stressed conditions, despite the fact that x-ray-induced chemical breakdown of the lipid occurred regardless of hydration level. Of the fully hydrated lipid systems studied, saturated diacyl-phosphatidylcholines were most sensitive to radiation damage compared to the ester- and ether-linked phosphatidylethanolamines and the ether-linked phosphatidylcholines. The inclusion of buffers or inorganic salts in the dispersing medium had only a minor effect in reducing damage development. A small inverse dose-rate effect was found when the x-ray beam intensity was changed 15-fold. These results contribute to our understanding of the mechanism of radiation damage, to our appreciation of the importance of monitoring both structure and composition when evaluating biomaterials radiation sensitivity, and to the development of strategies for eliminating or reducing the severity of damage due to an increasingly important source of x rays, synchrotron radiation. Because damage is shown to be free radical mediated, these results have an important bearing on age-related accumulation of free radicals in cells and how these might compromise membrane integrity, culminating in cell death.
Full text
PDF


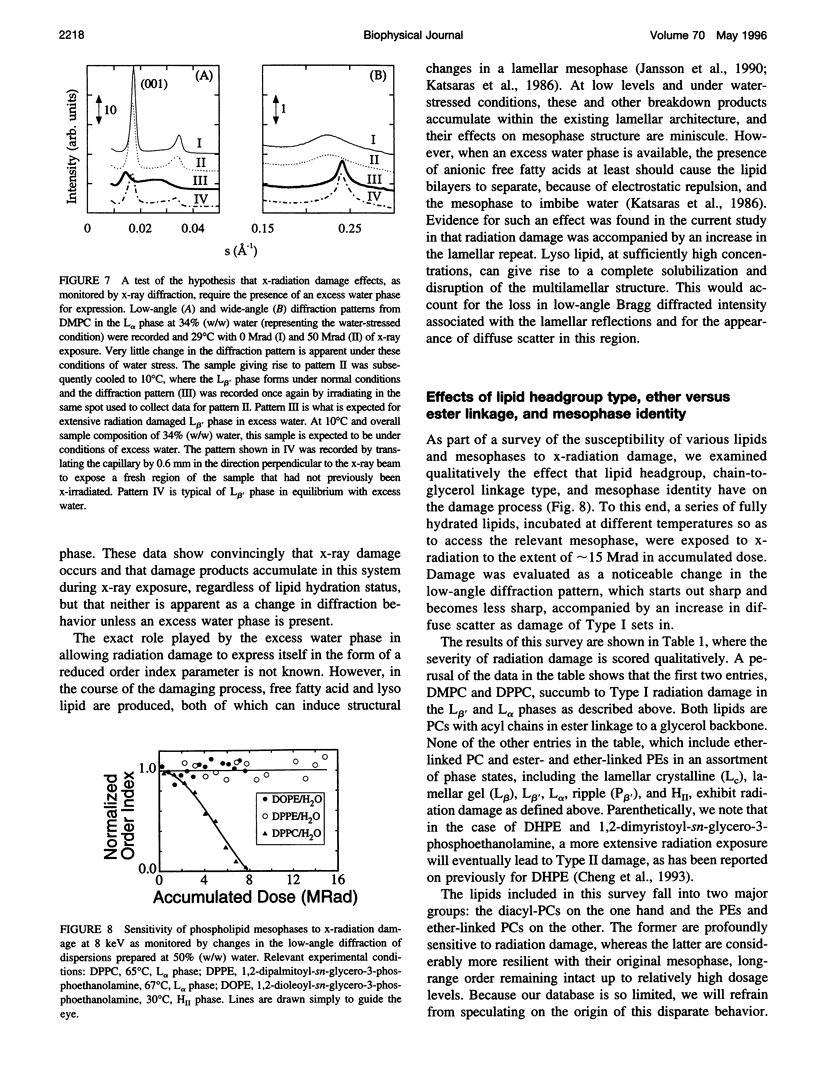
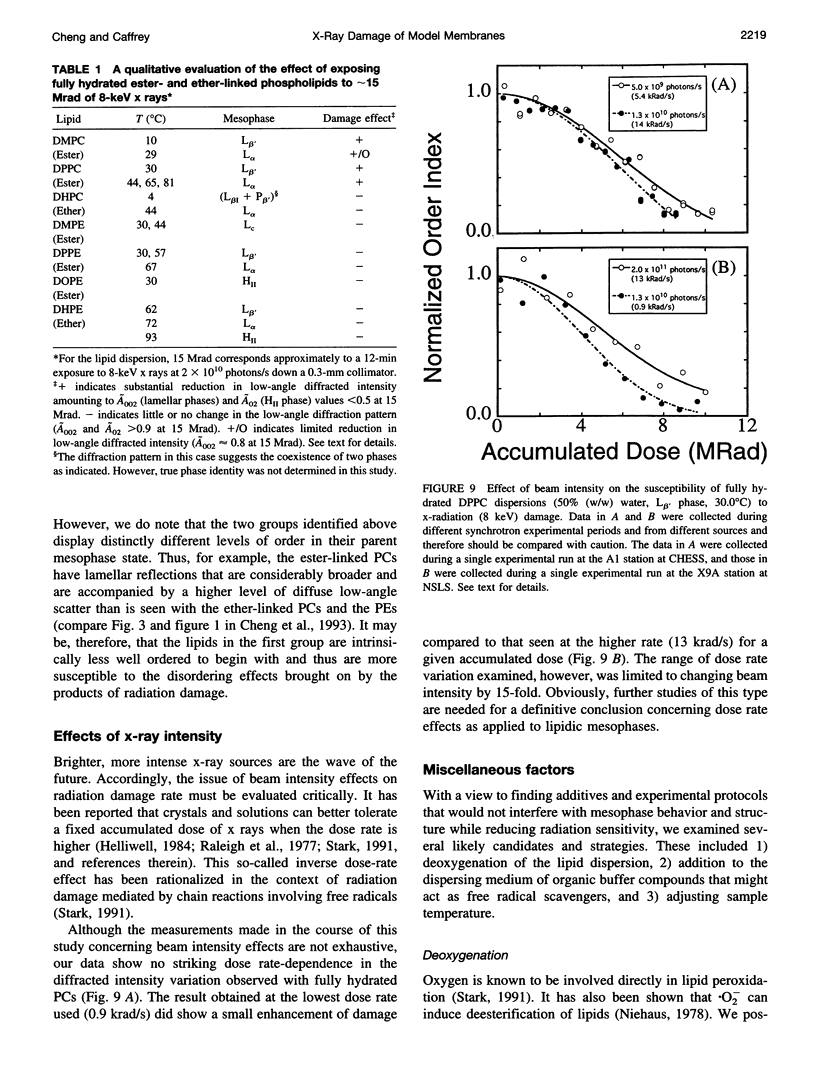
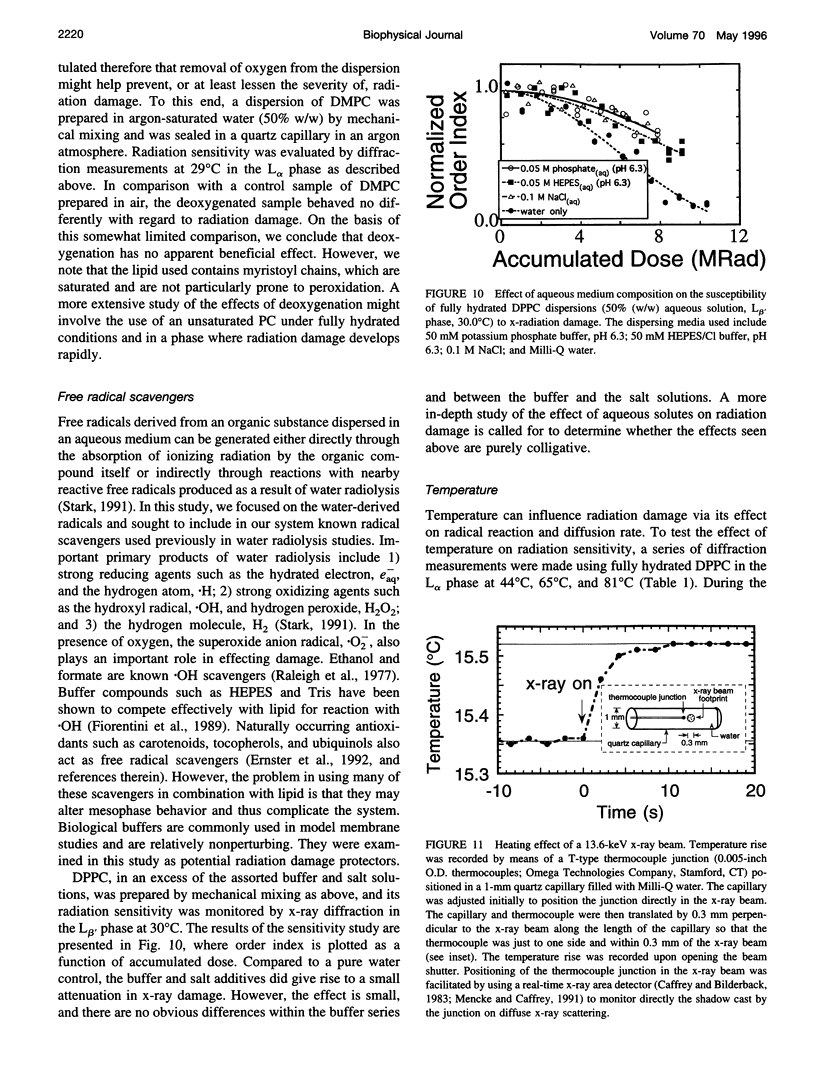


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaurock A. E. Evidence of bilayer structure and of membrane interactions from X-ray diffraction analysis. Biochim Biophys Acta. 1982 May 12;650(4):167–207. doi: 10.1016/0304-4157(82)90016-8. [DOI] [PubMed] [Google Scholar]
- Briggs J., Caffrey M. The temperature-composition phase diagram of monomyristolein in water: equilibrium and metastability aspects. Biophys J. 1994 Mar;66(3 Pt 1):573–587. doi: 10.1016/s0006-3495(94)80847-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey M., Bilderback D. H. Kinetics of the main phase transition of hydrated lecithin monitored by real-time X-ray diffraction. Biophys J. 1984 Mar;45(3):627–631. doi: 10.1016/S0006-3495(84)84201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey M., Feigenson G. W. Fluorescence quenching in model membranes. 