Abstract
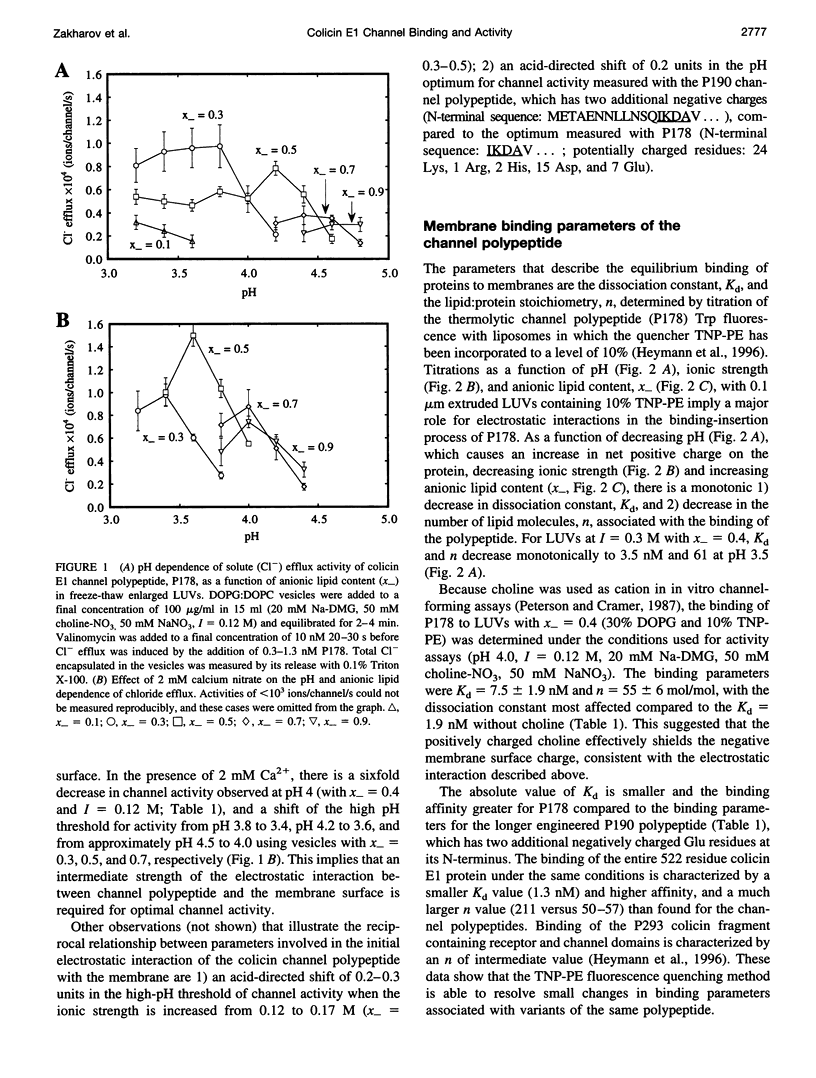
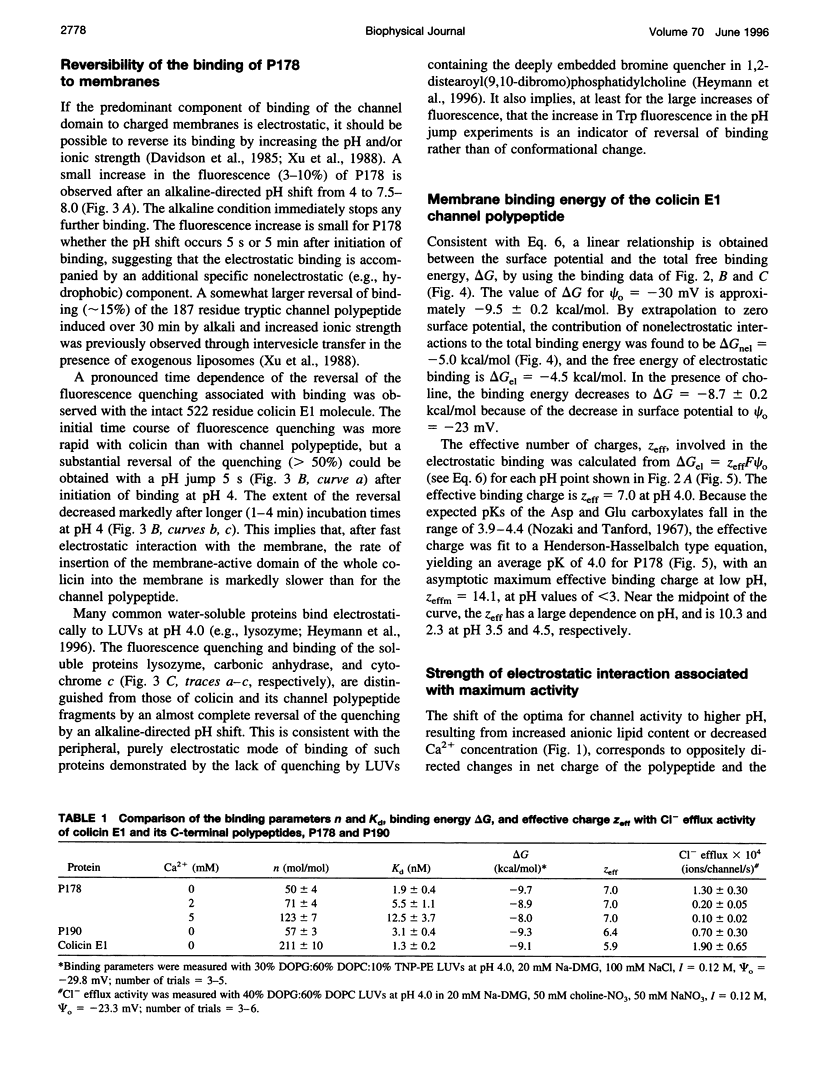
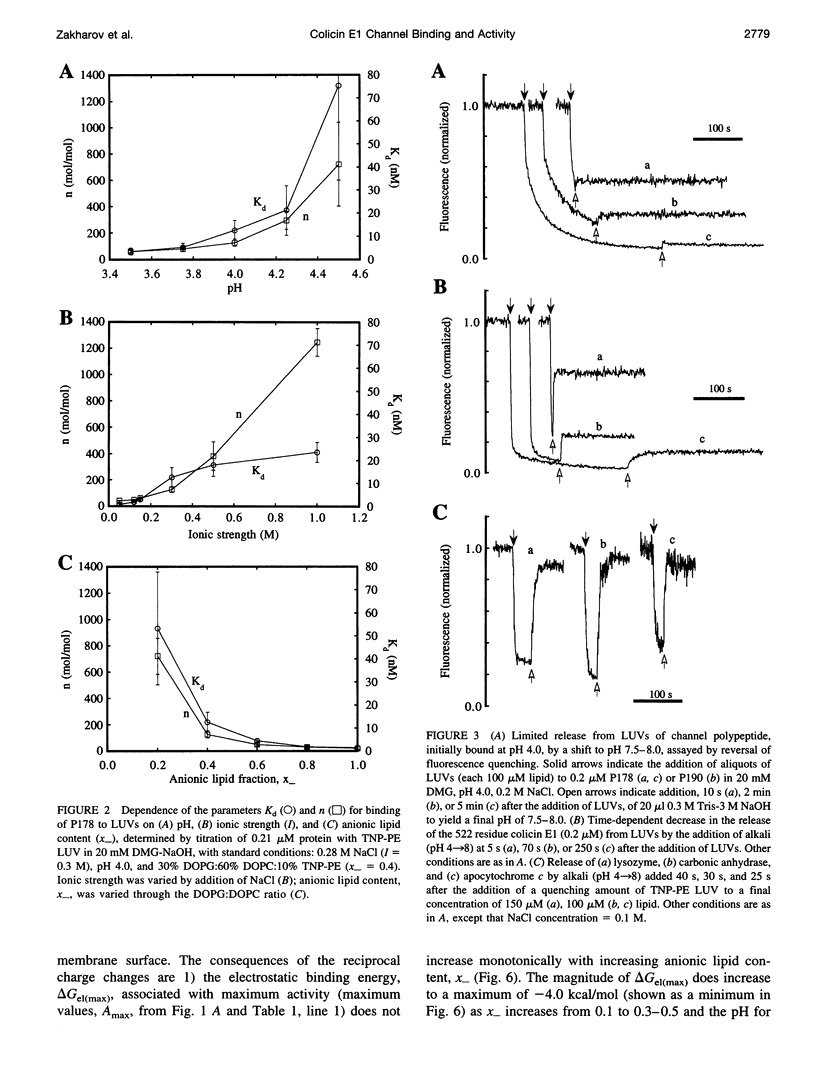
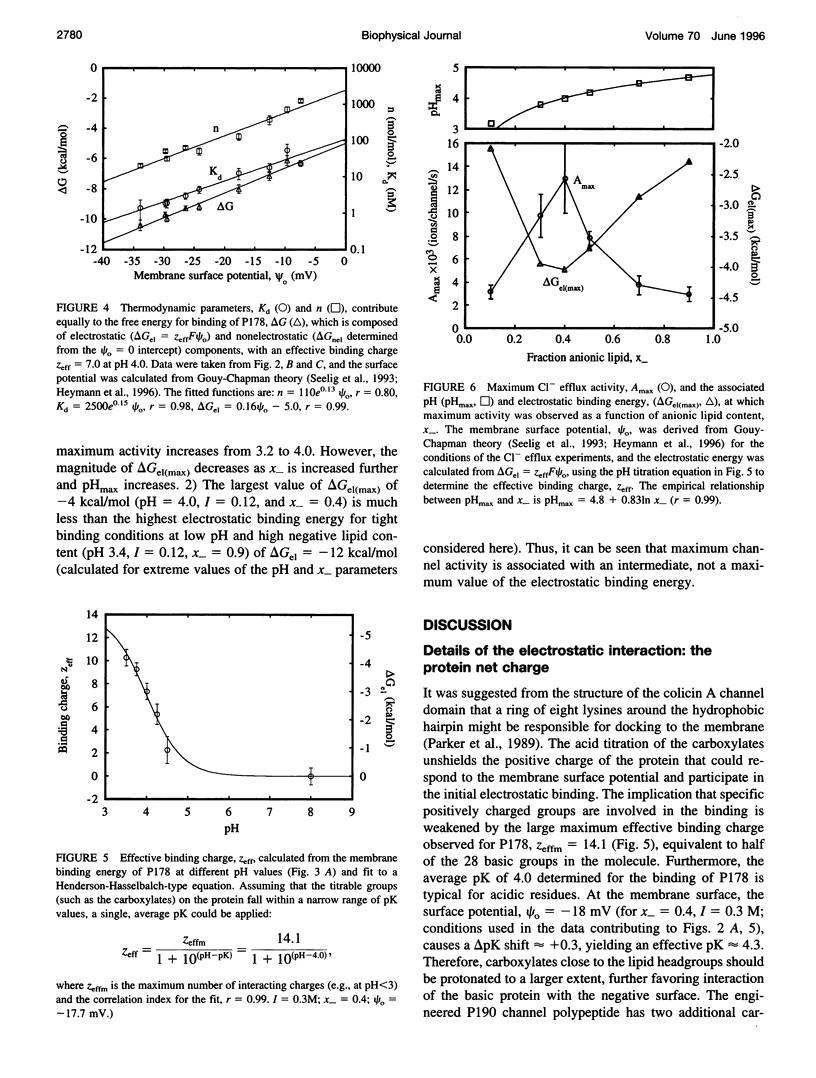
In vitro channel activity of the C-terminal colicin E1 channel polypeptide under conditions of variable electrostatic interaction with synthetic lipid membranes showed distinct maxima with respect to pH and membrane surface potential. The membrane binding energy was determined from fluorescence quenching of the intrinsic tryptophans of the channel polypeptide by liposomes containing N-trinitrophenyl-phosphatidylethanolamine. Maximum in vitro colicin channel activity correlated with an intermediate magnitude of the electrostatic interaction. For conditions associated with maximum activity (40% anionic lipid, I = 0.12 M, pH 4.0), the free energy of binding was delta G approximately -9 kcal/mol, with nonelectrostatic and electrostatic components, delta Gnel approximately -5 kcal/mol and delta Gel approximately -4 kcal/mol, and an effective binding charge of +7 at pH 4.0. Binding of the channel polypeptide to negative membranes at pH 8 is minimal, whereas initial binding at pH 4 followed by a shift to pH 8 causes only 3-10% reversal of binding, implying that it is kinetically trapped, probably by a hydrophobic interaction. It was inferred that membrane binding and insertion involves an initial electrostatic interaction responsible for concentration and binding to the membrane surface. This is followed by insertion into the bilayer driven by hydrophobic forces, which are countered in the case of excessive electrostatic binding.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop L. J., Bjes E. S., Davidson V. L., Cramer W. A. Localization of the immunity protein-reactive domain in unmodified and chemically modified COOH-terminal peptides of colicin E1. J Bacteriol. 1985 Oct;164(1):237–244. doi: 10.1128/jb.164.1.237-244.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunden K. R., Cramer W. A., Cohen F. S. Purification of a small receptor-binding peptide from the central region of the colicin E1 molecule. J Biol Chem. 1984 Jan 10;259(1):190–196. [PubMed] [Google Scholar]
