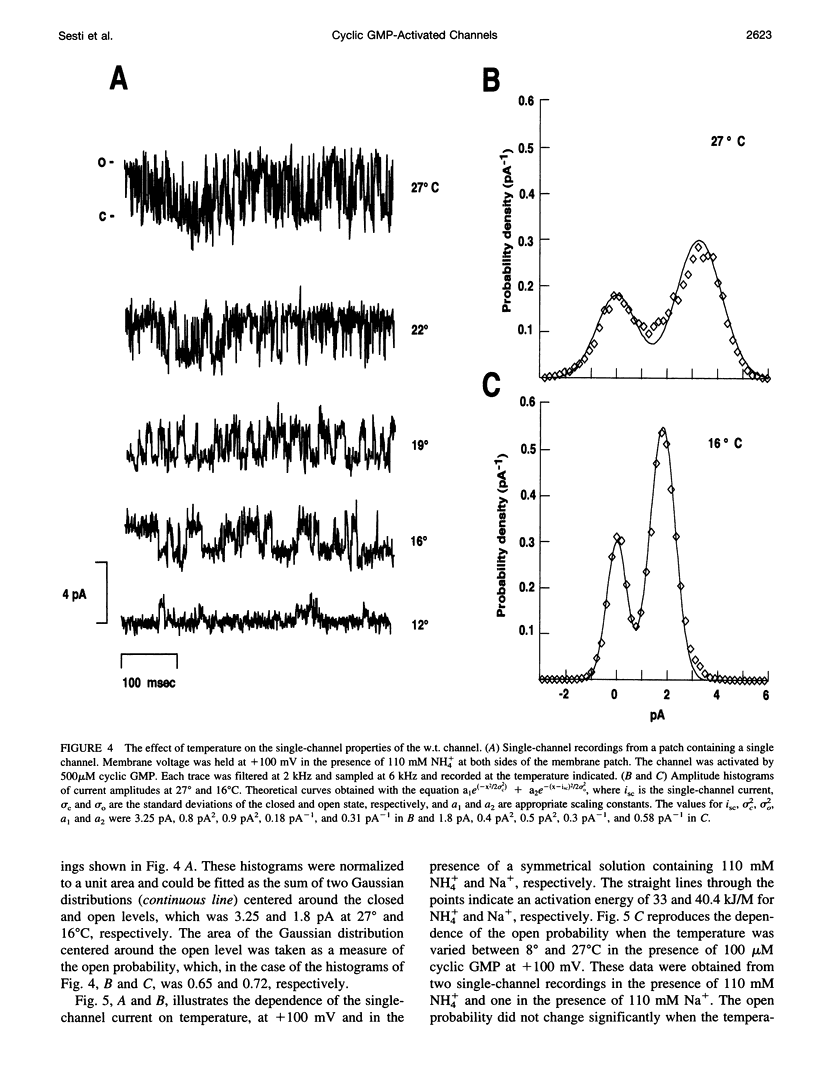
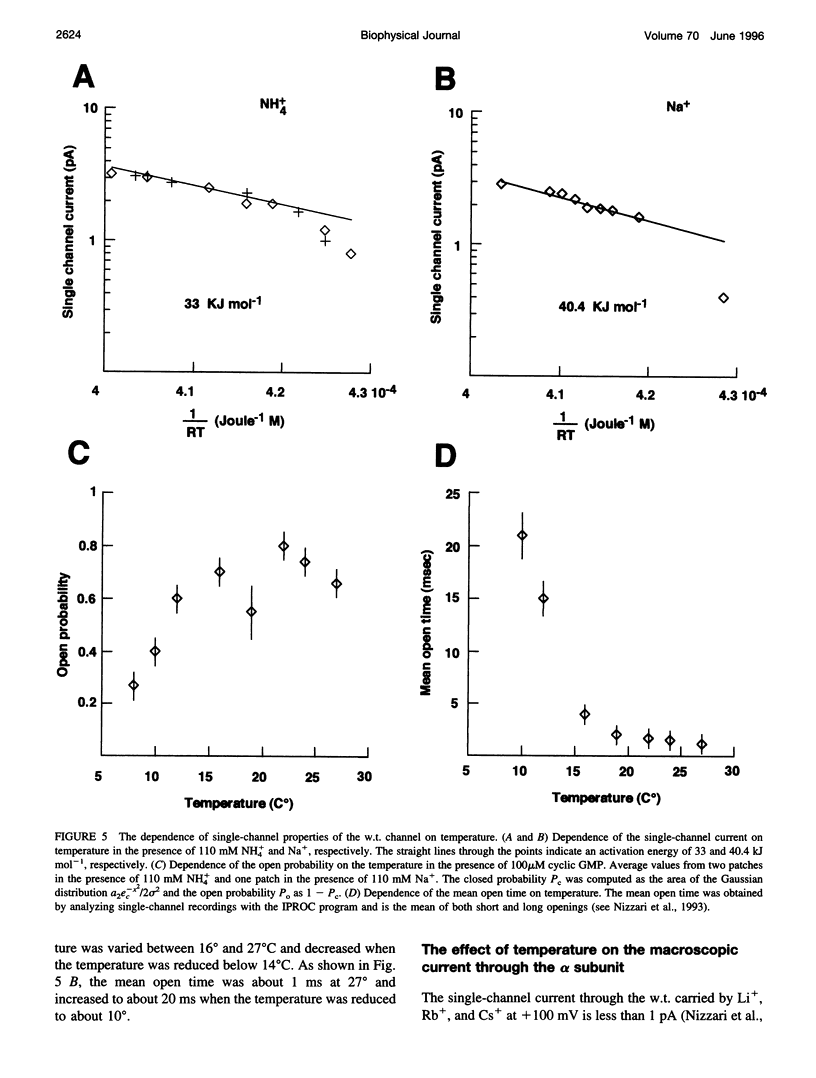
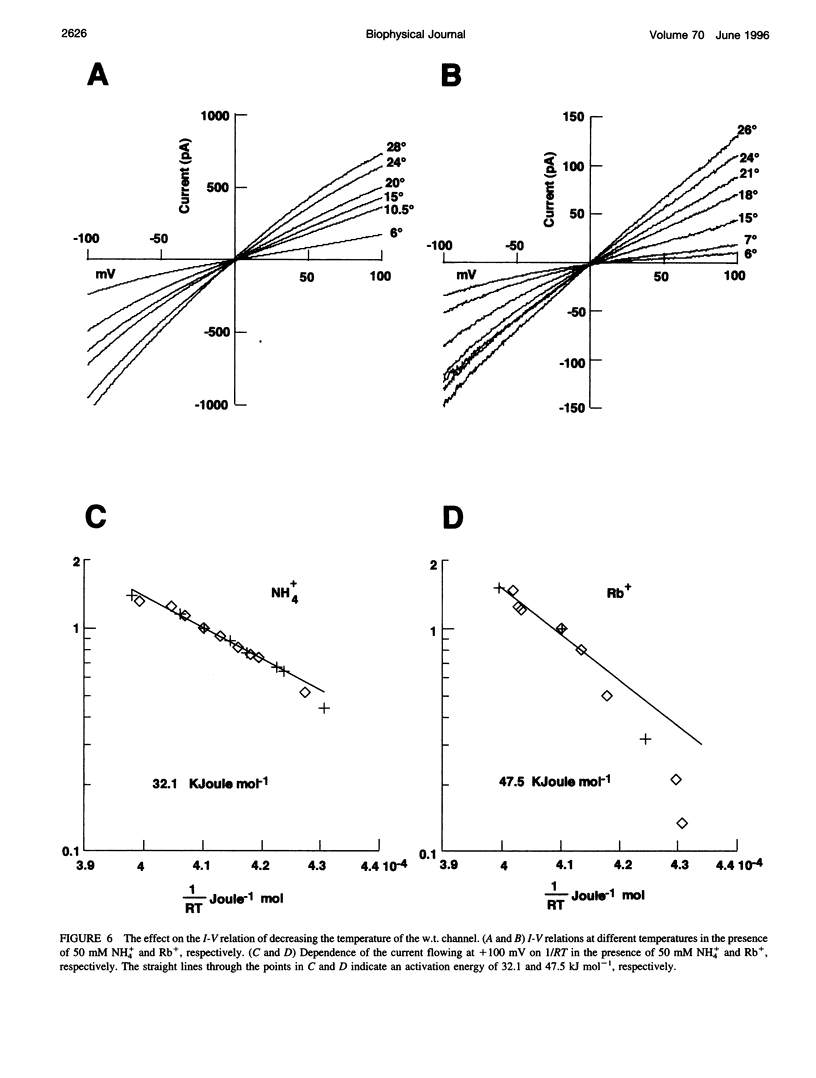
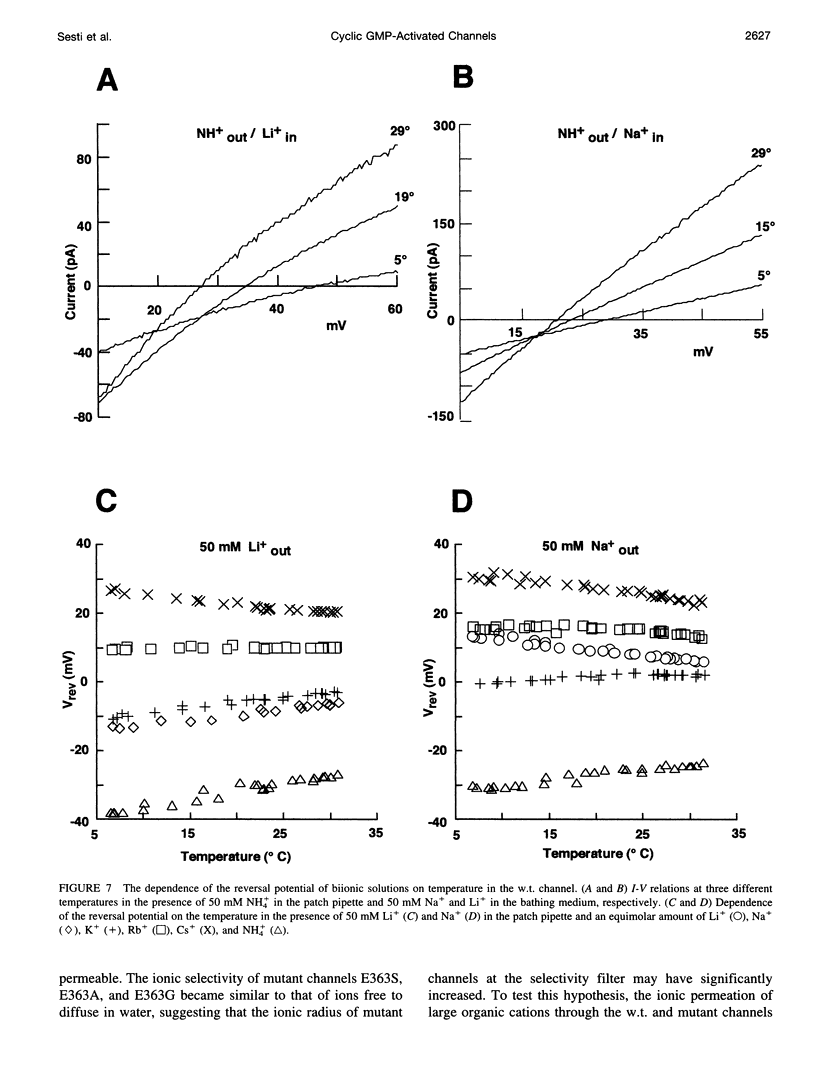
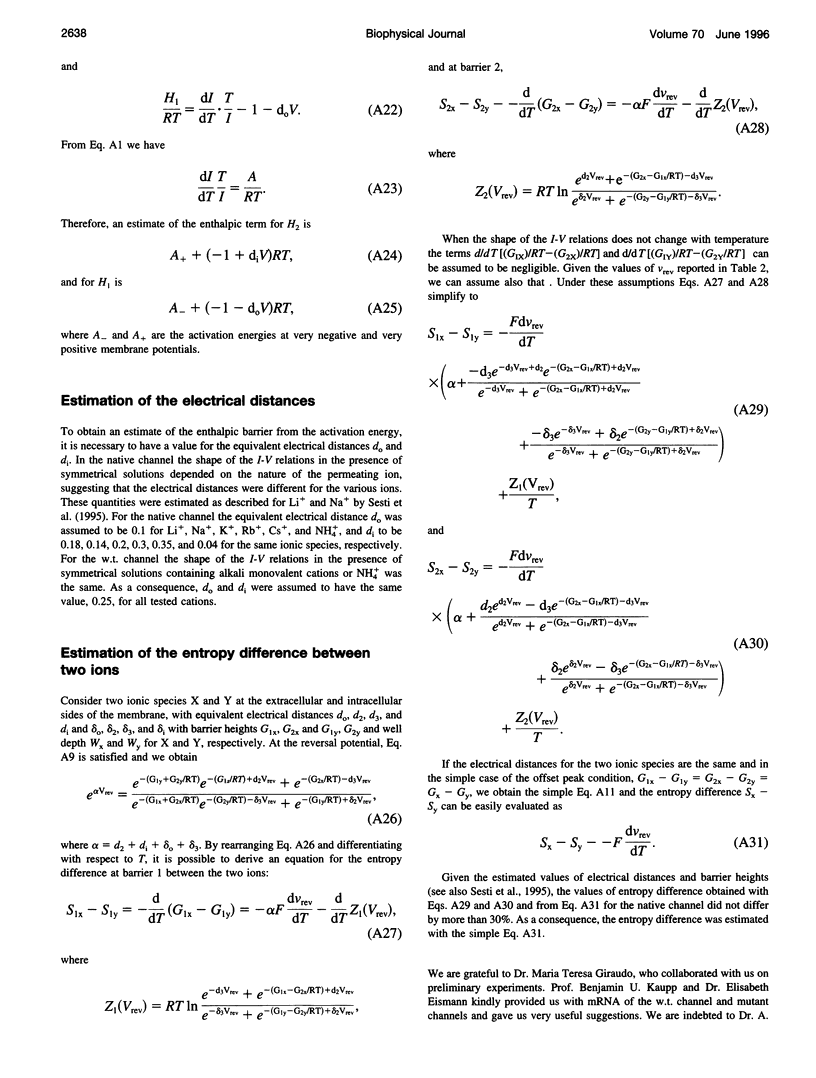
Abstract
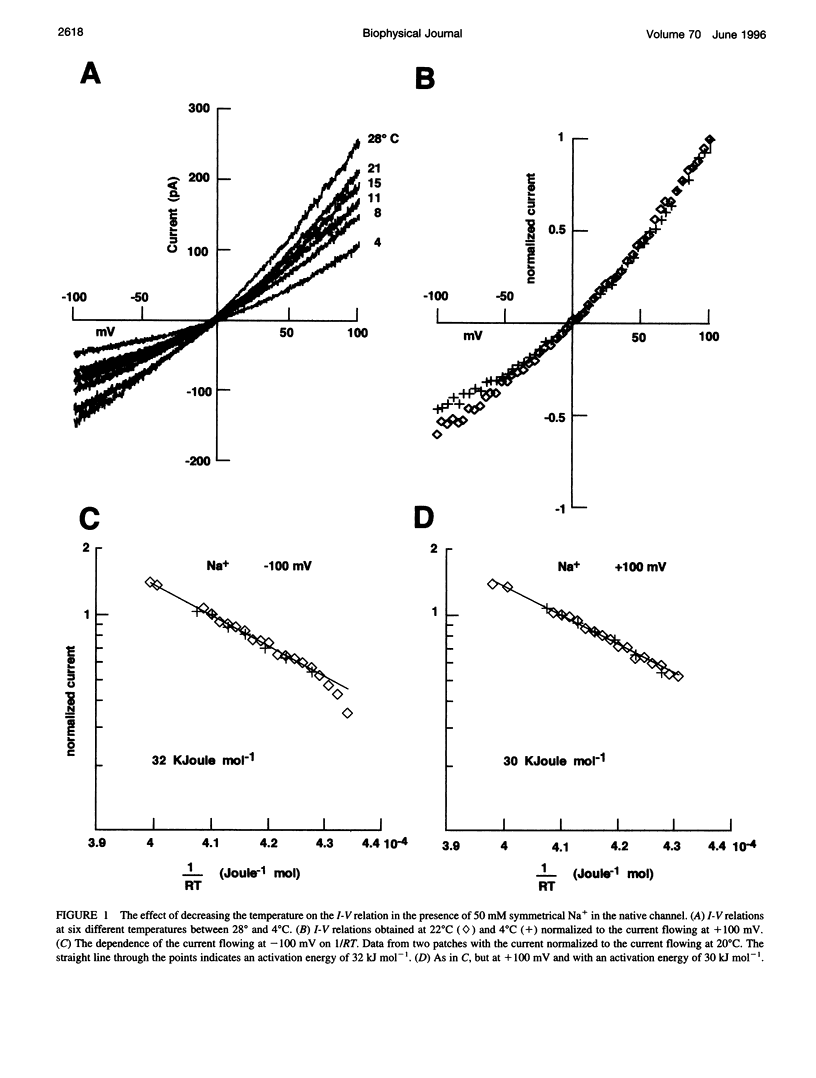
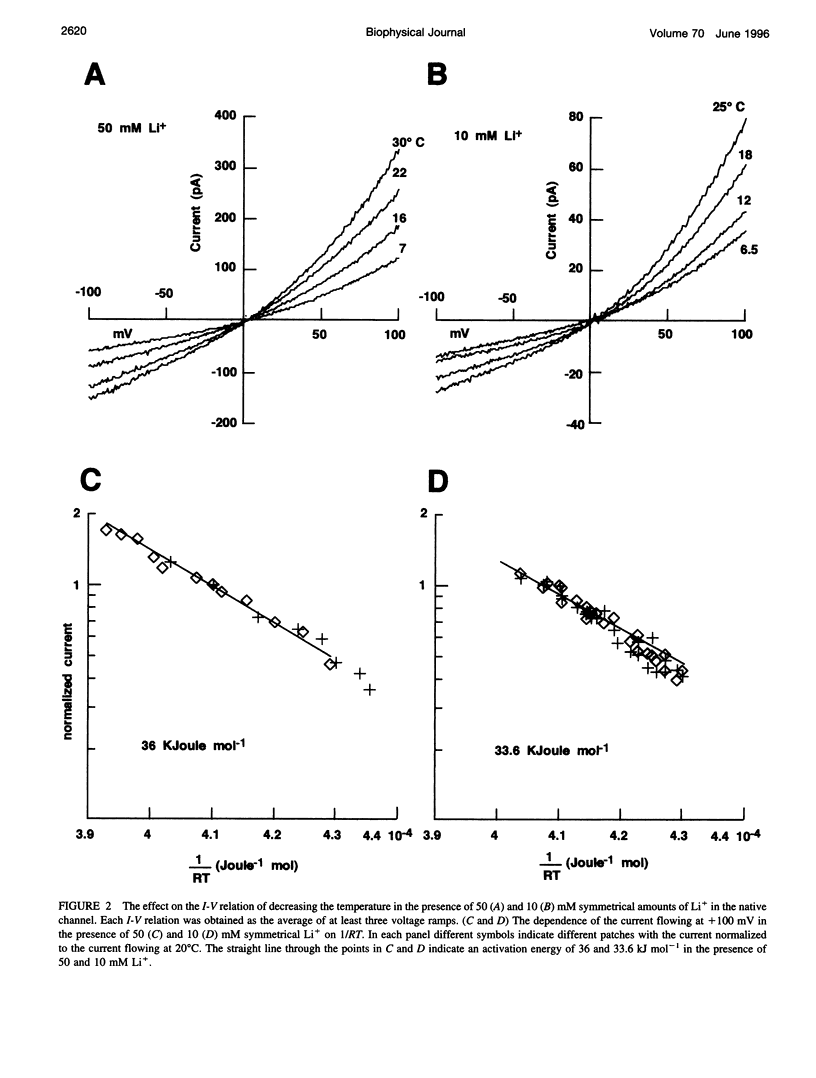
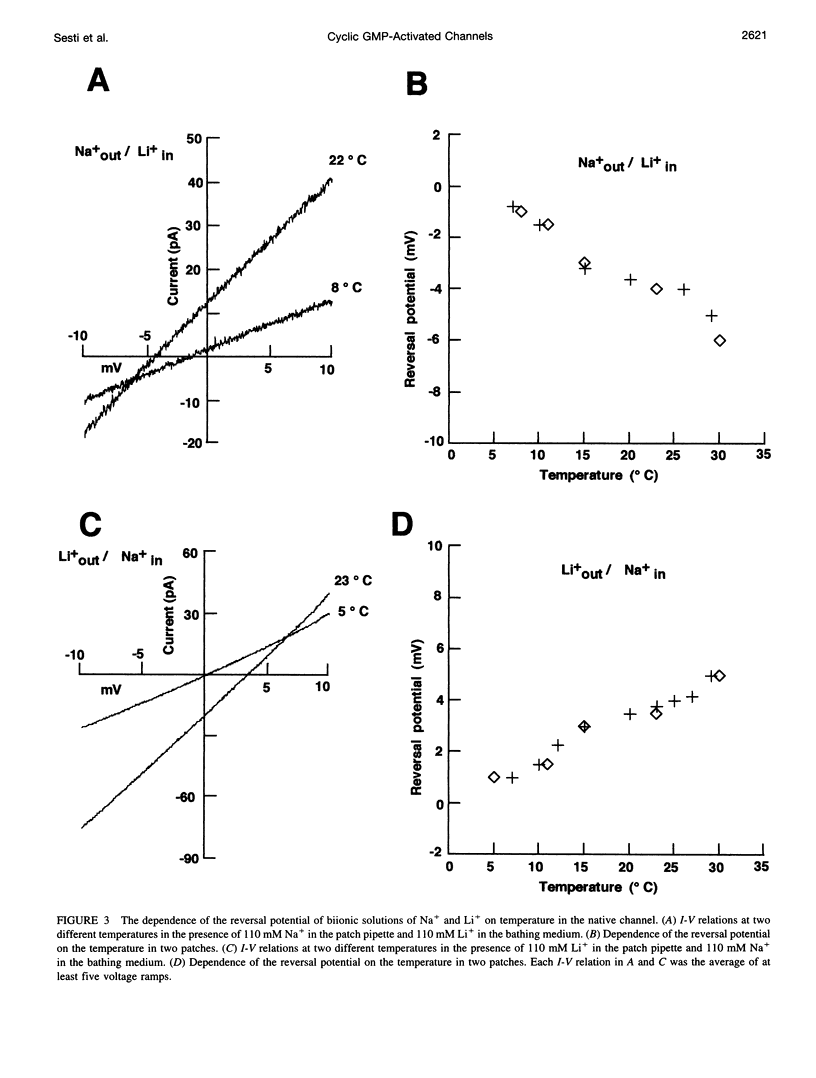
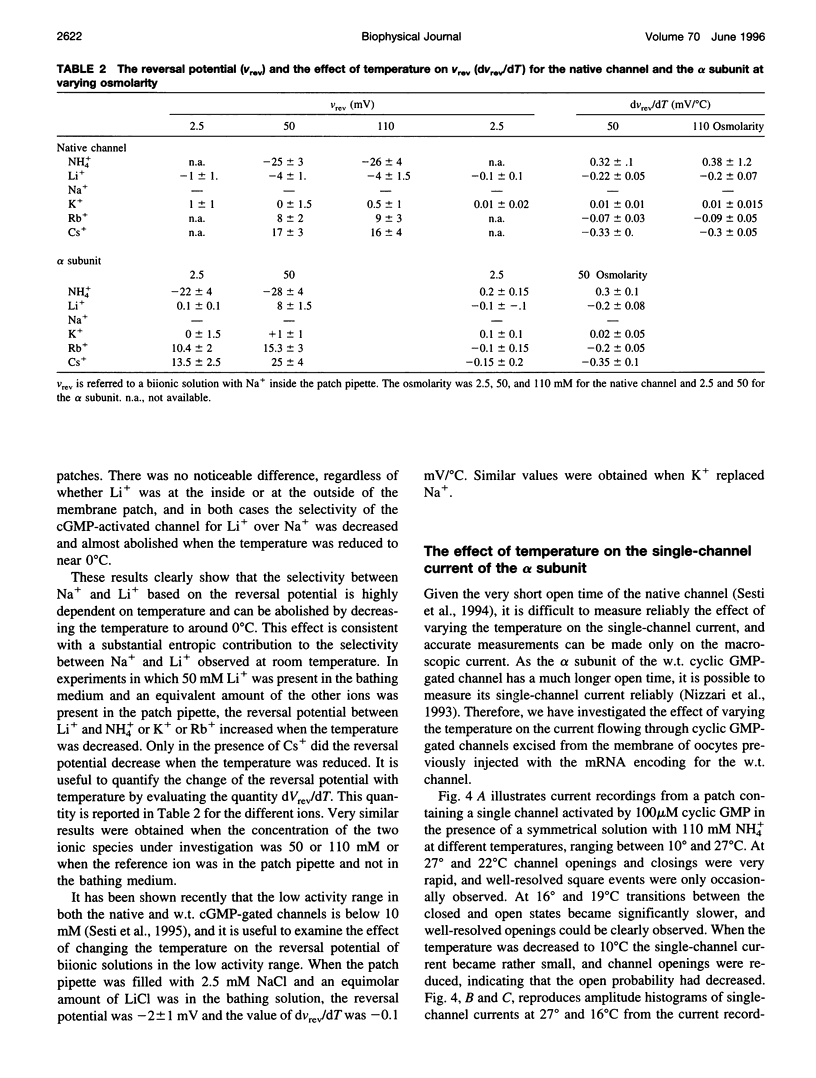
Native cGMP-gated channels were studied in rod outer segments of the larval tiger salamander Ambystoma tigrinum. The alpha subunit of the cGMP-gated channel from bovine rods, here referred to as the wild type (w.t.), and mutant channels were heterologously expressed in Xenopus laevis oocytes. These channels were studied in excised membrane patches in the inside-out configuration and were activated