Abstract
We have developed a photolytic method to determine the concentration of reactive hemes in a solution in the presence of a trace amount of CO. By measurement of the bimolecular rate of CO binding, and by calibration of the rate constant under equivalent conditions, the concentration of the reactive hemes can be determined. In a solution of sickle hemoglobin, the molecules in the gel contribute negligibly to the recombination rate, allowing the concentration of the molecules in the solution phase to be determined. To optimize signal to noise, modulated excitation methods were employed, although the method could also be used with pulse techniques and suitable signal averaging. Because the optical method employs a microspectrophotometer, only a few microliters of concentrated Hb solution is required to reproduce the entire temperature dependence of the solubility previously determined by centrifugation using milliliter quantities of solutions of the same concentration. This should be especially useful for studies of site-directed mutants, and we present results obtained on one such HbS in which Leu 88 beta has been replaced by Ala. The free energy difference in the polymerization of the Leu 88 beta double mutant is consistent with known differences in the amino acid hydrophobicities. The calibration required for these experiments also provides an excellent determination of the activation energy for binding the first CO to deoxy Hb.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi K., Asakura T. Nucleation-controlled aggregation of deoxyhemoglobin S. Possible difference in the size of nuclei in different phosphate concentrations. J Biol Chem. 1979 Aug 25;254(16):7765–7771. [PubMed] [Google Scholar]
- Benesch R. E., Edalji R., Kwong S., Benesch R. Oxygen affinity as an index of hemoglobin S polymerization: a new micromethod. Anal Biochem. 1978 Aug 15;89(1):162–173. doi: 10.1016/0003-2697(78)90737-6. [DOI] [PubMed] [Google Scholar]
- Benesch R. E., Kwong S., Benesch R. The effects of alpha chain mutations cis and trans to the beta6 mutation on the polymerization of sickle cell haemoglobin. Nature. 1982 Sep 16;299(5880):231–234. doi: 10.1038/299231a0. [DOI] [PubMed] [Google Scholar]
- Bookchin R. M., Nagel R. L. Ligand-induced conformational dependence of hemoglobin in sickling interactios. J Mol Biol. 1971 Sep 14;60(2):263–270. doi: 10.1016/0022-2836(71)90292-0. [DOI] [PubMed] [Google Scholar]
- Briehl R. W. Nucleation, fiber growth and melting, and domain formation and structure in sickle cell hemoglobin gels. J Mol Biol. 1995 Feb 3;245(5):710–723. doi: 10.1006/jmbi.1994.0057. [DOI] [PubMed] [Google Scholar]
