Abstract
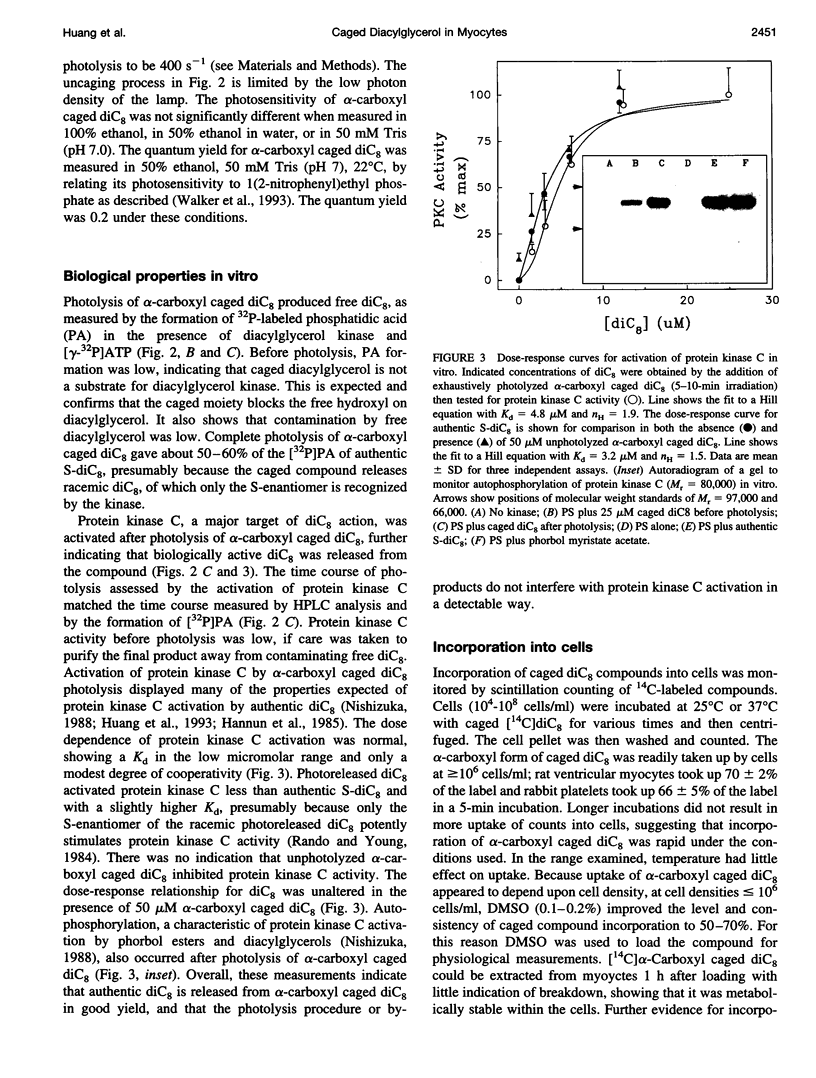
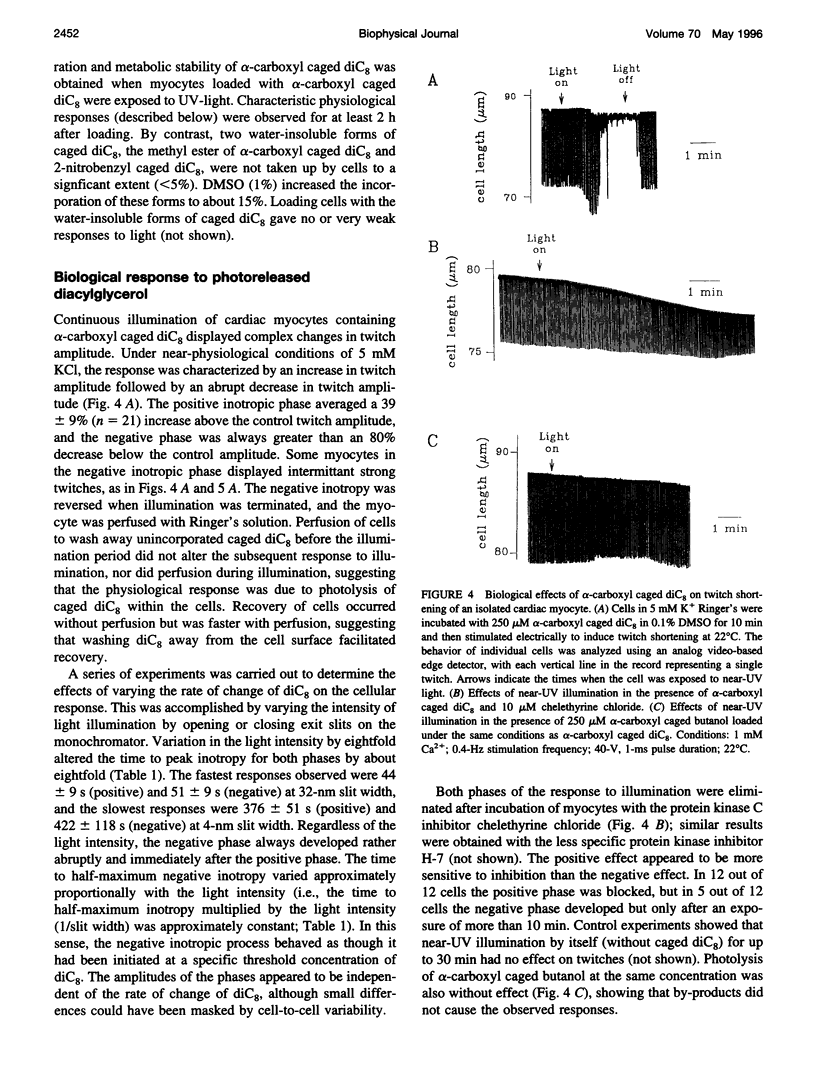
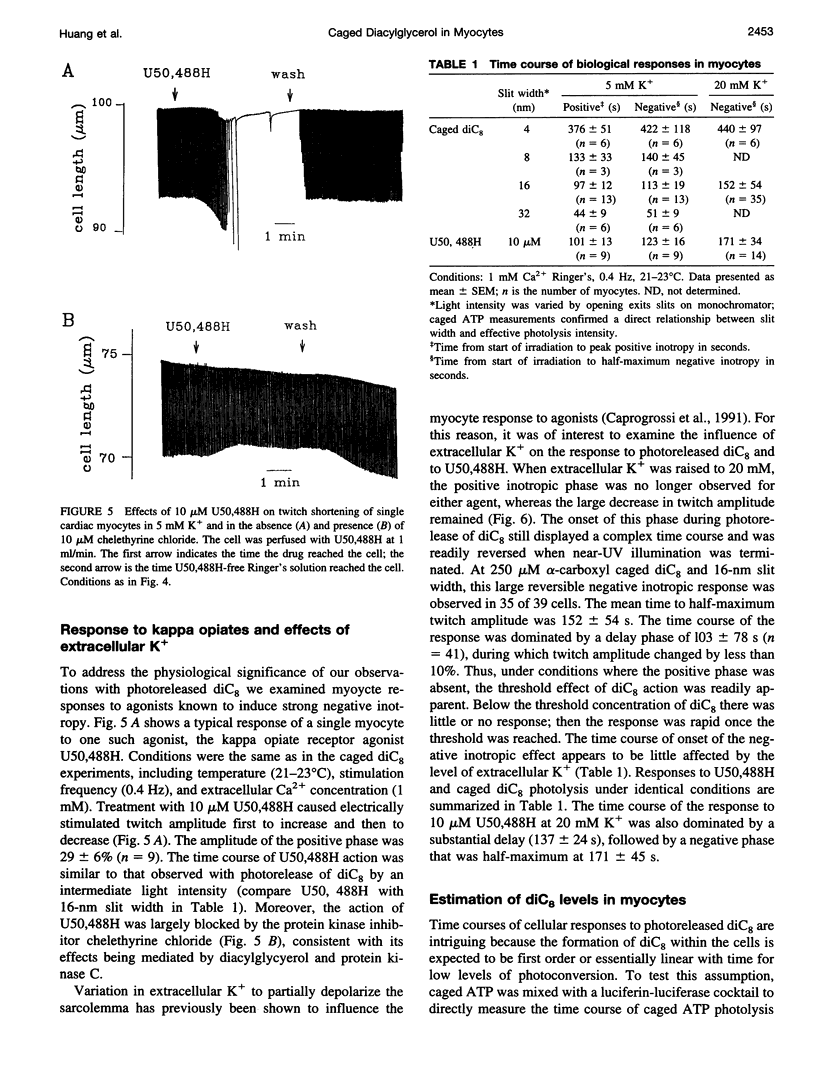
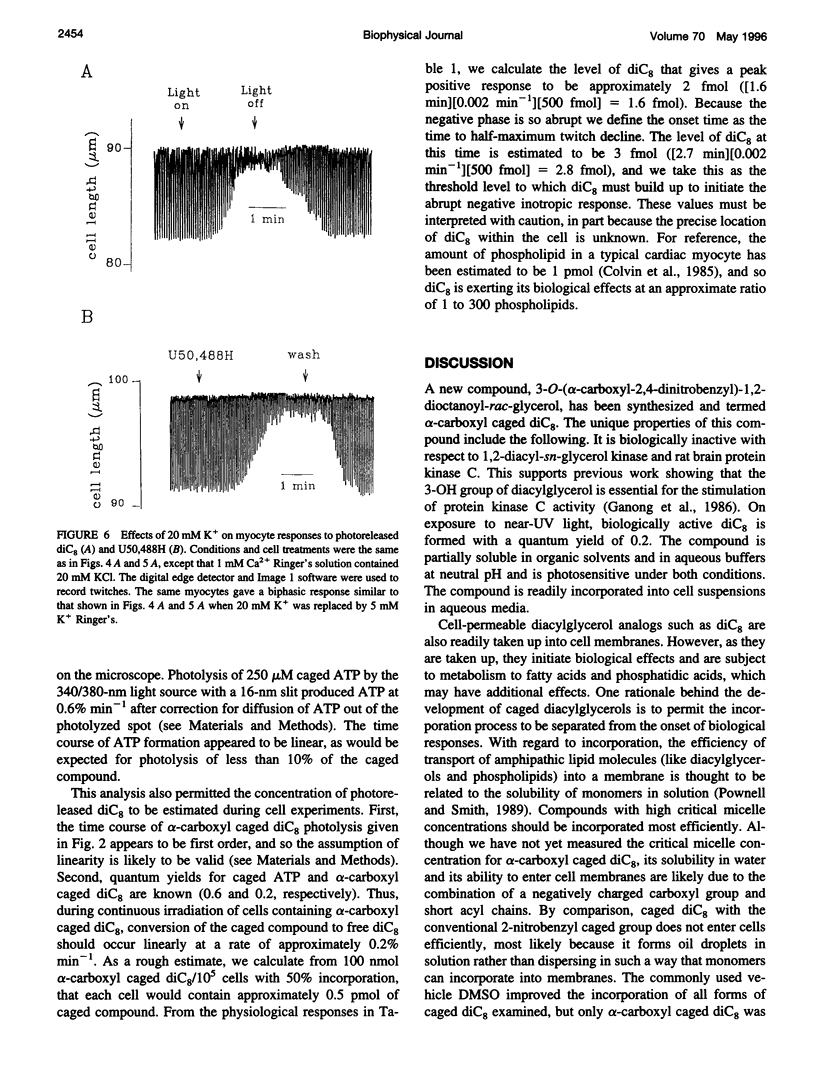
To test the responsiveness of living cells to the intracellular messenger diacylglycerol, we developed a prototype caged diacylglycerol compound, 3-O-(alpha-carboxyl-2,4-dinitrobenzyl)-1 ,2-dioctanoyl-rac-glycerol (designated alpha-carboxyl caged diC(8)), that produces dioctanoylglycerol (diC(8)) on photolysis. Alpha-Carboxyl caged diC(8) is biologically inert toward diacylglycerol kinase and protein kinase C in vitro and is readily incorporated into cardiac myocyte membranes, where it has no effect before irradiation. Exposure to near-UV light releases biologically active diC8 in good yield (quantum efficiency = 0.2). Here we examine a cellular response to controlled elevation of diC8 within single cardiac myocytes. Twitch amplitude was monitored in electrically stimulated myocytes, and a ramp increase in the concentration of diC(8) was generated by continuous irradiation of cells loaded with the caged compound. The myocyte response was biphasic with a positive inotropic phase (39% increase in twitch amplitude), followed by a large negative inotropic phase (>80% decrease). The time to peak inotropy for both phases depended on the light intensity, decreasing from 376 +/- 51 S to 44 +/- 5 s (positive phase) and 422 +/- 118 S to 51 +/- 9 S (negative phase) as the light intensity was increased eightfold. Both phases were inhibited by the protein kinase C inhibitor chelethyrine chloride. An increase in extracellular K+ from 5 mM to 20 mM to partially depolarize the cell membrane eliminated the positive inotropic phase, but the negative inotropic response was largely unaltered. The results reveal new features in the response of cardiac muscle to diacylglycerol, including a positive inotropic phase and a complex responsiveness to a simple linear increase in diacylglycerol. The effects of photoreleased diC(8) were similar to the effects of opiate agonists selective for kappa receptors, consistent with a major role for diacylglycerol in these responses.