by the addition of 100 or 500 microM cGMP. The effect of temperature on the ionic permeation was studied. The macroscopic current flowing through the native channel at +100 mV had an activation energy of 35.8, 30, 31.8, 34.5, 41.3, and 22.4 kJ mol-1 in the presence of Li+, Na+, K+, Rb+, Cs+, and NH4+, respectively. The macroscopic current flowing through the w.t. channel at +100 mV had an activation energy of 45.2, 38.2, 37.5, 47.3, 49.4, and 38.9 kJ mol-1 in the presence of Li+, Na+, K+, Rb+, Cs+, and NH4+, respectively. The activation energy of the macroscopic current flowing through the native and w.t. channels did not vary significantly when the ionic concentration of the permeant ion was changed between 2.5 and 110 mM. The activation energy of the single-channel current of the w.t. channel at +100 mV was 40.4 and 33 kJ mol-1 for Na+ and NH4+, respectively. The reversal potential of biionic solutions changed significantly with temperature. These results can be used to obtain an estimate of the enthalpic and entropic contributions to the barrier of the Gibbs free energy experienced by an ion during its permeation through the open channel. These estimates indicate that the ionic permeation and selectivity of the cGMP-gated channel are controlled both by enthalpic and entropic factors and that the selectivity of the native channel for Li+ over Na+ is primarily caused by entropic effects.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen T. Y., Peng Y. W., Dhallan R. S., Ahamed B., Reed R. R., Yau K. W. A new subunit of the cyclic nucleotide-gated cation channel in retinal rods. Nature. 