- Bullock J. O., Cohen F. S., Dankert J. R., Cramer W. A. Comparison of the macroscopic and single channel conductance properties of colicin E1 and its COOH-terminal tryptic peptide. J Biol Chem. 1983 Aug 25;258(16):9908–9912. [PubMed] [Google Scholar]
- Cleveland M. V., Slatin S., Finkelstein A., Levinthal C. Structure-function relationships for a voltage-dependent ion channel: properties of COOH-terminal fragments of colicin E1. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3706–3710. doi: 10.1073/pnas.80.12.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer W. A., Zhang Y. L., Schendel S., Merrill A. R., Song H. Y., Stauffacher C. V., Cohen F. S. Dynamic properties of the colicin E1 ion channel. FEMS Microbiol Immunol. 1992 Sep;5(1-3):71–81. doi: 10.1111/j.1574-6968.1992.tb05889.x. [DOI] [PubMed] [Google Scholar]
- Dankert J. R., Uratani Y., Grabau C., Cramer W. A., Hermodson M. On a domain structure of colicin E1. A COOH-terminal peptide fragment active in membrane depolarization. J Biol Chem. 1982 Apr 10;257(7):3857–3863. [PubMed] [Google Scholar]
- Dankert J., Hammond S. M., Cramer W. A. Reversal by trypsin of the inhibition of active transport by colicin E1. J Bacteriol. 1980 Aug;143(2):594–602. doi: 10.1128/jb.143.2.594-602.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson V. L., Brunden K. R., Cramer W. A. Acidic pH requirement for insertion of colicin E1 into artificial membrane vesicles: relevance to the mechanism of action of colicins and certain toxins. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1386–1390. doi: 10.1073/pnas.82.5.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins P. A., Song H. Y., Cramer W. A., Stauffacher C. V. Crystallization and characterization of colicin E1 channel-forming polypeptides. Proteins. 1994 Jun;19(2):150–157. doi: 10.1002/prot.340190208. [DOI] [PubMed] [Google Scholar]