3. Relationship between calcium adenosinetriphosphatase enzyme activity and the affinity of the protein for phosphatidylcholines with different acyl chain characteristics. Biochemistry. 1981 Mar 31;20(7):1949–1961. doi: 10.1021/bi00510a034. [DOI] [PubMed] [Google Scholar]
- Caffrey M. Kinetics and mechanism of the lamellar gel/lamellar liquid-crystal and lamellar/inverted hexagonal phase transition in phosphatidylethanolamine: a real-time X-ray diffraction study using synchrotron radiation. Biochemistry. 1985 Aug 27;24(18):4826–4844. doi: 10.1021/bi00339a017. [DOI] [PubMed] [Google Scholar]
- Cheng A. C., Hogan J. L., Caffrey M. X-rays destroy the lamellar structure of model membranes. J Mol Biol. 1993 Jan 20;229(2):291–294. doi: 10.1006/jmbi.1993.1034. [DOI] [PubMed] [Google Scholar]
- Cheng A., Hummel B., Mencke A., Caffrey M. Kinetics and mechanism of the barotropic lamellar gel/lamellar liquid crystal phase transition in fully hydrated dihexadecylphosphatidylethanolamine: a time-resolved x-ray diffraction study using pressure jump. Biophys J. 1994 Jul;67(1):293–303. doi: 10.1016/S0006-3495(94)80480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Ernster L., Forsmark P., Nordenbrand K. The mode of action of lipid-soluble antioxidants in biological membranes: relationship between the effects of ubiquinol and vitamin E as inhibitors of lipid peroxidation in submitochondrial particles. Biofactors. 1992 Apr;3(4):241–248. [PubMed] [Google Scholar]
- Fiorentini D., Landi L., Barzanti V., Cabrini L. Buffers can modulate the effect of sonication on egg lecithin liposomes. Free Radic Res Commun. 1989;6(4):243–250. doi: 10.3109/10715768909073477. [DOI] [PubMed] [Google Scholar]
- Hui S. W. Radiation damage of phosphatidylcholine bilayers effects of temperature and hydration. Ultramicroscopy. 1980;5(4):505–512. doi: 10.1016/s0304-3991(80)80007-6. [DOI] [PubMed] [Google Scholar]
