Abstract
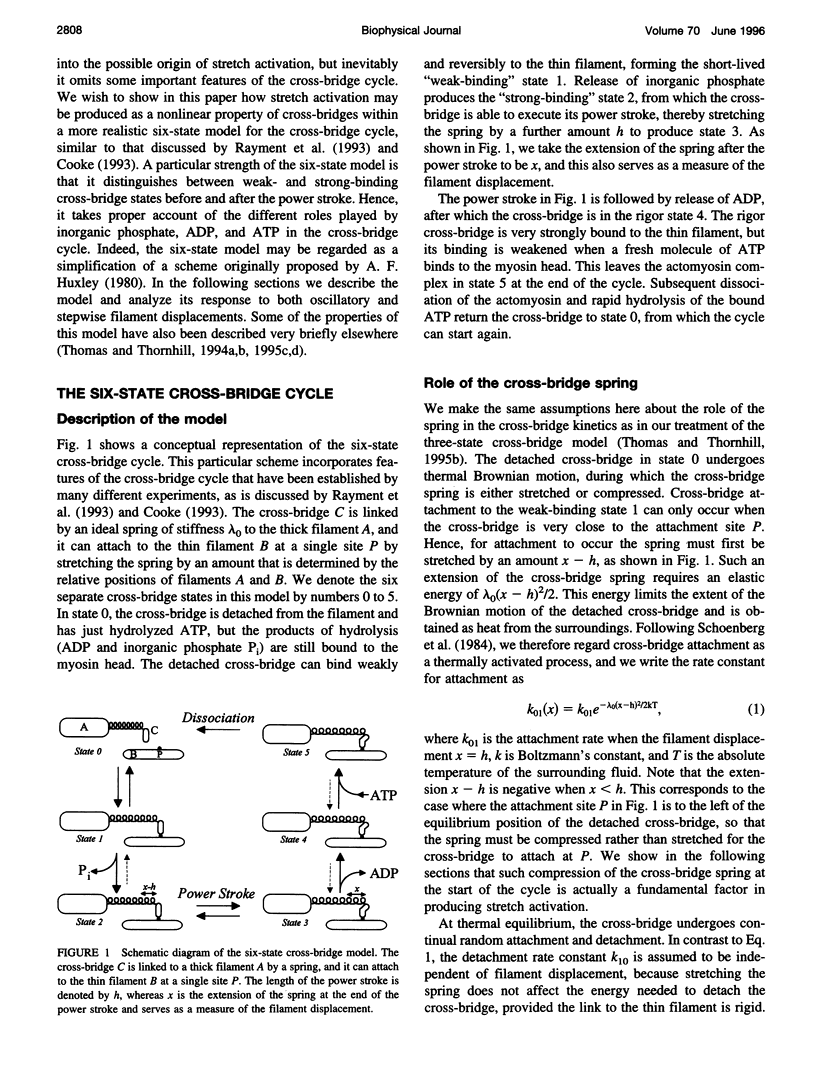
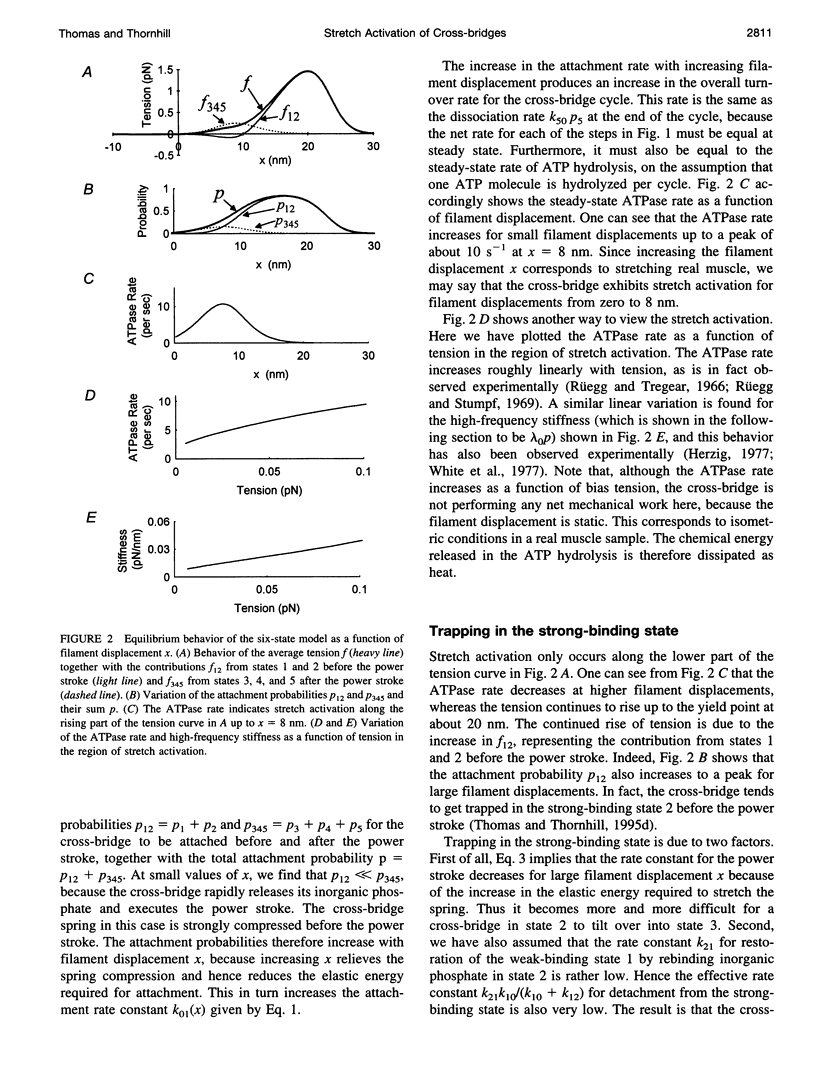
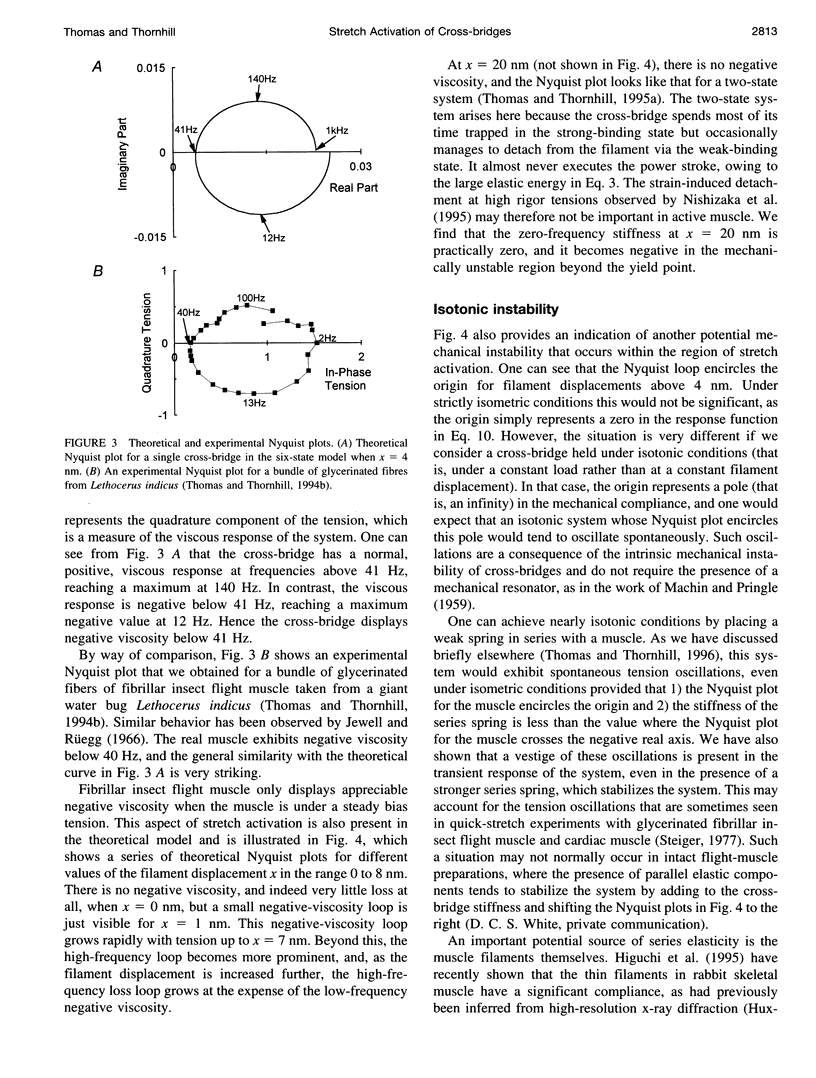
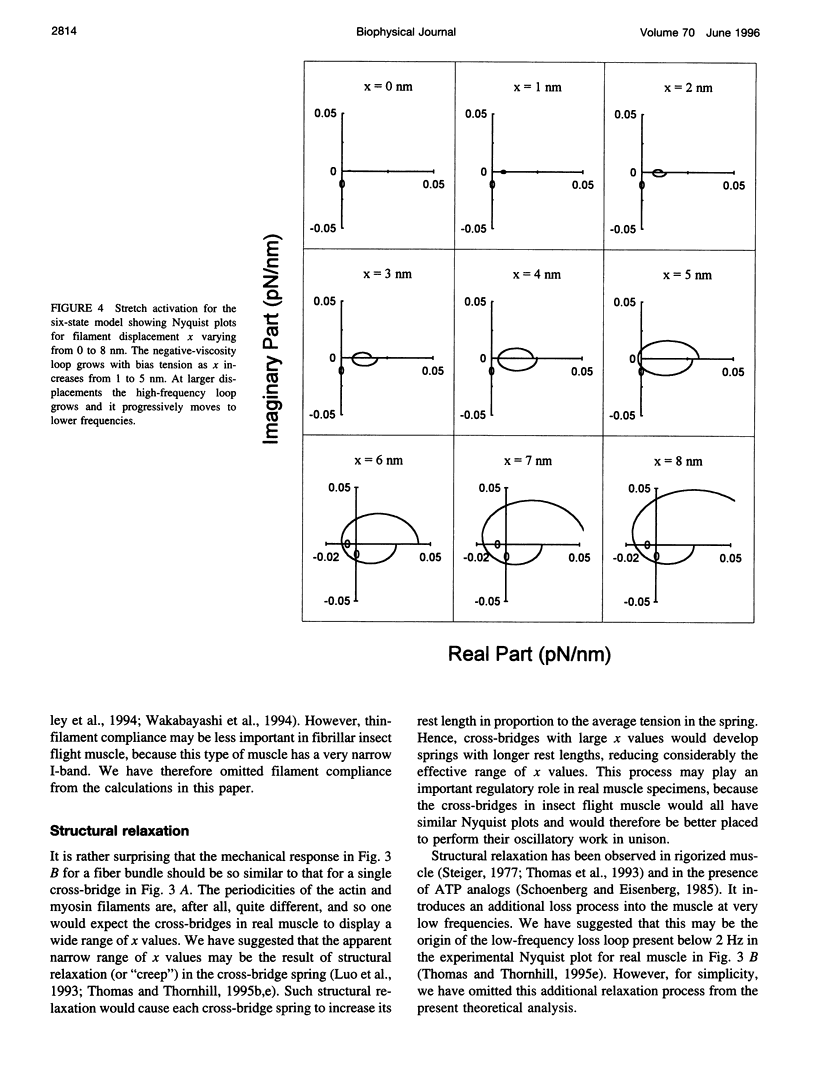
When active insect fibrillar flight muscle is stretched, its ATPase rate increases and it develops "negative viscosity," which allows it to perform oscillatory work. We use a six-state model for the cross-bridge cycle to show that such "stretch activation" may arise naturally as a nonlinear property of a cross-bridge interacting with a single attachment site on a thin filament. Attachment is treated as a thermally activated process in which elastic energy must be supplied to stretch or compress the cross-bridge spring. We find that stretch activation occurs at filament displacements where, before the power stroke, the spring is initially in compression rather than in tension. In that case, pulling the filaments relieves the initial compression and reduces the elastic energy required for attachment. The result is that the attachment rate is enhanced by stretching. The model also displays the "delayed tension" effect observed in length-step experiments. When the muscle is stretched suddenly, the power stroke responds very quickly, but there is a time lag before dissociation at the end of the cycle catches up with the increased attachment rate. This lag is responsible for the delayed tension and hence also for the negative viscosity.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen Y. D., Brenner B. On the regeneration of the actin-myosin power stroke in contracting muscle. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5148–5152. doi: 10.1073/pnas.90.11.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. The structure of a molecular motor. Curr Biol. 1993 Sep 1;3(9):590–592. doi: 10.1016/0960-9822(93)90005-9. [DOI] [PubMed] [Google Scholar]
