Abstract
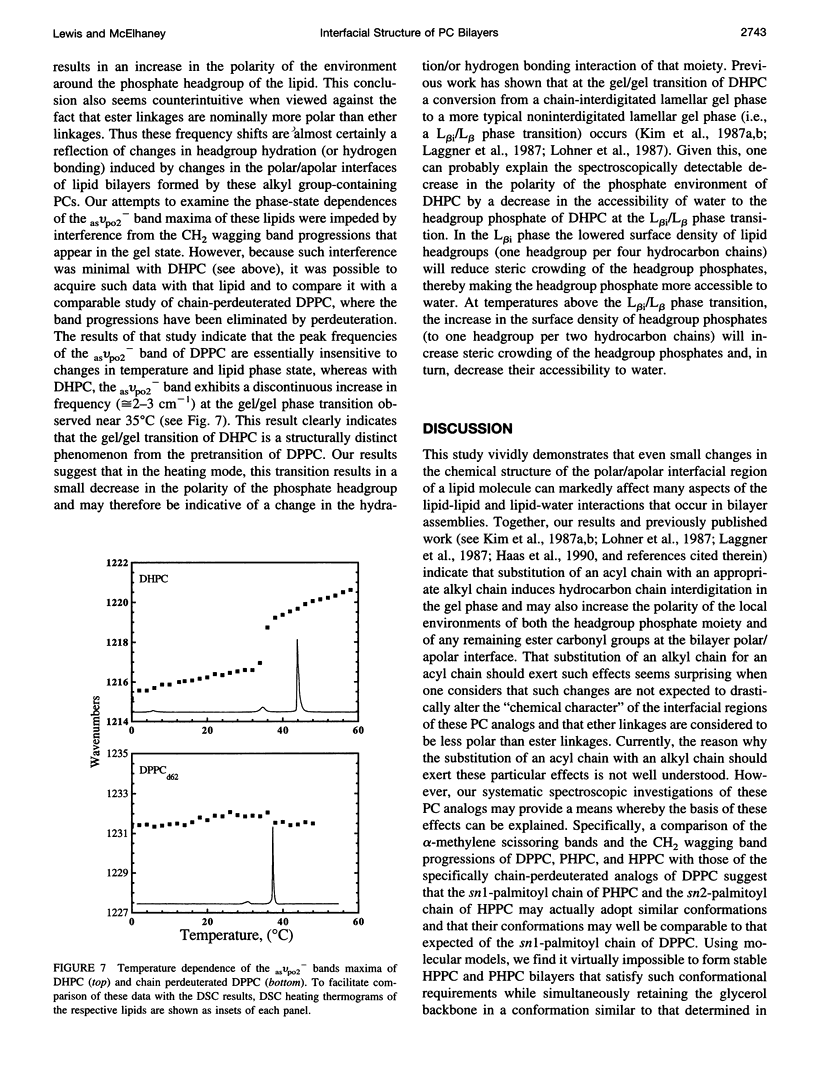
The thermotropic phase behavior of aqueous dispersions of dipalmitoylphosphatidylcholine (DPPC) and its 1,2-dialkyl, 1-acyl 2-alkyl and 1-alkyl 2-acyl analogs was examined by differential scanning calorimetry, and the organization of these molecules in those hydrated bilayers was studied by Fourier transform infrared spectroscopy. The calorimetric data indicate that substitution of either or both of the acyl chains of DPPC with the corresponding ether-linked hydrocarbon chain results in relatively small increases in the temperature (< 4 degrees C) and enthalpy (< 1 kcal/mol) of the lipid chain-melting phase transition. The spectroscopic data reveal that replacement of one or both of the ester-linked hydrocarbon chains of DPPC with its ether-linked analog causes structural changes in the bilayer assembly, which result in an increase in the polarity of the local environments of the phosphate headgroups and of the ester carbonyl groups at the bilayer polar/apolar interface. The latter observation is unexpected, given that ester linkages are considered to be intrinsically more polar that ether linkages. This finding cannot be satisfactorily rationalized unless the conformation of the glycerol backbones of the analogs containing ether-linked hydrocarbon chains differs significantly from that of diacyl glycerolipids such as DPPC. A comparison of the alpha-methylene scissoring bands and the methylene wagging band progressions of these lipids with the corresponding absorption bands of specifically chain-perdeuterated analogs of DPPC also supports the conclusion that replacement of the ester-linked hydrocarbon chains of DPPC with the corresponding ether-linked analog induces conformational changes in the lipid glycerol backbone. The suggestion that the conformation of glycerol backbones in the alkyl-acyl and dialkyl derivatives of DPPC differs from that of the naturally occurring 1,2-diacyl glycerolipid suggests that mono- and di-alkyl glycerolipids may not be good models of their diacyl analogs. These results, and previously published evidence that DPPC analogs with ether-linked hydrocarbon chains spontaneously form chain-interdigitated gel phases at low temperatures, clearly indicate that the properties of lipid bilayers can be substantially altered by small changes in the chemical structures of their polar/polar interfaces, and highlight the critical role of the interfacial region as a determinant of the structure and organization of lipid assemblies.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blume A., Eibl H. A calorimetric study of the thermotropic behaviour of 1,2-dipentadecylmethylidene phospholipids. Biochim Biophys Acta. 1981 Jan 22;640(2):609–618. doi: 10.1016/0005-2736(81)90484-3. [DOI] [PubMed] [Google Scholar]
- Blume A., Hübner W., Messner G. Fourier transform infrared spectroscopy of 13C = O-labeled phospholipids hydrogen bonding to carbonyl groups. Biochemistry. 1988 Oct 18;27(21):8239–8249. doi: 10.1021/bi00421a038. [DOI] [PubMed] [Google Scholar]
- Cameron D. G., Mantsch H. H. Metastability and polymorphism in the gel phase of 1,2-dipalmitoyl-3-SN-phosphatidylcholine. A Fourier transform infrared study of the subtransition. Biophys J. 1982 May;38(2):175–184. doi: 10.1016/S0006-3495(82)84544-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curatolo W., Bali A., Gupta C. M. Metastable phase behavior of a sphingolipid analogue. Biochim Biophys Acta. 1982 Aug 25;690(1):89–94. doi: 10.1016/0005-2736(82)90242-5. [DOI] [PubMed] [Google Scholar]
