Abstract
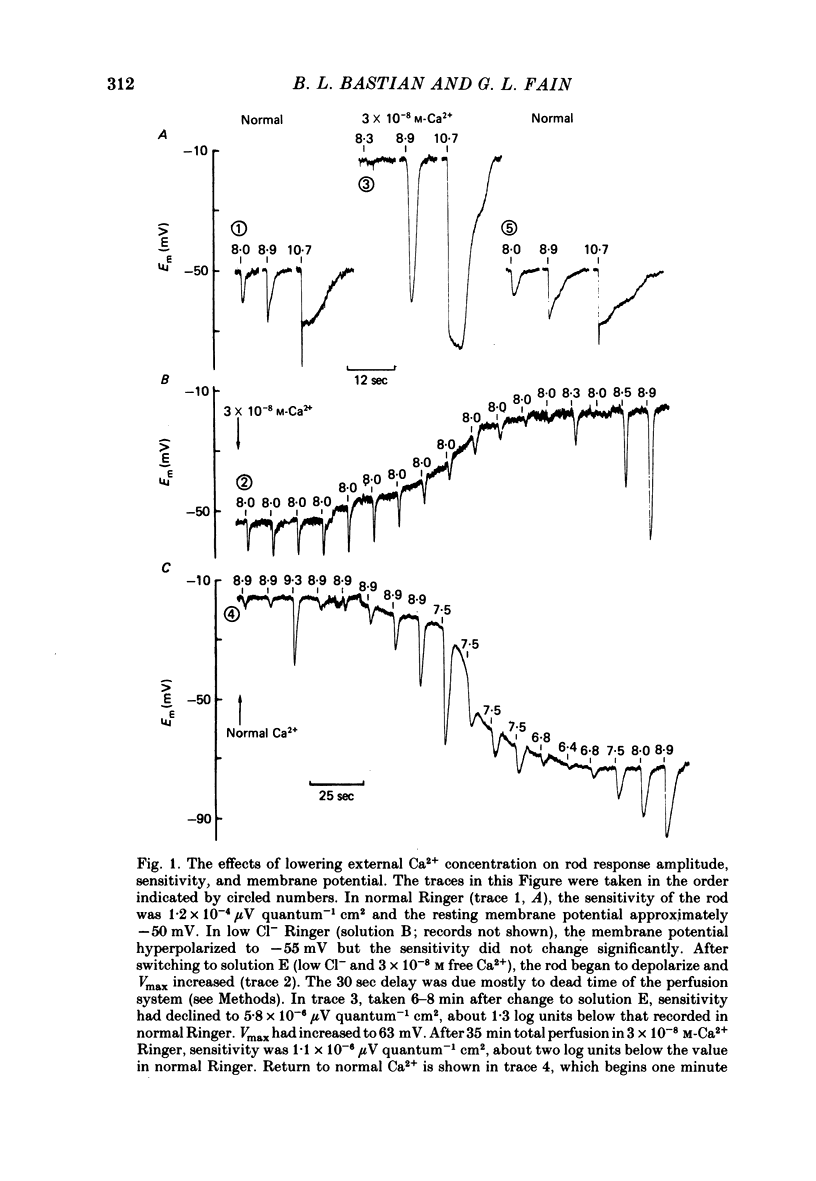
1. We have examined the effects of decreases in extracellular Ca2+ concentration on the intracellularly recorded light responses of rods from the toad, Bufo marinus. In agreement with previous results (Brown & Pinto, 1974; Lipton, Ostroy & Dowling, 1977), Ca2+ concentrations below 10-6 M produced a depolarization of rod resting membrane potential of approximately 30-40 mV and a corresponding increase in the maximum amplitude of the rod's light responses, so that saturating flashes in normal and low Ca2+ Ringer produced hyperpolarizations to approximately the same membrane potential.