Full text
PDF

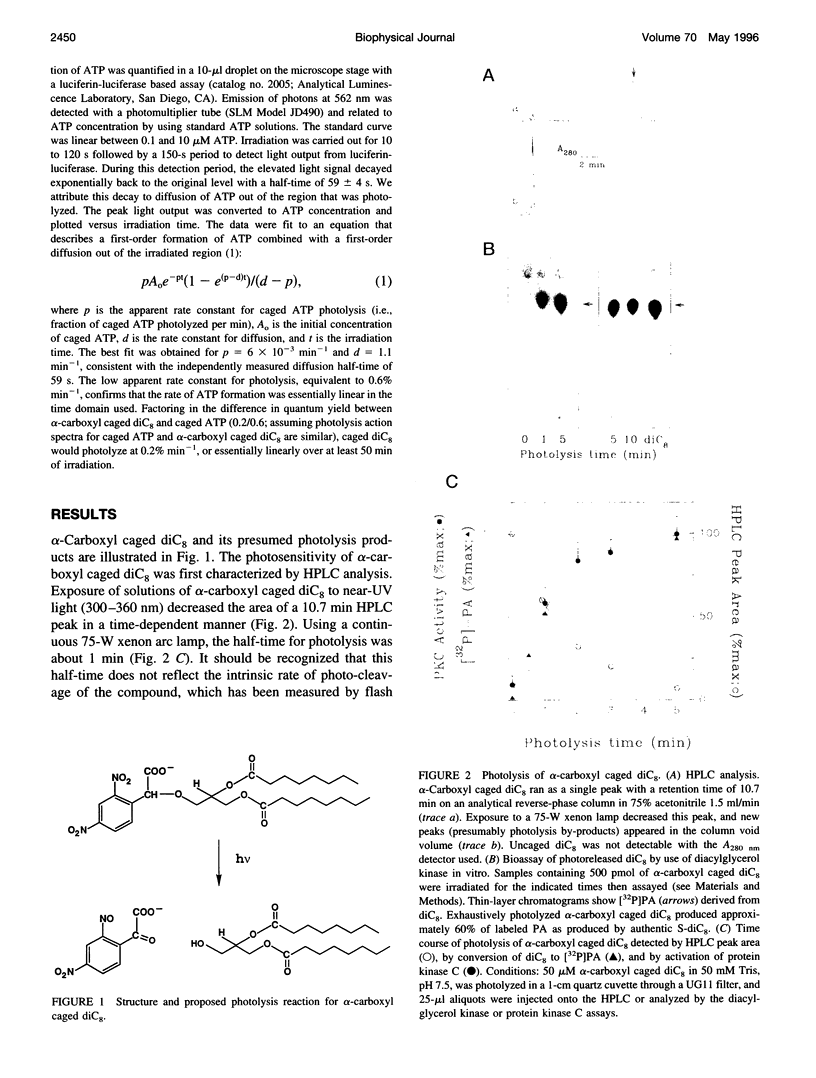



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apkon M., Nerbonne J. M. Alpha 1-adrenergic agonists selectively suppress voltage-dependent K+ current in rat ventricular myocytes. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8756–8760. doi: 10.1073/pnas.85.22.8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Capogrossi M. C., Kachadorian W. A., Gambassi G., Spurgeon H. A., Lakatta E. G. Ca2+ dependence of alpha-adrenergic effects on the contractile properties and Ca2+ homeostasis of cardiac myocytes. Circ Res. 1991 Aug;69(2):540–550. doi: 10.1161/01.res.69.2.540. [DOI] [PubMed] [Google Scholar]
- Capogrossi M. C., Kaku T., Filburn C. R., Pelto D. J., Hansford R. G., Spurgeon H. A., Lakatta E. G. Phorbol ester and dioctanoylglycerol stimulate membrane association of protein kinase C and have a negative inotropic effect mediated by changes in cytosolic Ca2+ in adult rat cardiac myocytes. Circ Res. 1990 Apr;66(4):1143–1155. doi: 10.1161/01.res.66.4.1143. [DOI] [PubMed] [Google Scholar]
- Chuang M., Severson D. L. Inhibition of diacylglycerol metabolism in isolated cardiac myocytes by U-57 908 (RHC 80267), a diacylglycerol lipase inhibitor. J Mol Cell Cardiol. 1990 Sep;22(9):1009–1016. doi: 10.1016/0022-2828(90)91040-e. [DOI] [PubMed] [Google Scholar]
- Colvin R. A., Ashavaid T. F., Herbette L. G. Structure-function studies of canine cardiac sarcolemmal membranes. I. Estimation of receptor site densities. Biochim Biophys Acta. 1985 Feb 14;812(3):601–608. doi: 10.1016/0005-2736(85)90253-6. [DOI] [PubMed] [Google Scholar]
- Da Silva C., Fan X. T., Martelly I., Castagna M. Phorbol esters mediate phospholipid-free activation of rat brain protein kinase C. Cancer Res. 1990 Apr 1;50(7):2081–2087. [PubMed] [Google Scholar]
- Davis R. J., Ganong B. R., Bell R. M., Czech M. P. sn-1,2-Dioctanoylglycerol. A cell-permeable diacylglycerol that mimics phorbol diester action on the epidermal growth factor receptor and mitogenesis. J Biol Chem. 1985 Feb 10;260(3):1562–1566. [PubMed] [Google Scholar]
- Döemeci A., Dhallan R. S., Cohen N. M., Lederer W. J., Rogers T. B. Phorbol ester increases calcium current and simulates the effects of angiotensin II on cultured neonatal rat heart myocytes. Circ Res. 1988 Feb;62(2):347–357. doi: 10.1161/01.res.62.2.347. [DOI] [PubMed] [Google Scholar]