- Carragher B., Bluemke D. A., Gabriel B., Potel M. J., Josephs R. Structural analysis of polymers of sickle cell hemoglobin. I. Sickle hemoglobin fibers. J Mol Biol. 1988 Jan 20;199(2):315–331. doi: 10.1016/0022-2836(88)90316-6. [DOI] [PubMed] [Google Scholar]
- Cho M. R., Ferrone F. A. Monomer diffusion and polymer alignment in domains of sickle hemoglobin. Biophys J. 1992 Jul;63(1):205–214. doi: 10.1016/S0006-3495(92)81595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cretegny I., Edelstein S. J. Double strand packing in hemoglobin S fibers. J Mol Biol. 1993 Apr 5;230(3):733–738. doi: 10.1006/jmbi.1993.1195. [DOI] [PubMed] [Google Scholar]
- Dykes G. W., Crepeau R. H., Edelstein S. J. Three-dimensional reconstruction of the 14-filament fibers of hemoglobin S. J Mol Biol. 1979 Jun 5;130(4):451–472. doi: 10.1016/0022-2836(79)90434-0. [DOI] [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J. Sickle cell hemoglobin polymerization. Adv Protein Chem. 1990;40:63–279. doi: 10.1016/s0065-3233(08)60287-9. [DOI] [PubMed] [Google Scholar]
- Ferrone F. A., Hofrichter J., Eaton W. A. Kinetics of sickle hemoglobin polymerization. I. Studies using temperature-jump and laser photolysis techniques. J Mol Biol. 1985 Jun 25;183(4):591–610. doi: 10.1016/0022-2836(85)90174-3. [DOI] [PubMed] [Google Scholar]
- Ferrone F. A., Hofrichter J., Eaton W. A. Kinetics of sickle hemoglobin polymerization. II. A double nucleation mechanism. J Mol Biol. 1985 Jun 25;183(4):611–631. doi: 10.1016/0022-2836(85)90175-5. [DOI] [PubMed] [Google Scholar]
- Ferrone F. A. Modulated excitation spectroscopy in hemoglobin. Methods Enzymol. 1994;232:292–321. doi: 10.1016/0076-6879(94)32053-5. [DOI] [PubMed] [Google Scholar]
- Gros G., Rollema H. S., Jelkmann W., Gros H., Bauer C., Moll W. Net charge and oxygen affinity of human hemoglobin are independent of hemoglobin concentration. J Gen Physiol. 1978 Dec;72(6):765–773. doi: 10.1085/jgp.72.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J. Ligand binding and the gelation of sickle cell hemoglobin. J Mol Biol. 1979 Mar 5;128(3):335–369. doi: 10.1016/0022-2836(79)90092-5. [DOI] [PubMed] [Google Scholar]
