Abstract
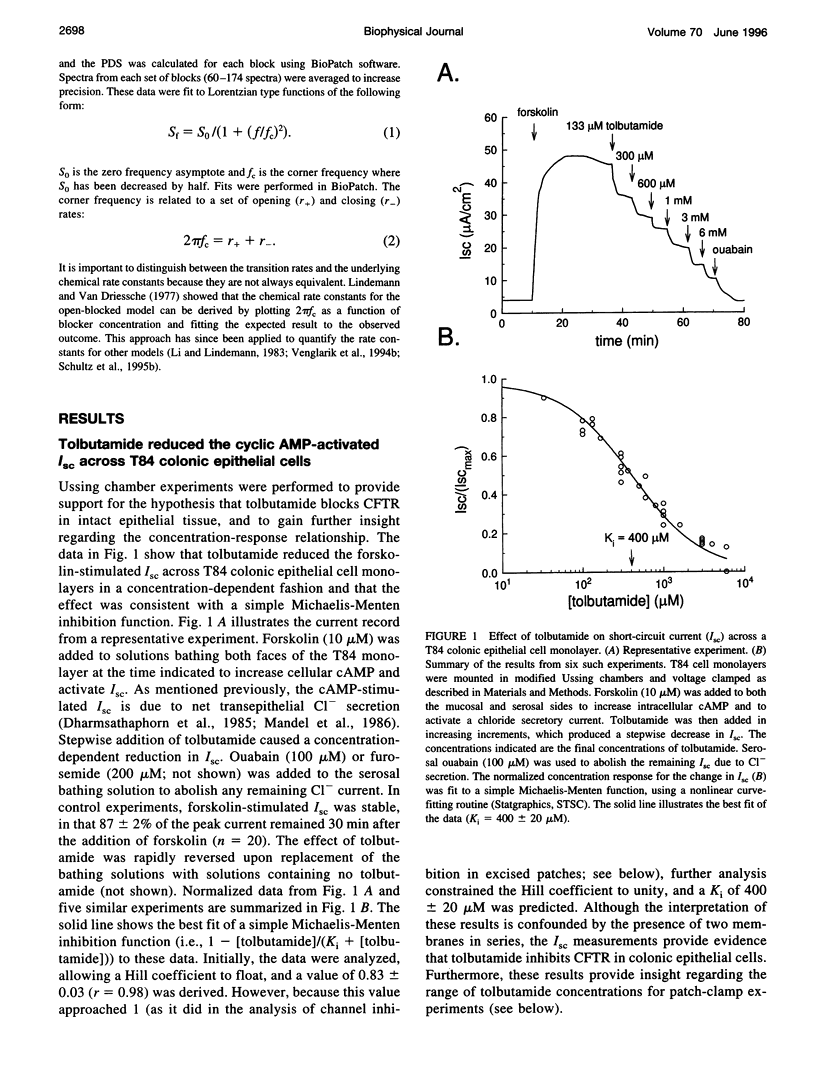
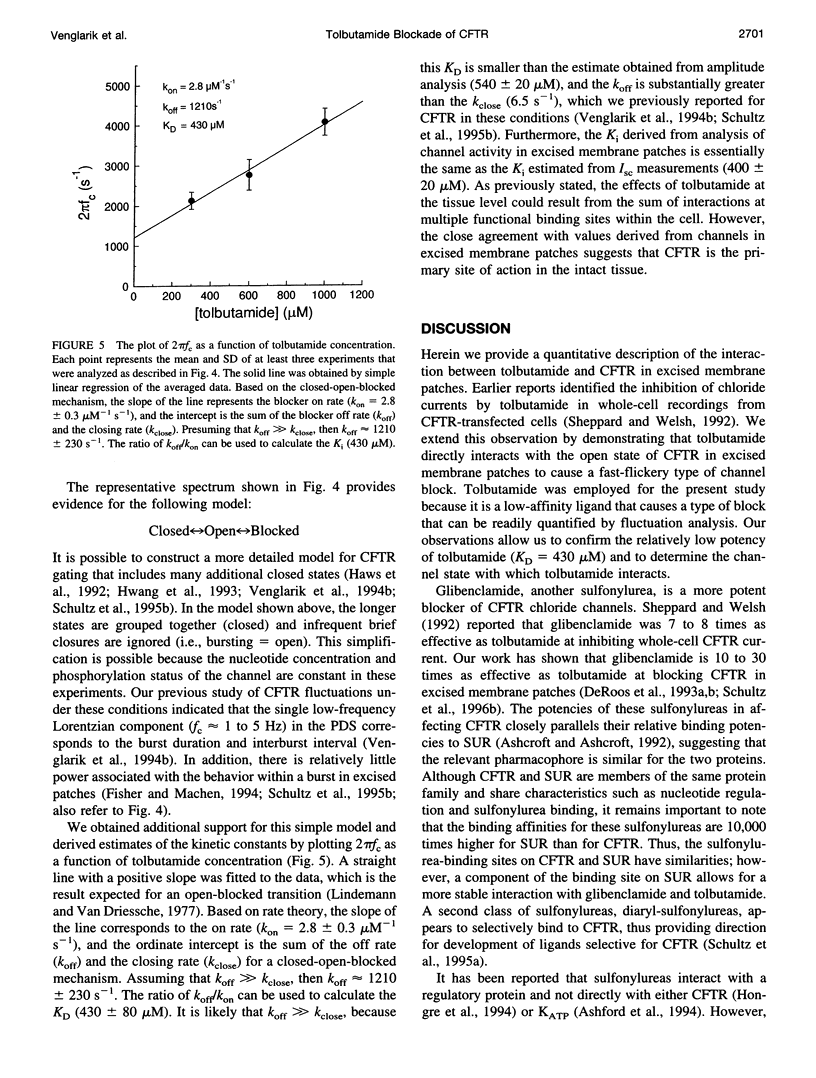
Cystic fibrosis transmembrane conductance regulator (CFTR) is an epithelial Cl- channel that is regulated by protein kinase A and cytosolic nucleotides. Previously, Sheppard and Welsh reported that the sulfonylureas glibenclamide and tolbutamide reduced CFTR whole cell currents. The aim of this study was to quantify the effects of tolbutamide on CFTR gating in excised membrane patches containing multiple channels. We chose tolbutamide because weak (i.e., fast-type) open channel blockers introduce brief events into multichannel recordings that can be readily quantified by current fluctuation analysis. Inspection of current records revealed that the addition of tolbutamide reduced the apparent single-channel current amplitude and increased the open-channel noise, as expected for a fast-type open channel blocker. The apparent decrease in unitary current amplitude provides a measure of open probability within a burst (P0 Burst), and the resulting concentration-response relationship was described by a simple Michaelis-Menten inhibition function. The concentration of tolbutamide causing a 50% reduction of Po Burst (540 +/- 20 microM) was similar to the concentration producing a 50% inhibition of short-circuit current across T84 colonic epithelial cell monolayers (400 +/- 20 microM). Changes in CFTR gating were then quantified by analyzing current fluctuations. Tolbutamide caused a high-frequency Lorentzian (corner frequency, fc > 300 Hz) to appear in the power density spectrum. The fc of this Lorentzian component increased as a linear function of tolbutamide concentration, as expected for a pseudo-first-order open-blocked mechanism and yielded estimates of the on rate (koff = 2.8 +/- 0.3 microM-1 s-1), the off rate (kon = 1210 +/- 225 s-1), and the dissociation constant (KD = 430 +/- 80 microM). Based on these observations, we propose that there is a bimolecular interaction between tolbutamide and CFTR, causing open channel blockade.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar-Bryan L., Nichols C. G., Wechsler S. W., Clement J. P., 4th, Boyd A. E., 3rd, González G., Herrera-Sosa H., Nguy K., Bryan J., Nelson D. A. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995 Apr 21;268(5209):423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- Anderson M. P., Berger H. A., Rich D. P., Gregory R. J., Smith A. E., Welsh M. J. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell. 1991 Nov 15;67(4):775–784. doi: 10.1016/0092-8674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- Ashcroft S. J., Ashcroft F. M. The sulfonylurea receptor. Biochim Biophys Acta. 1992 Dec 15;1175(1):45–59. doi: 10.1016/0167-4889(92)90008-y. [DOI] [PubMed] [Google Scholar]