- Curatolo W., Bali A., Gupta C. M. Phase behavior of carbamyloxyphosphatidylcholine, a sphingolipid analogue. J Pharm Sci. 1985 Dec;74(12):1255–1258. doi: 10.1002/jps.2600741203. [DOI] [PubMed] [Google Scholar]
- Dluhy R. A., Chowdhry B. Z., Cameron D. G. Infrared characterization of conformational differences in the lamellar phases of 1,3-dipalmitoyl-sn-glycero-2-phosphocholine. Biochim Biophys Acta. 1985 Dec 19;821(3):437–444. doi: 10.1016/0005-2736(85)90048-3. [DOI] [PubMed] [Google Scholar]
- Eibl H., Blume A. The influence of charge on phosphatidic acid bilayer membranes. Biochim Biophys Acta. 1979 Jun 2;553(3):476–488. doi: 10.1016/0005-2736(79)90303-1. [DOI] [PubMed] [Google Scholar]
- Füldner H. H. Characterization of a third phase transition in multilamellar dipalmitoyllecithin liposomes. Biochemistry. 1981 Sep 29;20(20):5707–5710. doi: 10.1021/bi00523a011. [DOI] [PubMed] [Google Scholar]
- Haas N. S., Sripada P. K., Shipley G. G. Effect of chain-linkage on the structure of phosphatidyl choline bilayers. Hydration studies of 1-hexadecyl 2-palmitoyl-sn-glycero-3-phosphocholine. Biophys J. 1990 Jan;57(1):117–124. doi: 10.1016/S0006-3495(90)82512-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Sundell S. Preferred conformation and dynamics of the glycerol backbone in phospholipids. An NMR and X-ray single-crystal analysis. Biochemistry. 1988 Dec 27;27(26):9166–9174. doi: 10.1021/bi00426a014. [DOI] [PubMed] [Google Scholar]
- Hing F. S., Maulik P. R., Shipley G. G. Structure and interactions of ether- and ester-linked phosphatidylethanolamines. Biochemistry. 1991 Sep 17;30(37):9007–9015. doi: 10.1021/bi00101a014. [DOI] [PubMed] [Google Scholar]
- Hinz H. J., Kuttenreich H., Meyer R., Renner M., Fründ R., Koynova R., Boyanov A. I., Tenchov B. G. Stereochemistry and size of sugar head groups determine structure and phase behavior of glycolipid membranes: densitometric, calorimetric, and X-ray studies. Biochemistry. 1991 May 28;30(21):5125–5138. doi: 10.1021/bi00235a003. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. B., Mason R., Thomas K. M., Shipley G. G. Structural chemistry of 1,2 dilauroyl-DL-phosphatidylethanolamine: molecular conformation and intermolecular packing of phospholipids. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3036–3040. doi: 10.1073/pnas.71.8.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. T., Mattai J., Shipley G. G. Bilayer interactions of ether- and ester-linked phospholipids: dihexadecyl- and dipalmitoylphosphatidylcholines. Biochemistry. 1987 Oct 20;26(21):6599–6603. doi: 10.1021/bi00395a006. [DOI] [PubMed] [Google Scholar]
- Kim J. T., Mattai J., Shipley G. G. Gel phase polymorphism in ether-linked dihexadecylphosphatidylcholine bilayers. Biochemistry. 1987 Oct 20;26(21):6592–6598. doi: 10.1021/bi00395a005. [DOI] [PubMed] [Google Scholar]