- Ebeling J. G., Vandenbark G. R., Kuhn L. J., Ganong B. R., Bell R. M., Niedel J. E. Diacylglycerols mimic phorbol diester induction of leukemic cell differentiation. Proc Natl Acad Sci U S A. 1985 Feb;82(3):815–819. doi: 10.1073/pnas.82.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganong B. R., Loomis C. R., Hannun Y. A., Bell R. M. Specificity and mechanism of protein kinase C activation by sn-1,2-diacylglycerols. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1184–1188. doi: 10.1073/pnas.83.5.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. A., Bhamidipati S. P., Kodali D. R., Small D. M. The interfacial conformation and transbilayer movement of diacylglycerols in phospholipid bilayers. J Biol Chem. 1991 Jan 15;266(2):1177–1186. [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Bell R. M. Activation of protein kinase C by Triton X-100 mixed micelles containing diacylglycerol and phosphatidylserine. J Biol Chem. 1985 Aug 25;260(18):10039–10043. [PubMed] [Google Scholar]
- Haworth R. A., Goknur A. B., Warner T. F., Berkoff H. A. Some determinants of quality and yield in the isolation of adult heart cells from rat. Cell Calcium. 1989 Jan;10(1):57–62. doi: 10.1016/0143-4160(89)90044-4. [DOI] [PubMed] [Google Scholar]
- Hockberger P., Toselli M., Swandulla D., Lux H. D. A diacylglycerol analogue reduces neuronal calcium currents independently of protein kinase C activation. Nature. 1989 Mar 23;338(6213):340–342. doi: 10.1038/338340a0. [DOI] [PubMed] [Google Scholar]
- Huang X. P., Da Silva C., Fan X. T., Castagna M. Characteristics of arachidonic-acid-mediated brain protein kinase C activation: evidence for concentration-dependent heterogeneity. Biochim Biophys Acta. 1993 Feb 17;1175(3):351–356. doi: 10.1016/0167-4889(93)90228-h. [DOI] [PubMed] [Google Scholar]
- Lacerda A. E., Rampe D., Brown A. M. Effects of protein kinase C activators on cardiac Ca2+ channels. Nature. 1988 Sep 15;335(6187):249–251. doi: 10.1038/335249a0. [DOI] [PubMed] [Google Scholar]
- Leatherman G. F., Kim D., Smith T. W. Effect of phorbol esters on contractile state and calcium flux in cultured chick heart cells. Am J Physiol. 1987 Jul;253(1 Pt 2):H205–H209. doi: 10.1152/ajpheart.1987.253.1.H205. [DOI] [PubMed] [Google Scholar]
- MacLeod K. T., Harding S. E. Effects of phorbol ester on contraction, intracellular pH and intracellular Ca2+ in isolated mammalian ventricular myocytes. J Physiol. 1991 Dec;444:481–498. doi: 10.1113/jphysiol.1991.sp018889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey C. V., Kohout T. A., Gaa S. T., Lederer W. J., Rogers T. B. Molecular and cellular actions of platelet-activating factor in rat heart cells. J Clin Invest. 