- Ashford M. L., Bond C. T., Blair T. A., Adelman J. P. Cloning and functional expression of a rat heart KATP channel. Nature. 1994 Aug 11;370(6489):456–459. doi: 10.1038/370456a0. [DOI] [PubMed] [Google Scholar]
- Bahinski A., Nairn A. C., Greengard P., Gadsby D. C. Chloride conductance regulated by cyclic AMP-dependent protein kinase in cardiac myocytes. Nature. 1989 Aug 31;340(6236):718–721. doi: 10.1038/340718a0. [DOI] [PubMed] [Google Scholar]
- Bridges R. J., Rummel W., Simon B. Forskolin induced chloride secretion across the isolated mucosa of rat colon descendens. Naunyn Schmiedebergs Arch Pharmacol. 1983 Aug;323(4):355–360. doi: 10.1007/BF00512476. [DOI] [PubMed] [Google Scholar]
- Caro J. F. Effects of glyburide on carbohydrate metabolism and insulin action in the liver. Am J Med. 1990 Aug 20;89(2A):17S–53S. doi: 10.1016/0002-9343(90)90332-8. [DOI] [PubMed] [Google Scholar]
- Devor D. C., Forrest J. N., Jr, Suggs W. K., Frizzell R. A. cAMP-activated Cl- channels in primary cultures of spiny dogfish (Squalus acanthias) rectal gland. Am J Physiol. 1995 Jan;268(1 Pt 1):C70–C79. doi: 10.1152/ajpcell.1995.268.1.C70. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Mandel K. G., Masui H., McRoberts J. A. Vasoactive intestinal polypeptide-induced chloride secretion by a colonic epithelial cell line. Direct participation of a basolaterally localized Na+,K+,Cl- cotransport system. J Clin Invest. 1985 Feb;75(2):462–471. doi: 10.1172/JCI111721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G., Weston A. H. Induction of a glibenclamide-sensitive K-current by modification of a delayed rectifier channel in rat portal vein in insulinoma cells. Br J Pharmacol. 1993 Dec;110(4):1280–1281. doi: 10.1111/j.1476-5381.1993.tb13955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehara T., Ishihara K. Anion channels activated by adrenaline in cardiac myocytes. Nature. 1990 Sep 20;347(6290):284–286. doi: 10.1038/347284a0. [DOI] [PubMed] [Google Scholar]
- Fischer H., Machen T. E. CFTR displays voltage dependence and two gating modes during stimulation. J Gen Physiol. 1994 Sep;104(3):541–566. doi: 10.1085/jgp.104.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. A., Greenwell J. R., Argent B. E. Secretin-regulated chloride channel on the apical plasma membrane of pancreatic duct cells. J Membr Biol. 1988 Oct;105(2):131–142. doi: 10.1007/BF02009166. [DOI] [PubMed] [Google Scholar]
- Gunderson K. L., Kopito R. R. Conformational states of CFTR associated with channel gating: the role ATP binding and hydrolysis. Cell. 1995 Jul 28;82(2):231–239. doi: 10.1016/0092-8674(95)90310-0. [DOI] [PubMed] [Google Scholar]