1993 Apr 22;362(6422):764–767. doi: 10.1038/362764a0. [DOI] [PubMed] [Google Scholar]
- EISENMAN G. Cation selective glass electrodes and their mode of operation. Biophys J. 1962 Mar;2(2 Pt 2):259–323. doi: 10.1016/s0006-3495(62)86959-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman G., Horn R. Ionic selectivity revisited: the role of kinetic and equilibrium processes in ion permeation through channels. J Membr Biol. 1983;76(3):197–225. doi: 10.1007/BF01870364. [DOI] [PubMed] [Google Scholar]
- Eismann E., Müller F., Heinemann S. H., Kaupp U. B. A single negative charge within the pore region of a cGMP-gated channel controls rectification, Ca2+ blockage, and ionic selectivity. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1109–1113. doi: 10.1073/pnas.91.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Furman R. E., Tanaka J. C. Monovalent selectivity of the cyclic guanosine monophosphate-activated ion channel. J Gen Physiol. 1990 Jul;96(1):57–82. doi: 10.1085/jgp.96.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B., Niidome T., Tanabe T., Terada S., Bönigk W., Stühmer W., Cook N. J., Kangawa K., Matsuo H., Hirose T. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 1989 Dec 14;342(6251):762–766. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- Körschen H. G., Illing M., Seifert R., Sesti F., Williams A., Gotzes S., Colville C., Müller F., Dosé A., Godde M. A 240 kDa protein represents the complete beta subunit of the cyclic nucleotide-gated channel from rod photoreceptor. Neuron. 