- Dantzig J. A., Goldman Y. E., Millar N. C., Lacktis J., Homsher E. Reversal of the cross-bridge force-generating transition by photogeneration of phosphate in rabbit psoas muscle fibres. J Physiol. 1992;451:247–278. doi: 10.1113/jphysiol.1992.sp019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshcherevskii V. I. A kinetic theory of striated muscle contraction. Biorheology. 1971 Jan;7(3):147–170. [PubMed] [Google Scholar]
- Finer J. T., Simmons R. M., Spudich J. A. Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature. 1994 Mar 10;368(6467):113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- HUXLEY A. F. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- Higuchi H., Yanagida T., Goldman Y. E. Compliance of thin filaments in skinned fibers of rabbit skeletal muscle. Biophys J. 1995 Sep;69(3):1000–1010. doi: 10.1016/S0006-3495(95)79975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuti K., Kagawa K., Yamada K. The initial contraction of skinned muscle fibers on photorelease of ATP in the presence of ADP. Jpn J Physiol. 1994;44(6):675–691. doi: 10.2170/jjphysiol.44.675. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Stewart A., Sosa H., Irving T. X-ray diffraction measurements of the extensibility of actin and myosin filaments in contracting muscle. Biophys J. 1994 Dec;67(6):2411–2421. doi: 10.1016/S0006-3495(94)80728-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Ishijima A., Harada Y., Kojima H., Funatsu T., Higuchi H., Yanagida T. Single-molecule analysis of the actomyosin motor using nano-manipulation. Biochem Biophys Res Commun. 1994 Mar 15;199(2):1057–1063. doi: 10.1006/bbrc.1994.1336. [DOI] [PubMed] [Google Scholar]