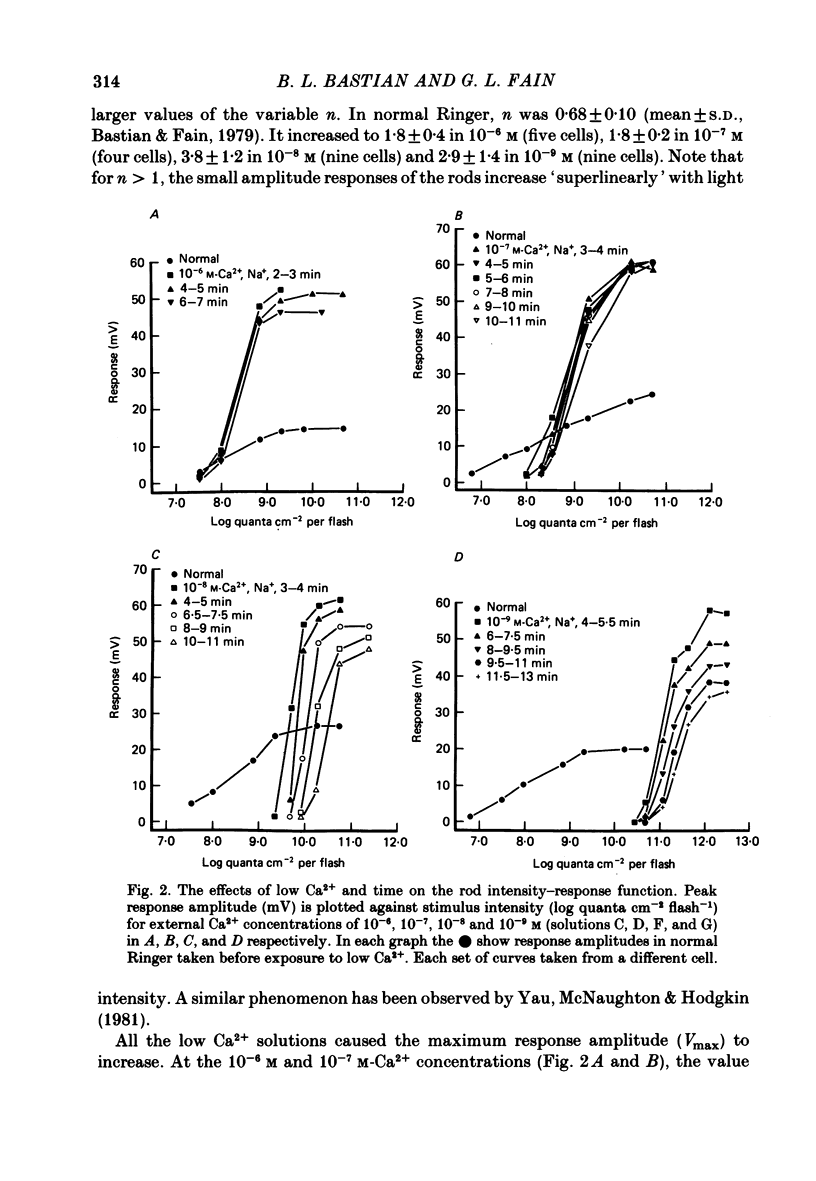
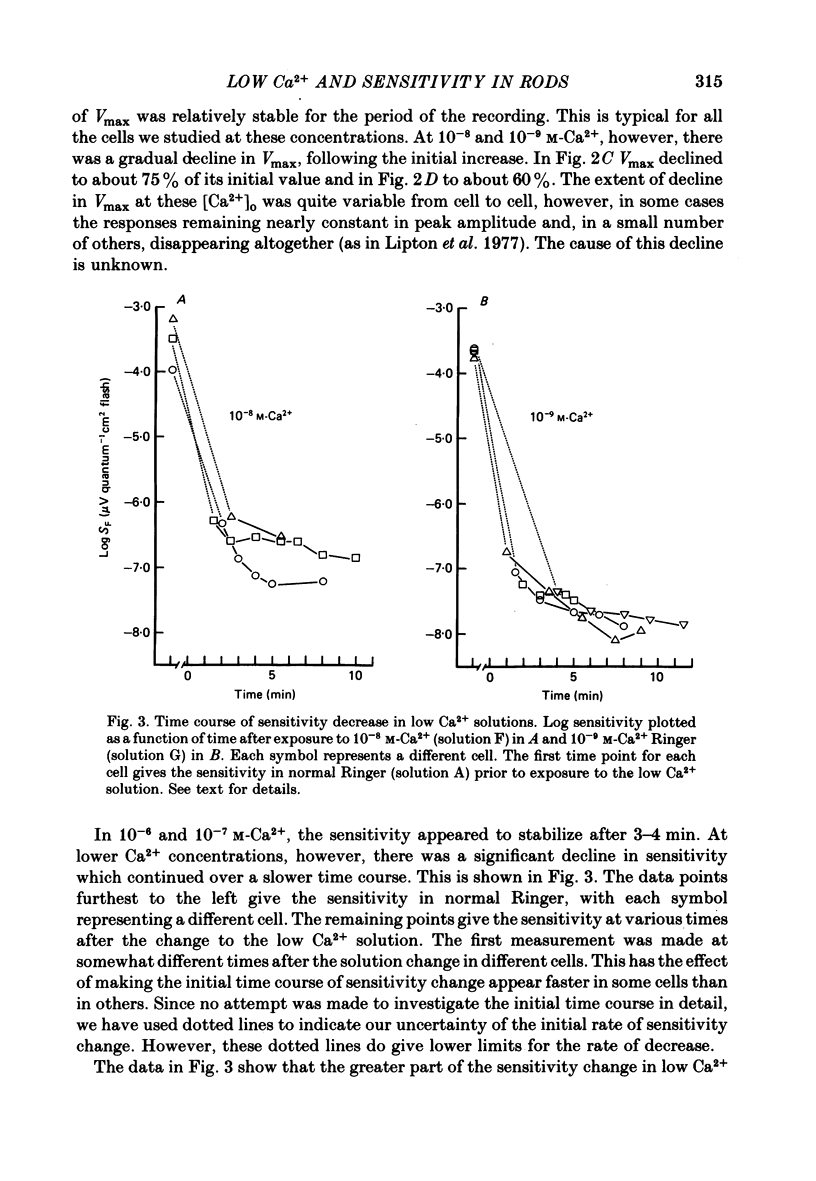
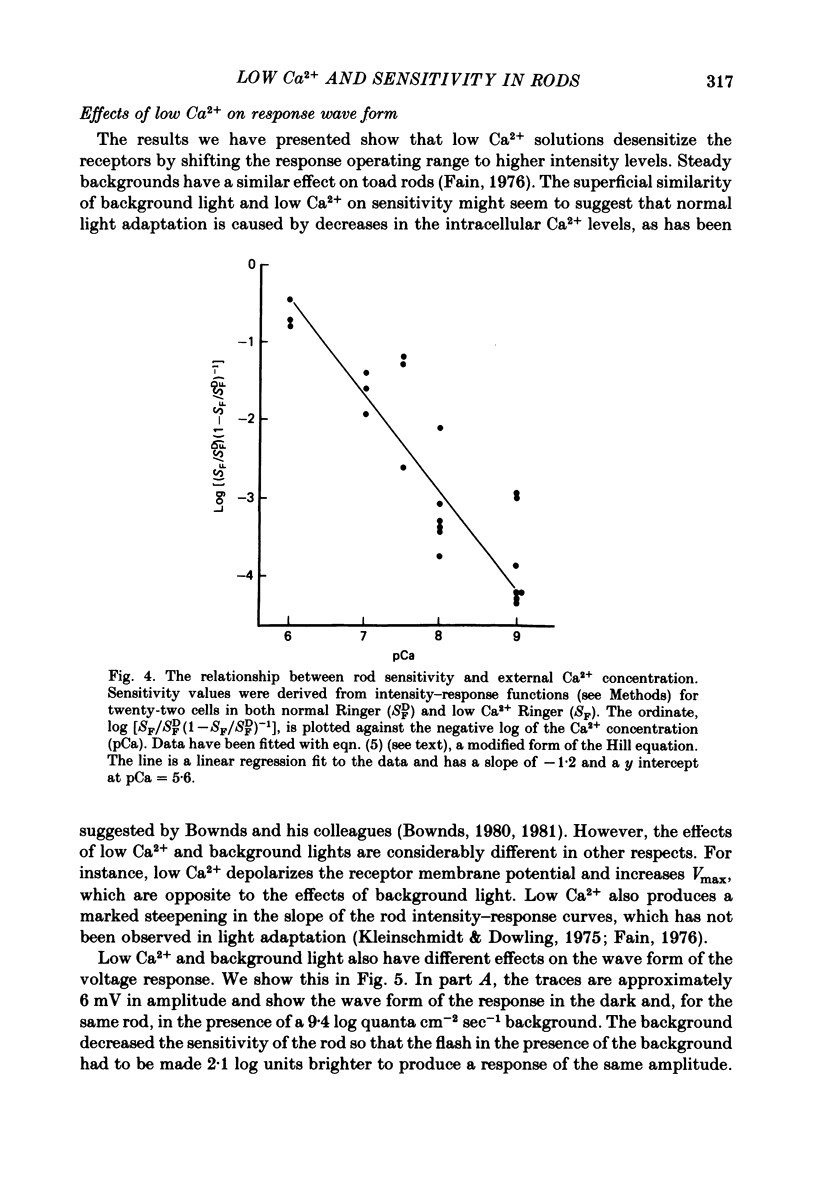
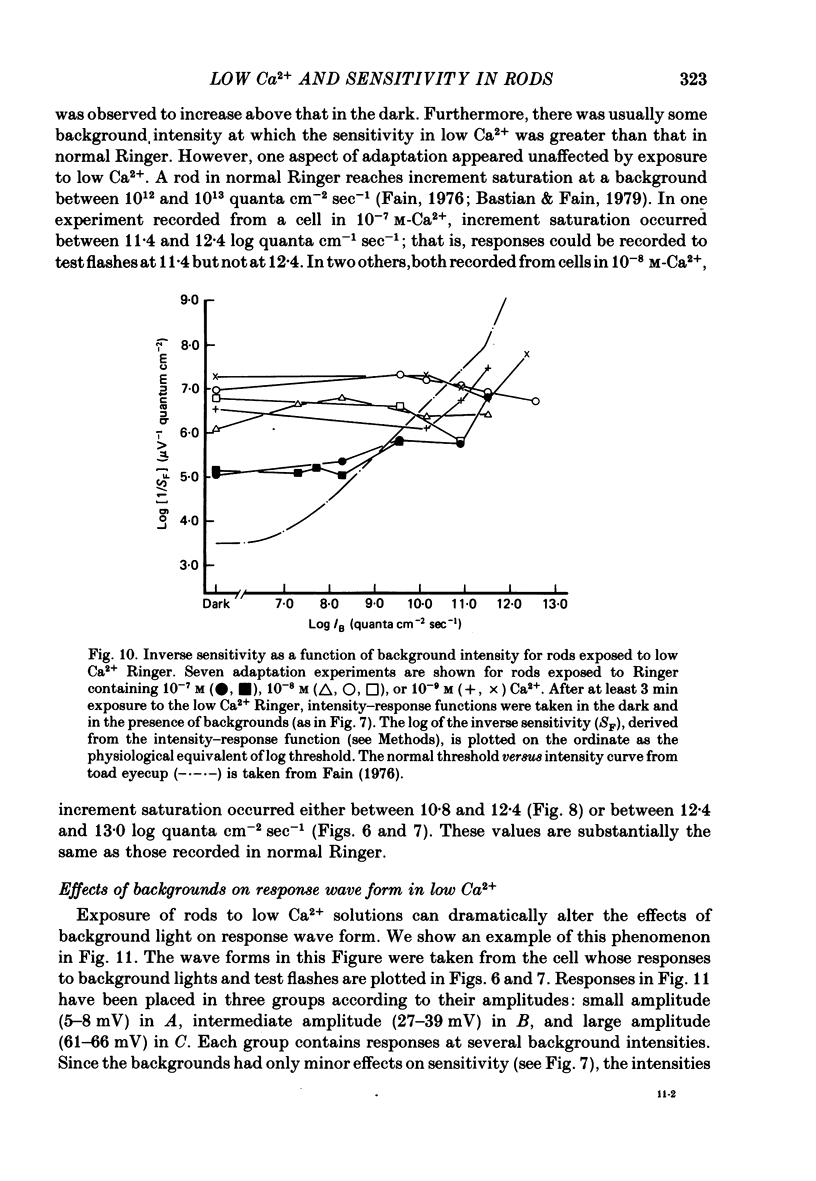
2. The rod's sensitivity was reduced in low Ca2+ Ringer by an amount dependent upon the extracellular Ca2+ concentration. At 10-6 M-Ca2+, sensitivity was approximately 0·6 log units below normal. Thereafter, it dropped nearly linearly with [Ca2+]o to a value approximately 4·0 log units below normal at 10-9 M-Ca2+. Most of the decline occurred within 1-2 min after the solution change as the membrane potential depolarized, but sensitivity continued to fall slowly with time at the lowest Ca2+ concentrations. Exposure to low Ca2+ solutions altered the kinetics of the receptor response to brief flashes, delaying response onset and time-to-peak but affecting the time course of decay very little.