- Engelman D. M., Steitz T. A. The spontaneous insertion of proteins into and across membranes: the helical hairpin hypothesis. Cell. 1981 Feb;23(2):411–422. doi: 10.1016/0092-8674(81)90136-7. [DOI] [PubMed] [Google Scholar]
- González-Mañas J. M., Lakey J. H., Pattus F. Interaction of the colicin-A pore-forming domain with negatively charged phospholipids. Eur J Biochem. 1993 Feb 1;211(3):625–633. doi: 10.1111/j.1432-1033.1993.tb17590.x. [DOI] [PubMed] [Google Scholar]
- Gould J. M., Cramer W. A. Studies on the depolarization of the Escherichia coli cell membrane by colicin E1. J Biol Chem. 1977 Aug 10;252(15):5491–5497. [PubMed] [Google Scholar]
- Guihard G., Bénédetti H., Besnard M., Letellier L. Phosphate efflux through the channels formed by colicins and phage T5 in Escherichia coli cells is responsible for the fall in cytoplasmic ATP. J Biol Chem. 1993 Aug 25;268(24):17775–17780. [PubMed] [Google Scholar]
- Heymann J. B., Zakharov S. D., Zhang Y. L., Cramer W. A. Characterization of electrostatic and nonelectrostatic components of protein--membrane binding interactions. Biochemistry. 1996 Feb 27;35(8):2717–2725. doi: 10.1021/bi951535l. [DOI] [PubMed] [Google Scholar]
- Hille J. D., Donné-Op den Kelder G. M., Sauve P., de Haas G. H., Egmond M. R. Physicochemical studies on the interaction of pancreatic phospholipase A2 with a micellar substrate analogue. Biochemistry. 1981 Jul 7;20(14):4068–4073. doi: 10.1021/bi00517a019. [DOI] [PubMed] [Google Scholar]
- Janin J., Chothia C. Role of hydrophobicity in the binding of coenzymes. Appendix. Translational and rotational contribution to the free energy of dissociation. Biochemistry. 1978 Jul 25;17(15):2943–2948. doi: 10.1021/bi00608a001. [DOI] [PubMed] [Google Scholar]
- Jeanteur D., Pattus F., Timmins P. A. Membrane-bound form of the pore-forming domain of colicin A. A neutron scattering study. J Mol Biol. 1994 Jan 21;235(3):898–907. doi: 10.1006/jmbi.1994.1047. [DOI] [PubMed] [Google Scholar]
- Kayalar C., Düzgüneş N. Membrane action of colicin E1: detection by the release of carboxyfluorescein and calcein from liposomes. Biochim Biophys Acta. 1986 Aug 7;860(1):51–56. doi: 10.1016/0005-2736(86)90497-9. [DOI] [PubMed] [Google Scholar]