- Haws C., Krouse M. E., Xia Y., Gruenert D. C., Wine J. J. CFTR channels in immortalized human airway cells. Am J Physiol. 1992 Dec;263(6 Pt 1):L692–L707. doi: 10.1152/ajplung.1992.263.6.L692. [DOI] [PubMed] [Google Scholar]
- Hongre A. S., Baró I., Berthon B., Escande D. Effects of sulphonylureas on cAMP-stimulated Cl- transport via the cystic fibrosis gene product in human epithelial cells. Pflugers Arch. 1994 Feb;426(3-4):284–287. doi: 10.1007/BF00374783. [DOI] [PubMed] [Google Scholar]
- Hwang T. C., Horie M., Gadsby D. C. Functionally distinct phospho-forms underlie incremental activation of protein kinase-regulated Cl- conductance in mammalian heart. J Gen Physiol. 1993 May;101(5):629–650. doi: 10.1085/jgp.101.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang T. C., Nagel G., Nairn A. C., Gadsby D. C. Regulation of the gating of cystic fibrosis transmembrane conductance regulator C1 channels by phosphorylation and ATP hydrolysis. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4698–4702. doi: 10.1073/pnas.91.11.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N., Gonoi T., Clement J. P., 4th, Namba N., Inazawa J., Gonzalez G., Aguilar-Bryan L., Seino S., Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995 Nov 17;270(5239):1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Li J. H., Lindemann B. Competitive blocking of epithelial sodium channels by organic cations: the relationship between macroscopic and microscopic inhibition constants. J Membr Biol. 1983;76(3):235–251. doi: 10.1007/BF01870366. [DOI] [PubMed] [Google Scholar]
- Lindemann B., Van Driessche W. Sodium-specific membrane channels of frog skin are pores: current fluctuations reveal high turnover. Science. 1977 Jan 21;195(4275):292–294. doi: 10.1126/science.299785. [DOI] [PubMed] [Google Scholar]
- Mandel K. G., Dharmsathaphorn K., McRoberts J. A. Characterization of a cyclic AMP-activated Cl-transport pathway in the apical membrane of a human colonic epithelial cell line. J Biol Chem. 1986 Jan 15;261(2):704–712. [PubMed] [Google Scholar]
- Miller C. Bis-quaternary ammonium blockers as structural probes of the sarcoplasmic reticulum K+ channel. J Gen Physiol. 1982 May;79(5):869–891. doi: 10.1085/jgp.79.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G., Hwang T. C., Nastiuk K. L., Nairn A. C., Gadsby D. C. The protein kinase A-regulated cardiac Cl- channel resembles the cystic fibrosis transmembrane conductance regulator. Nature. 1992 Nov 5;360(6399):81–84. doi: 10.1038/360081a0. [DOI] [PubMed] [Google Scholar]