- Hofrichter J., Ross P. D., Eaton W. A. Supersaturation in sickle cell hemoglobin solutions. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3035–3039. doi: 10.1073/pnas.73.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D., Jiang J., Zhao M., Ferrone F. A. Modulated excitation of singly ligated carboxyhemoglobin. Biophys J. 1993 Nov;65(5):2059–2067. doi: 10.1016/S0006-3495(93)81268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin de Llano J. J., Manning J. M. Properties of a recombinant human hemoglobin double mutant: sickle hemoglobin with Leu-88(beta) at the primary aggregation site substituted by Ala. Protein Sci. 1994 Aug;3(8):1206–1212. doi: 10.1002/pro.5560030806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin de Llano J. J., Schneewind O., Stetler G., Manning J. M. Recombinant human sickle hemoglobin expressed in yeast. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):918–922. doi: 10.1073/pnas.90.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki Y., Tanford C. The solubility of amino acids and two glycine peptides in aqueous ethanol and dioxane solutions. Establishment of a hydrophobicity scale. J Biol Chem. 1971 Apr 10;246(7):2211–2217. [PubMed] [Google Scholar]
- Padlan E. A., Love W. E. Refined crystal structure of deoxyhemoglobin S. II. Molecular interactions in the crystal. J Biol Chem. 1985 Jul 15;260(14):8280–8291. [PubMed] [Google Scholar]
- Ross P. D., Hofrichter J., Eaton W. A. Thermodynamics of gelation of sickle cell deoxyhemoglobin. J Mol Biol. 1977 Sep 15;115(2):111–134. doi: 10.1016/0022-2836(77)90093-6. [DOI] [PubMed] [Google Scholar]
- Shapiro D. B., Esquerra R. M., Goldbeck R. A., Ballas S. K., Mohandas N., Kliger D. S. Carbon monoxide religation kinetics to hemoglobin S polymers following ligand photolysis. J Biol Chem. 1995 Nov 3;270(44):26078–26085. doi: 10.1074/jbc.270.44.26078. [DOI] [PubMed] [Google Scholar]
- Sunshine H. R., Hofrichter J., Ferrone F. A., Eaton W. A. Oxygen binding by sickle cell hemoglobin polymers. J Mol Biol. 1982 Jun 25;158(2):251–273. doi: 10.1016/0022-2836(82)90432-6. [DOI] [PubMed] [Google Scholar]
- Watowich S. J., Gross L. J., Josephs R. Analysis of the intermolecular contacts within sickle hemoglobin fibers: effect of site-specific substitutions, fiber pitch, and double-strand disorder. J Struct Biol. 1993 Nov-Dec;111(3):161–179. doi: 10.1006/jsbi.1993.1047. [DOI] [PubMed] [Google Scholar]
- Watowich S. J., Gross L. J., Josephs R. Intermolecular contacts within sickle hemoglobin fibers. J Mol Biol. 1989 Oct 20;209(4):821–828. doi: 10.1016/0022-2836(89)90610-4. [DOI] [PubMed] [Google Scholar]
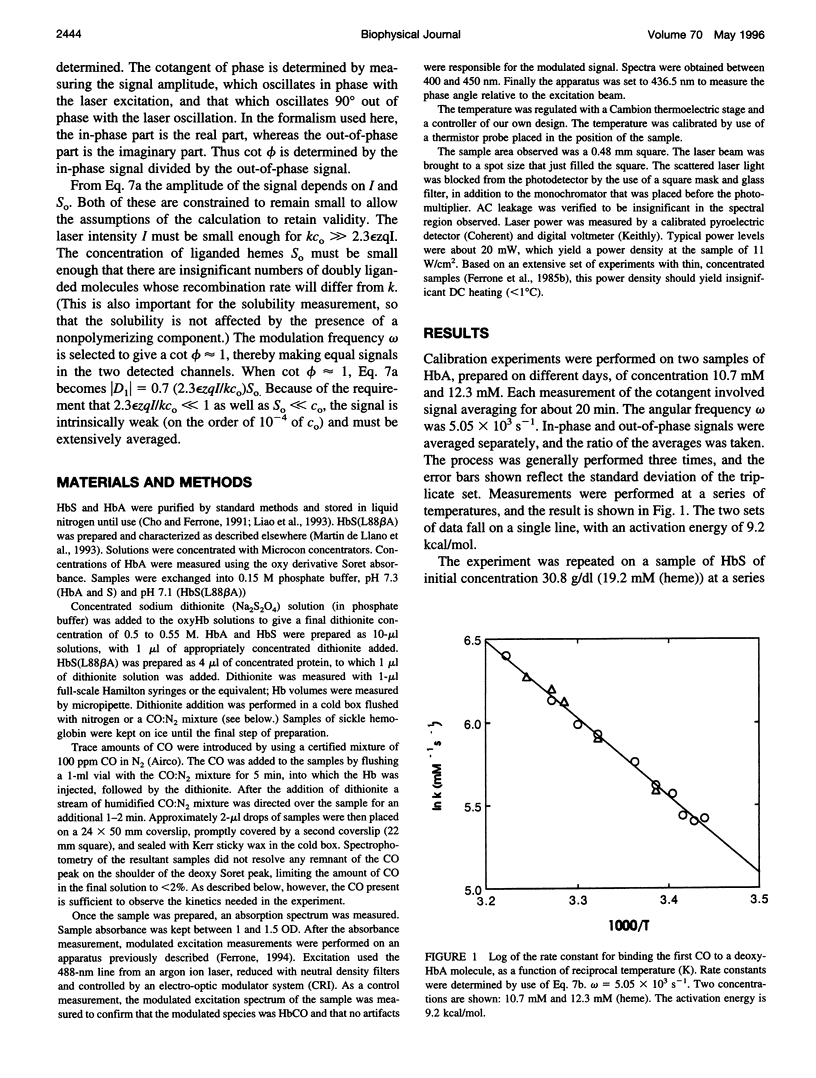
- Wishner B. C., Ward K. B., Lattman E. E., Love W. E. Crystal structure of sickle-cell deoxyhemoglobin at 5 A resolution. J Mol Biol. 1975 Oct 15;98(1):179–194. doi: 10.1016/s0022-2836(75)80108-2. [DOI] [PubMed] [Google Scholar]