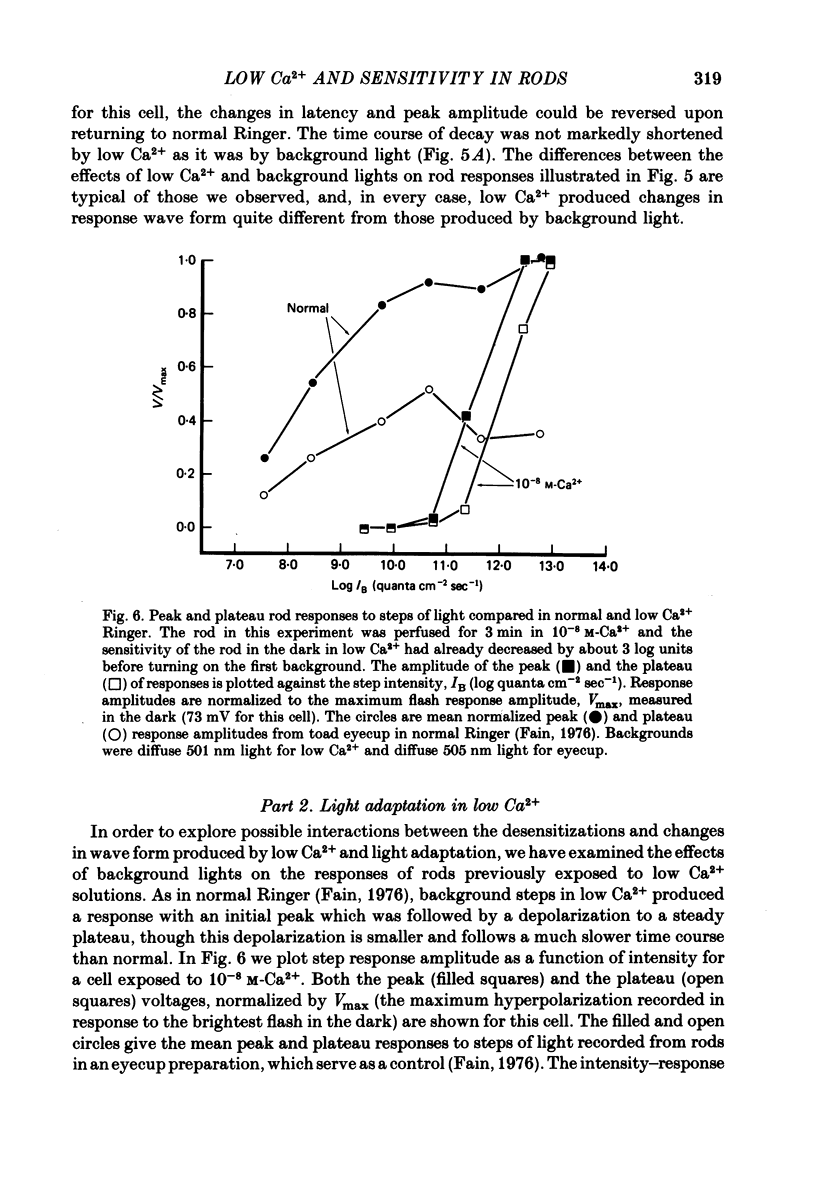
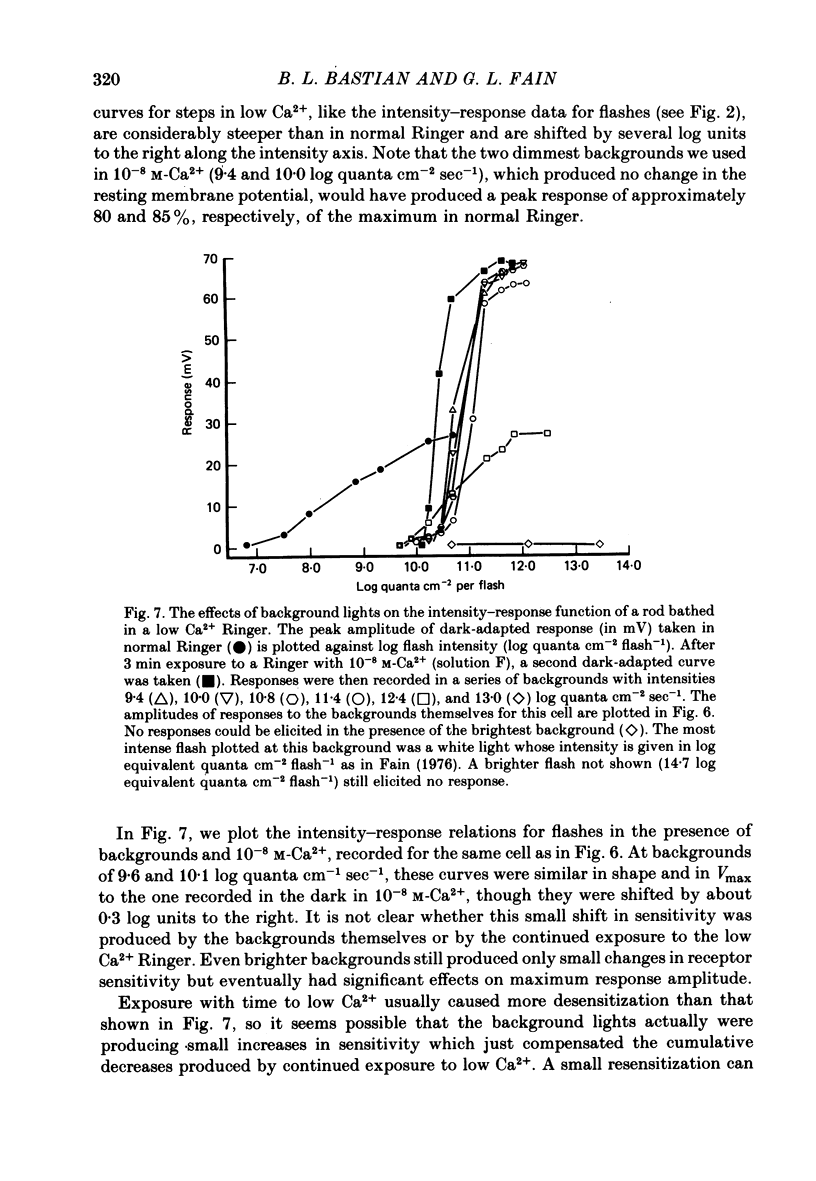
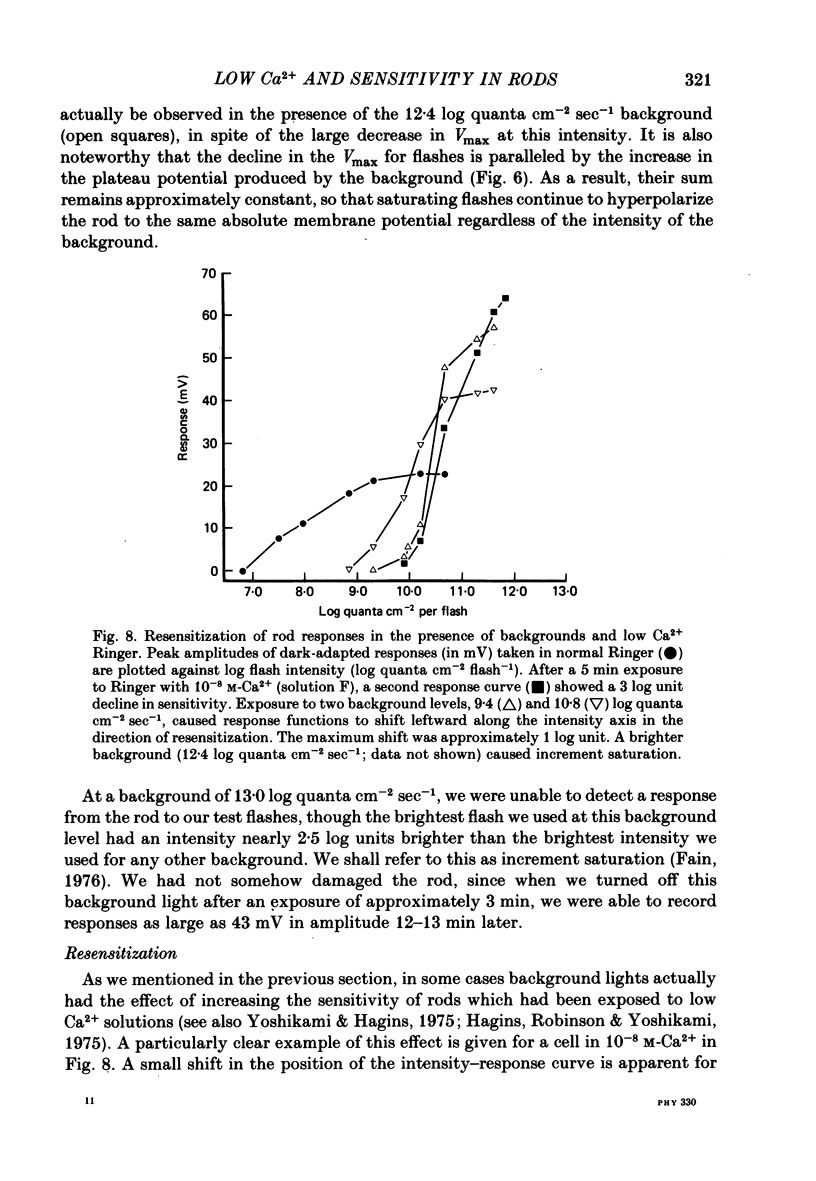
3. The sensitivity of the rod to maintained steps of light was also reduced in low Ca2+. Furthermore, the changes in sensitivity produced by background illumination were very much smaller in low Ca2+ than in normal Ringer. In some cases backgrounds actually increased sensitivity.