- Okuno S., Inaba M., Nishizawa Y., Inoue A., Morii H. Effect of tolbutamide and glyburide on cAMP-dependent protein kinase activity in rat liver cytosol. Diabetes. 1988 Jul;37(7):857–861. doi: 10.2337/diab.37.7.857. [DOI] [PubMed] [Google Scholar]
- Quinton P. M. Cystic fibrosis: a disease in electrolyte transport. FASEB J. 1990 Jul;4(10):2709–2717. doi: 10.1096/fasebj.4.10.2197151. [DOI] [PubMed] [Google Scholar]
- Rabe A., Disser J., Frömter E. Cl- channel inhibition by glibenclamide is not specific for the CFTR-type Cl- channel. Pflugers Arch. 1995 Mar;429(5):659–662. doi: 10.1007/BF00373986. [DOI] [PubMed] [Google Scholar]
- Schultz B. D., Venglarik C. J., Bridges R. J., Frizzell R. A. Regulation of CFTR Cl- channel gating by ADP and ATP analogues. J Gen Physiol. 1995 Mar;105(3):329–361. doi: 10.1085/jgp.105.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D. N., Welsh M. J. Effect of ATP-sensitive K+ channel regulators on cystic fibrosis transmembrane conductance regulator chloride currents. J Gen Physiol. 1992 Oct;100(4):573–591. doi: 10.1085/jgp.100.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabcharani J. A., Chang X. B., Riordan J. R., Hanrahan J. W. Phosphorylation-regulated Cl- channel in CHO cells stably expressing the cystic fibrosis gene. Nature. 1991 Aug 15;352(6336):628–631. doi: 10.1038/352628a0. [DOI] [PubMed] [Google Scholar]
- Tabcharani J. A., Low W., Elie D., Hanrahan J. W. Low-conductance chloride channel activated by cAMP in the epithelial cell line T84. FEBS Lett. 1990 Sep 17;270(1-2):157–164. doi: 10.1016/0014-5793(90)81257-o. [DOI] [PubMed] [Google Scholar]
- Venglarik C. J., Dawson D. C. Cholinergic regulation of Na absorption by turtle colon: role of basolateral K conductance. Am J Physiol. 1986 Oct;251(4 Pt 1):C563–C570. doi: 10.1152/ajpcell.1986.251.4.C563. [DOI] [PubMed] [Google Scholar]
- Venglarik C. J., Schultz B. D., Frizzell R. A., Bridges R. J. ATP alters current fluctuations of cystic fibrosis transmembrane conductance regulator: evidence for a three-state activation mechanism. J Gen Physiol. 1994 Jul;104(1):123–146. doi: 10.1085/jgp.104.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venglarik C. J., Singh A. K., Wang R., Bridges R. J. Trinitrophenyl-ATP blocks colonic Cl- channels in planar phospholipid bilayers. Evidence for two nucleotide binding sites. J Gen Physiol. 1993 Apr;101(4):545–569. doi: 10.1085/jgp.101.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Devor D. C., Engelhardt J. F., Ernst S. A., Strong T. V., Collins F. S., Cohn J. A., Frizzell R. A., Wilson J. M. Molecular basis of defective anion transport in L cells expressing recombinant forms of CFTR. Hum Mol Genet. 1993 Aug;2(8):1253–1261. doi: 10.1093/hmg/2.8.1253. [DOI] [PubMed] [Google Scholar]