1991 Dec;88(6):2106–2116. doi: 10.1172/JCI115540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Otani H., Otani H., Uriu T., Hara M., Inoue M., Omori K., Cragoe E. J., Jr, Inagaki C. Effects of inhibitors of protein kinase C and Na+/H+ exchange on alpha 1-adrenoceptor-mediated inotropic responses in the rat left ventricular papillary muscle. Br J Pharmacol. 1990 Jun;100(2):207–210. doi: 10.1111/j.1476-5381.1990.tb15783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pownall H. J., Smith L. C. Pyrene-labeled lipids: versatile probes of membrane dynamics in vitro and in living cells. Chem Phys Lipids. 1989 Jun;50(3-4):191–211. doi: 10.1016/0009-3084(89)90050-9. [DOI] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Rando R. R., Young N. The stereospecific activation of protein kinase C. Biochem Biophys Res Commun. 1984 Jul 31;122(2):818–823. doi: 10.1016/s0006-291x(84)80107-2. [DOI] [PubMed] [Google Scholar]
- Rogers T. B., Gaa S. T., Massey C., Dösemeci A. Protein kinase C inhibits Ca2+ accumulation in cardiac sarcoplasmic reticulum. J Biol Chem. 1990 Mar 15;265(8):4302–4308. [PubMed] [Google Scholar]
- Tseng G. N., Boyden P. A. Different effects of intracellular Ca and protein kinase C on cardiac T and L Ca currents. Am J Physiol. 1991 Aug;261(2 Pt 2):H364–H379. doi: 10.1152/ajpheart.1991.261.2.H364. [DOI] [PubMed] [Google Scholar]
- Venema R. C., Kuo J. F. Protein kinase C-mediated phosphorylation of troponin I and C-protein in isolated myocardial cells is associated with inhibition of myofibrillar actomyosin MgATPase. J Biol Chem. 1993 Feb 5;268(4):2705–2711. [PubMed] [Google Scholar]
- Ventura C., Spurgeon H., Lakatta E. G., Guarnieri C., Capogrossi M. C. Kappa and delta opioid receptor stimulation affects cardiac myocyte function and Ca2+ release from an intracellular pool in myocytes and neurons. Circ Res. 1992 Jan;70(1):66–81. doi: 10.1161/01.res.70.1.66. [DOI] [PubMed] [Google Scholar]
- Walker J. W., Martin H., Schmitt F. R., Barsotti R. J. Rapid release of an alpha-adrenergic receptor ligand from photolabile analogues. Biochemistry. 1993 Feb 9;32(5):1338–1345. doi: 10.1021/bi00056a020. [DOI] [PubMed] [Google Scholar]
- Ward C. A., Moffat M. P. Positive and negative inotropic effects of phorbol 12-myristate 13-acetate: relationship to PKC-dependence and changes in [Ca2+]i. J Mol Cell Cardiol. 1992 Sep;24(9):937–948. doi: 10.1016/0022-2828(92)91861-x. [DOI] [PubMed] [Google Scholar]
- Yuan S. H., Sunahara F. A., Sen A. K. Tumor-promoting phorbol esters inhibit cardiac functions and induce redistribution of protein kinase C in perfused beating rat heart. Circ Res. 1987 Sep;61(3):372–378. doi: 10.1161/01.res.61.3.372. [DOI] [PubMed] [Google Scholar]