- Kopecky A. L., Copeland D. P., Lusk J. E. Viability of Escherichia coli treated with colicin K. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4631–4634. doi: 10.1073/pnas.72.11.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakey J. H., Duché D., González-Mañas J. M., Baty D., Pattus F. Fluorescence energy transfer distance measurements. The hydrophobic helical hairpin of colicin A in the membrane bound state. J Mol Biol. 1993 Apr 5;230(3):1055–1067. doi: 10.1006/jmbi.1993.1218. [DOI] [PubMed] [Google Scholar]
- Lakey J. H., González-Mañas J. M., van der Goot F. G., Pattus F. The membrane insertion of colicins. FEBS Lett. 1992 Jul 27;307(1):26–29. doi: 10.1016/0014-5793(92)80895-n. [DOI] [PubMed] [Google Scholar]
- Lakey J. H., Parker M. W., González-Mañas J. M., Duché D., Vriend G., Baty D., Pattus F. The role of electrostatic charge in the membrane insertion of colicin A. Calculation and mutation. Eur J Biochem. 1994 Feb 15;220(1):155–163. doi: 10.1111/j.1432-1033.1994.tb18610.x. [DOI] [PubMed] [Google Scholar]
- Massotte D., Yamamoto M., Scianimanico S., Sorokine O., van Dorsselaer A., Nakatani Y., Ourisson G., Pattus F. Structure of the membrane-bound form of the pore-forming domain of colicin A: a partial proteolysis and mass spectrometry study. Biochemistry. 1993 Dec 21;32(50):13787–13794. doi: 10.1021/bi00213a006. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- Mel S. F., Stroud R. M. Colicin Ia inserts into negatively charged membranes at low pH with a tertiary but little secondary structural change. Biochemistry. 1993 Mar 2;32(8):2082–2089. doi: 10.1021/bi00059a028. [DOI] [PubMed] [Google Scholar]
- Merrill A. R., Cramer W. A. Identification of a voltage-responsive segment of the potential-gated colicin E1 ion channel. Biochemistry. 1990 Sep 18;29(37):8529–8534. doi: 10.1021/bi00489a004. [DOI] [PubMed] [Google Scholar]
- Palmer L. R., Merrill A. R. Mapping the membrane topology of the closed state of the colicin E1 channel. J Biol Chem. 1994 Feb 11;269(6):4187–4193. [PubMed] [Google Scholar]