- Julian F. J. Activation in a skeletal muscle contraction model with a modification for insect fibrillar muscle. Biophys J. 1969 Apr;9(4):547–570. doi: 10.1016/S0006-3495(69)86403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Brandt P. W. Sinusoidal analysis: a high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscles of rabbit, frog and crayfish. J Muscle Res Cell Motil. 1980 Sep;1(3):279–303. doi: 10.1007/BF00711932. [DOI] [PubMed] [Google Scholar]
- Murase M., Tanaka H., Nishiyama K., Shimizu H. A three-state model for oscillation in muscle: sinusoidal analysis. J Muscle Res Cell Motil. 1986 Feb;7(1):2–10. doi: 10.1007/BF01756196. [DOI] [PubMed] [Google Scholar]
- Nishizaka T., Miyata H., Yoshikawa H., Ishiwata S., Kinosita K., Jr Unbinding force of a single motor molecule of muscle measured using optical tweezers. Nature. 1995 Sep 21;377(6546):251–254. doi: 10.1038/377251a0. [DOI] [PubMed] [Google Scholar]
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993 Jul 2;261(5117):58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Rüegg J. C., Stumpf H. Activation of the myofibrillar ATPase activity by extension of glycerolextracted insect fibrillar muscle. Pflugers Arch. 1969;305(1):34–46. doi: 10.1007/BF00586394. [DOI] [PubMed] [Google Scholar]
- Rüegg J. C., Tregear R. T. Mechanical factors affecting the ATPase activity of glycerol-extracted insect fibrillar flight muscle. Proc R Soc Lond B Biol Sci. 1966 Oct 11;165(1001):497–512. doi: 10.1098/rspb.1966.0080. [DOI] [PubMed] [Google Scholar]
- Schoenberg M., Brenner B., Chalovich J. M., Greene L. E., Eisenberg E. Cross-bridge attachment in relaxed muscle. Adv Exp Med Biol. 1984;170:269–284. doi: 10.1007/978-1-4684-4703-3_24. [DOI] [PubMed] [Google Scholar]
- Schoenberg M., Eisenberg E. Muscle cross-bridge kinetics in rigor and in the presence of ATP analogues. Biophys J. 1985 Dec;48(6):863–871. doi: 10.1016/S0006-3495(85)83847-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawnych M. P., Seow C. Y., Huxley A. F., Ford L. E. A program for developing a comprehensive mathematical description of the crossbridge cycle of muscle. Biophys J. 1994 Oct;67(4):1669–1677. doi: 10.1016/S0006-3495(94)80639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire J. M. Muscle filament lattices and stretch-activation: the match-mismatch model reassessed. J Muscle Res Cell Motil. 