1995 Sep;15(3):627–636. doi: 10.1016/0896-6273(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Läuger P. Microscopic calculation of ion-transport rates in membrane channels. Biophys Chem. 1982 May;15(2):89–100. doi: 10.1016/0301-4622(82)80021-5. [DOI] [PubMed] [Google Scholar]
- Menini A. Currents carried by monovalent cations through cyclic GMP-activated channels in excised patches from salamander rods. J Physiol. 1990 May;424:167–185. doi: 10.1113/jphysiol.1990.sp018061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C., Stahl N., Barrol M. A thermodynamic analysis of monovalent cation permeation through a K(+)-selective ion channel. Neuron. 1988 Apr;1(2):159–164. doi: 10.1016/0896-6273(88)90200-0. [DOI] [PubMed] [Google Scholar]
- Nizzari M., Sesti F., Giraudo M. T., Virginio C., Cattaneo A., Torre V. Single-channel properties of cloned cGMP-activated channels from retinal rods. Proc Biol Sci. 1993 Oct 22;254(1339):69–74. doi: 10.1098/rspb.1993.0128. [DOI] [PubMed] [Google Scholar]
- Picco C., Menini A. The permeability of the cGMP-activated channel to organic cations in retinal rods of the tiger salamander. J Physiol. 1993 Jan;460:741–758. doi: 10.1113/jphysiol.1993.sp019497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root M. J., MacKinnon R. Identification of an external divalent cation-binding site in the pore of a cGMP-activated channel. Neuron. 1993 Sep;11(3):459–466. doi: 10.1016/0896-6273(93)90150-p. [DOI] [PubMed] [Google Scholar]
- Sesti F., Eismann E., Kaupp U. B., Nizzari M., Torre V. The multi-ion nature of the cGMP-gated channel from vertebrate rods. J Physiol. 1995 Aug 15;487(1):17–36. doi: 10.1113/jphysiol.1995.sp020858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesti F., Straforini M., Lamb T. D., Torre V. Gating, selectivity and blockage of single channels activated by cyclic GMP in retinal rods of the tiger salamander. J Physiol. 1994 Jan 15;474(2):203–222. doi: 10.1113/jphysiol.1994.sp020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. R., Baylor D. A. Conductance and kinetics of single cGMP-activated channels in salamander rod outer segments. J Physiol. 1995 Mar 15;483(Pt 3):567–582. doi: 10.1113/jphysiol.1995.sp020607. [DOI] [PMC free article] [PubMed] [Google Scholar]