- Parker M. W., Pattus F., Tucker A. D., Tsernoglou D. Structure of the membrane-pore-forming fragment of colicin A. Nature. 1989 Jan 5;337(6202):93–96. doi: 10.1038/337093a0. [DOI] [PubMed] [Google Scholar]
- Parker M. W., Postma J. P., Pattus F., Tucker A. D., Tsernoglou D. Refined structure of the pore-forming domain of colicin A at 2.4 A resolution. J Mol Biol. 1992 Apr 5;224(3):639–657. doi: 10.1016/0022-2836(92)90550-4. [DOI] [PubMed] [Google Scholar]
- Parker M. W., Tucker A. D., Tsernoglou D., Pattus F. Insights into membrane insertion based on studies of colicins. Trends Biochem Sci. 1990 Apr;15(4):126–129. doi: 10.1016/0968-0004(90)90205-p. [DOI] [PubMed] [Google Scholar]
- Peterson A. A., Cramer W. A. Voltage-dependent, monomeric channel activity of colicin E1 in artificial membrane vesicles. J Membr Biol. 1987;99(3):197–204. doi: 10.1007/BF01995700. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Phillips S. K., Cramer W. A. Properties of the fluorescence probe response associated with the transmission mechanism of colicin E1. Biochemistry. 1973 Mar 13;12(6):1170–1176. doi: 10.1021/bi00730a024. [DOI] [PubMed] [Google Scholar]
- Rath P., Bousché O., Merrill A. R., Cramer W. A., Rothschild K. J. Fourier transform infrared evidence for a predominantly alpha-helical structure of the membrane bound channel forming COOH-terminal peptide of colicin E1. Biophys J. 1991 Mar;59(3):516–522. doi: 10.1016/S0006-3495(91)82268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel S. L., Cramer W. A. On the nature of the unfolded intermediate in the in vitro transition of the colicin E1 channel domain from the aqueous to the membrane phase. Protein Sci. 1994 Dec;3(12):2272–2279. doi: 10.1002/pro.5560031212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig J., Nebel S., Ganz P., Bruns C. Electrostatic and nonpolar peptide-membrane interactions. Lipid binding and functional properties of somatostatin analogues of charge z = +1 to z = +3. Biochemistry. 1993 Sep 21;32(37):9714–9721. doi: 10.1021/bi00088a025. [DOI] [PubMed] [Google Scholar]