- Laggner P., Lohner K., Degovics G., Müller K., Schuster A. Structure and thermodynamics of the dihexadecylphosphatidylcholine-water system. Chem Phys Lipids. 1987 Jun;44(1):31–60. doi: 10.1016/0009-3084(87)90004-1. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., Mak N., McElhaney R. N. A differential scanning calorimetric study of the thermotropic phase behavior of model membranes composed of phosphatidylcholines containing linear saturated fatty acyl chains. Biochemistry. 1987 Sep 22;26(19):6118–6126. doi: 10.1021/bi00393a026. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N., Harper P. E., Turner D. C., Gruner S. M. Studies of the thermotropic phase behavior of phosphatidylcholines containing 2-alkyl substituted fatty acyl chains: a new class of phosphatidylcholines forming inverted nonlamellar phases. Biophys J. 1994 Apr;66(4):1088–1103. doi: 10.1016/S0006-3495(94)80890-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N., Pohle W., Mantsch H. H. Components of the carbonyl stretching band in the infrared spectra of hydrated 1,2-diacylglycerolipid bilayers: a reevaluation. Biophys J. 1994 Dec;67(6):2367–2375. doi: 10.1016/S0006-3495(94)80723-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Structures of the subgel phases of n-saturated diacyl phosphatidylcholine bilayers: FTIR spectroscopic studies of 13C = O and 2H labeled lipids. Biophys J. 1992 Jan;61(1):63–77. doi: 10.1016/S0006-3495(92)81816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Studies of mixed-chain diacyl phosphatidylcholines with highly asymmetric acyl chains: a Fourier transform infrared spectroscopic study of interfacial hydration and hydrocarbon chain packing in the mixed interdigitated gel phase. Biophys J. 1993 Nov;65(5):1866–1877. doi: 10.1016/S0006-3495(93)81251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Subgel phases of n-saturated diacylphosphatidylcholines: a Fourier-transform infrared spectroscopic study. Biochemistry. 1990 Aug 28;29(34):7946–7953. doi: 10.1021/bi00486a024. [DOI] [PubMed] [Google Scholar]
- Lewis R. N., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing iso-branched fatty acids. 1. Differential scanning calorimetric studies. Biochemistry. 1985 May 7;24(10):2431–2439. doi: 10.1021/bi00331a007. [DOI] [PubMed] [Google Scholar]
- Lohner K., Schuster A., Degovics G., Müller K., Laggner P. Thermal phase behaviour and structure of hydrated mixtures between dipalmitoyl- and dihexadecylphosphatidylcholine. Chem Phys Lipids. 1987 Jun;44(1):61–70. doi: 10.1016/0009-3084(87)90005-3. [DOI] [PubMed] [Google Scholar]
- Mannock D. A., Lewis R. N., McElhaney R. N., Akiyama M., Yamada H., Turner D. C., Gruner S. M. Effect of the chirality of the glycerol backbone on the bilayer and nonbilayer phase transitions in the diastereomers of di-dodecyl-beta-D-glucopyranosyl glycerol. Biophys J. 1992 Nov;63(5):1355–1368. doi: 10.1016/S0006-3495(92)81713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannock D. A., McElhaney R. N., Harper P. E., Gruner S. M. Differential scanning calorimetry and X-ray diffraction studies of the thermotropic phase behavior of the diastereomeric di-tetradecyl-beta-D-galactosyl glycerols and their mixture. Biophys J. 1994 Mar;66(3 Pt 1):734–740. doi: 10.1016/s0006-3495(94)80849-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch H. H., Madec C., Lewis R. N., McElhaney R. N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing iso-branched fatty acids. 2. Infrared and 31P NMR spectroscopic studies. Biochemistry. 1985 May 7;24(10):2440–2446. doi: 10.1021/bi00331a008. [DOI] [PubMed] [Google Scholar]
- Mendelsohn R., Liang G. L., Strauss H. L., Snyder R. G. IR spectroscopic determination of gel state miscibility in long-chain phosphatidylcholine mixtures. Biophys J. 1995 Nov;69(5):1987–1998. doi: 10.1016/S0006-3495(95)80069-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushayakarara E. C., Wong P. T., Mantsch H. H. Detection by high pressure infrared spectrometry of hydrogen-bonding between water and triacetyl glycerol. Biochem Biophys Res Commun. 1986 Jan 14;134(1):140–145. doi: 10.1016/0006-291x(86)90538-3. [DOI] [PubMed] [Google Scholar]