1992 Apr;13(2):183–189. doi: 10.1007/BF01874155. [DOI] [PubMed] [Google Scholar]
- Thirlwell H., Corrie J. E., Reid G. P., Trentham D. R., Ferenczi M. A. Kinetics of relaxation from rigor of permeabilized fast-twitch skeletal fibers from the rabbit using a novel caged ATP and apyrase. Biophys J. 1994 Dec;67(6):2436–2447. doi: 10.1016/S0006-3495(94)80730-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas N., Thornhill R. A. A theory of tension fluctuations due to muscle cross-bridges. Proc Biol Sci. 1995 Mar 22;259(1356):235–242. doi: 10.1098/rspb.1995.0035. [DOI] [PubMed] [Google Scholar]
- Thorson J., White D. C. Distributed representations for actin-myosin interaction in the oscillatory contraction of muscle. Biophys J. 1969 Mar;9(3):360–390. doi: 10.1016/S0006-3495(69)86392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorson J., White D. C. Role of cross-bridge distortion in the small-signal mechanical dynamics of insect and rabbit striated muscle. J Physiol. 1983 Oct;343:59–84. doi: 10.1113/jphysiol.1983.sp014881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K., Sugimoto Y., Tanaka H., Ueno Y., Takezawa Y., Amemiya Y. X-ray diffraction evidence for the extensibility of actin and myosin filaments during muscle contraction. Biophys J. 1994 Dec;67(6):2422–2435. doi: 10.1016/S0006-3495(94)80729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C., Thorson J. Phosphate starvation and the nonlinear dynamics of insect fibrillar flight muscle. J Gen Physiol. 1972 Sep;60(3):307–336. doi: 10.1085/jgp.60.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]