- Shibuya I. Metabolic regulations and biological functions of phospholipids in Escherichia coli. Prog Lipid Res. 1992;31(3):245–299. doi: 10.1016/0163-7827(92)90010-g. [DOI] [PubMed] [Google Scholar]
- Shin Y. K., Levinthal C., Levinthal F., Hubbell W. L. Colicin E1 binding to membranes: time-resolved studies of spin-labeled mutants. Science. 1993 Feb 12;259(5097):960–963. doi: 10.1126/science.8382373. [DOI] [PubMed] [Google Scholar]
- Slatin S. L., Qiu X. Q., Jakes K. S., Finkelstein A. Identification of a translocated protein segment in a voltage-dependent channel. Nature. 1994 Sep 8;371(6493):158–161. doi: 10.1038/371158a0. [DOI] [PubMed] [Google Scholar]
- Song H. Y., Cohen F. S., Cramer W. A. Membrane topography of ColE1 gene products: the hydrophobic anchor of the colicin E1 channel is a helical hairpin. J Bacteriol. 1991 May;173(9):2927–2934. doi: 10.1128/jb.173.9.2927-2934.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. E. The tol gene products and the import of macromolecules into Escherichia coli. Mol Microbiol. 1991 May;5(5):1005–1011. doi: 10.1111/j.1365-2958.1991.tb01873.x. [DOI] [PubMed] [Google Scholar]
- Xu S., Cramer W. A., Peterson A. A., Hermodson M., Montecucco C. Dynamic properties of membrane proteins: reversible insertion into membrane vesicles of a colicin E1 channel-forming peptide. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7531–7535. doi: 10.1073/pnas.85.20.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. L., Cramer W. A. Constraints imposed by protease accessibility on the trans-membrane and surface topography of the colicin E1 ion channel. Protein Sci. 1992 Dec;1(12):1666–1676. doi: 10.1002/pro.5560011215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Goot F. G., Didat N., Pattus F., Dowhan W., Letellier L. Role of acidic lipids in the translocation and channel activity of colicins A and N in Escherichia coli cells. Eur J Biochem. 1993 Apr 1;213(1):217–221. doi: 10.1111/j.1432-1033.1993.tb17751.x. [DOI] [PubMed] [Google Scholar]