- Pearson R. H., Pascher I. The molecular structure of lecithin dihydrate. Nature. 1979 Oct 11;281(5731):499–501. doi: 10.1038/281499a0. [DOI] [PubMed] [Google Scholar]
- Raheja R. K., Kaur C., Singh A., Bhatia I. S. New colorimetric method for the quantitative estimation of phospholipids without acid digestion. J Lipid Res. 1973 Nov;14(6):695–697. [PubMed] [Google Scholar]
- Ruocco M. J., Siminovitch D. J., Griffin R. G. Comparative study of the gel phases of ether- and ester-linked phosphatidylcholines. Biochemistry. 1985 May 7;24(10):2406–2411. doi: 10.1021/bi00331a003. [DOI] [PubMed] [Google Scholar]
- Seddon J. M., Cevc G., Kaye R. D., Marsh D. X-ray diffraction study of the polymorphism of hydrated diacyl- and dialkylphosphatidylethanolamines. Biochemistry. 1984 Jun 5;23(12):2634–2644. doi: 10.1021/bi00307a015. [DOI] [PubMed] [Google Scholar]
- Sen A., Hui S. W., Mannock D. A., Lewis R. N., McElhaney R. N. Physical properties of glycosyl diacylglycerols. 2. X-ray diffraction studies of a homologous series of 1,2-Di-O-acyl-3-O-(alpha-D-glucopyranosyl)-sn-glycerols. Biochemistry. 1990 Aug 28;29(34):7799–7804. doi: 10.1021/bi00486a004. [DOI] [PubMed] [Google Scholar]
- Serrallach E. N., Dijkman R., de Haas G. H., Shipley G. G. Structure and thermotropic properties of 1,3-dipalmitoyl-glycero-2-phosphocholine. J Mol Biol. 1983 Oct 15;170(1):155–174. doi: 10.1016/s0022-2836(83)80231-9. [DOI] [PubMed] [Google Scholar]
- Siminovitch D. J., Wong P. T., Mantsch H. H. High pressure infrared spectroscopy of lipid bilayers: new tests for interdigitation. Biochim Biophys Acta. 1987 Jun 12;900(1):163–167. doi: 10.1016/0005-2736(87)90289-6. [DOI] [PubMed] [Google Scholar]
- Siminovitch D. J., Wong P. T., Mantsch H. H. High-pressure infrared spectroscopy of ether- and ester-linked phosphatidylcholine aqueous dispersions. Biophys J. 1987 Mar;51(3):465–473. doi: 10.1016/S0006-3495(87)83368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon S. A., McIntosh T. J. Interdigitated hydrocarbon chain packing causes the biphasic transition behavior in lipid/alcohol suspensions. Biochim Biophys Acta. 1984 Jun 13;773(1):169–172. doi: 10.1016/0005-2736(84)90562-5. [DOI] [PubMed] [Google Scholar]
- Singer M. A., Jain M. K., Sable H. Z., Pownall H. J., Mantulin W. W., Lister M. D., Hancock A. J. The properties of membranes formed from cyclopentanoid analogues of phosphatidylcholine. Biochim Biophys Acta. 1983 Jun 10;731(2):373–377. doi: 10.1016/0005-2736(83)90030-5. [DOI] [PubMed] [Google Scholar]
- Stümpel J., Nicksch A., Eibl H. Calorimetric studies on saturated mixed-chain lecithin-water systems. Nonequivalence of acyl chains in the thermotropic phase transition. Biochemistry. 1981 Feb 3;20(3):662–665. doi: 10.1021/bi00506a033. [DOI] [PubMed] [Google Scholar]
- Tristram-Nagle S., Wiener M. C., Yang C. P., Nagle J. F. Kinetics of the subtransition in dipalmitoylphosphatidylcholine. Biochemistry. 1987 Jul 14;26(14):4288–4294. doi: 10.1021/bi00388a016. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. A., Tirrell D. A., Turek A. B., McIntosh T. J. Tris buffer causes acyl chain interdigitation in phosphatidylglycerol. Biochim Biophys Acta. 1987 Dec 11;905(2):447–453. doi: 10.1016/0005-2736(87)90474-3. [DOI] [PubMed] [Google Scholar]