- Janiak M. J., Small D. M., Shipley G. G. Nature of the Thermal pretransition of synthetic phospholipids: dimyristolyl- and dipalmitoyllecithin. Biochemistry. 1976 Oct 19;15(21):4575–4580. doi: 10.1021/bi00666a005. [DOI] [PubMed] [Google Scholar]
- Janiak M. J., Small D. M., Shipley G. G. Temperature and compositional dependence of the structure of hydrated dimyristoyl lecithin. J Biol Chem. 1979 Jul 10;254(13):6068–6078. [PubMed] [Google Scholar]
- Jansson M., Thurmond R. L., Trouard T. P., Brown M. F. Magnetic alignment and orientational order of dipalmitoylphosphatidylcholine bilayers containing palmitoyllysophosphatidylcholine. Chem Phys Lipids. 1990 Jun;54(3-4):157–170. doi: 10.1016/0009-3084(90)90009-g. [DOI] [PubMed] [Google Scholar]
- Mencke A. P., Caffrey M. Kinetics and mechanism of the pressure-induced lamellar order/disorder transition in phosphatidylethanolamine: a time-resolved X-ray diffraction study. Biochemistry. 1991 Mar 5;30(9):2453–2463. doi: 10.1021/bi00223a023. [DOI] [PubMed] [Google Scholar]
- Raleigh J. A., Kremers W., Gaboury B. Dose-rate and oxygen effects in models of lipid membranes: linoleic acid. Int J Radiat Biol Relat Stud Phys Chem Med. 1977 Mar;31(3):203–213. doi: 10.1080/09553007714550251. [DOI] [PubMed] [Google Scholar]
- Stark G. The effect of ionizing radiation on lipid membranes. Biochim Biophys Acta. 1991 Jul 22;1071(2):103–122. doi: 10.1016/0304-4157(91)90020-w. [DOI] [PubMed] [Google Scholar]
- Swartz H. M., Swartz S. M. Biochemical and biophysical applications of electron spin resonance. Methods Biochem Anal. 1983;29:207–323. doi: 10.1002/9780470110492.ch5. [DOI] [PubMed] [Google Scholar]
- Wiener M. C., White S. H. Fluid bilayer structure determination by the combined use of x-ray and neutron diffraction. I. Fluid bilayer models and the limits of resolution. Biophys J. 1991 Jan;59(1):162–173. doi: 10.1016/S0006-3495(91)82208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]