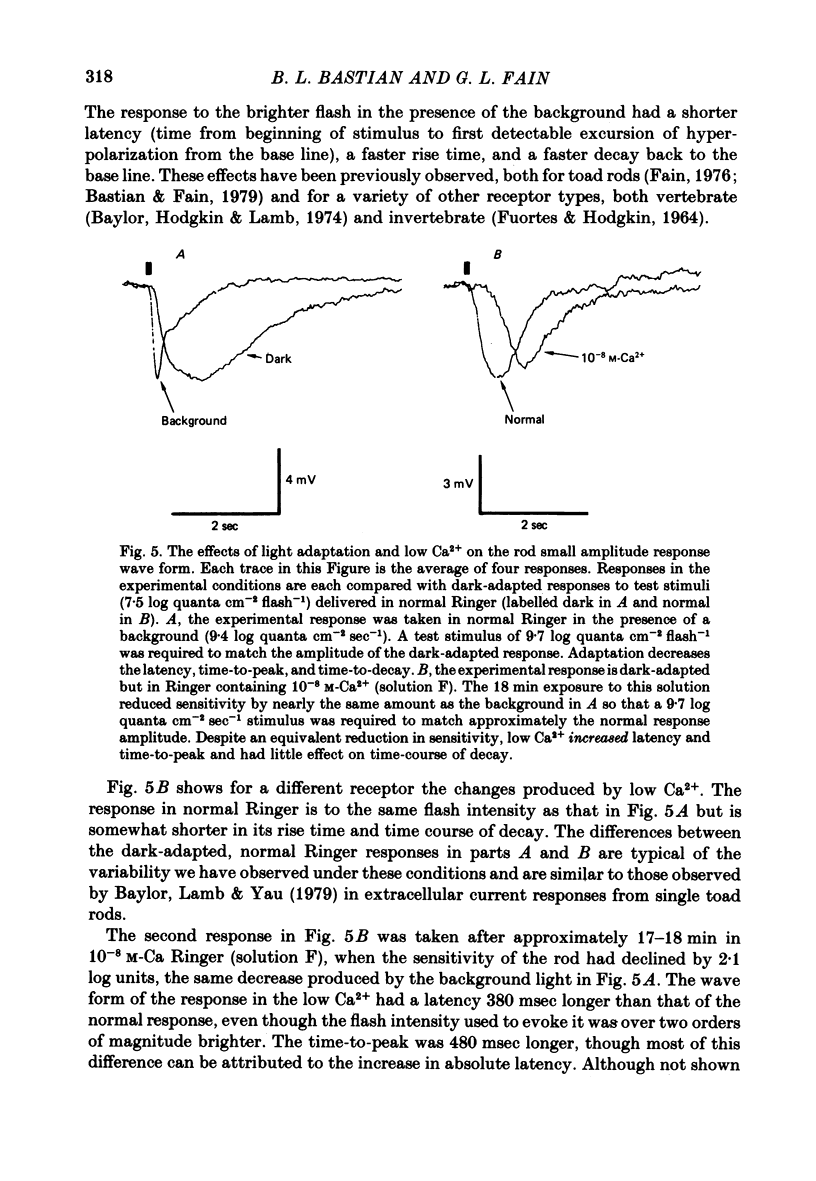
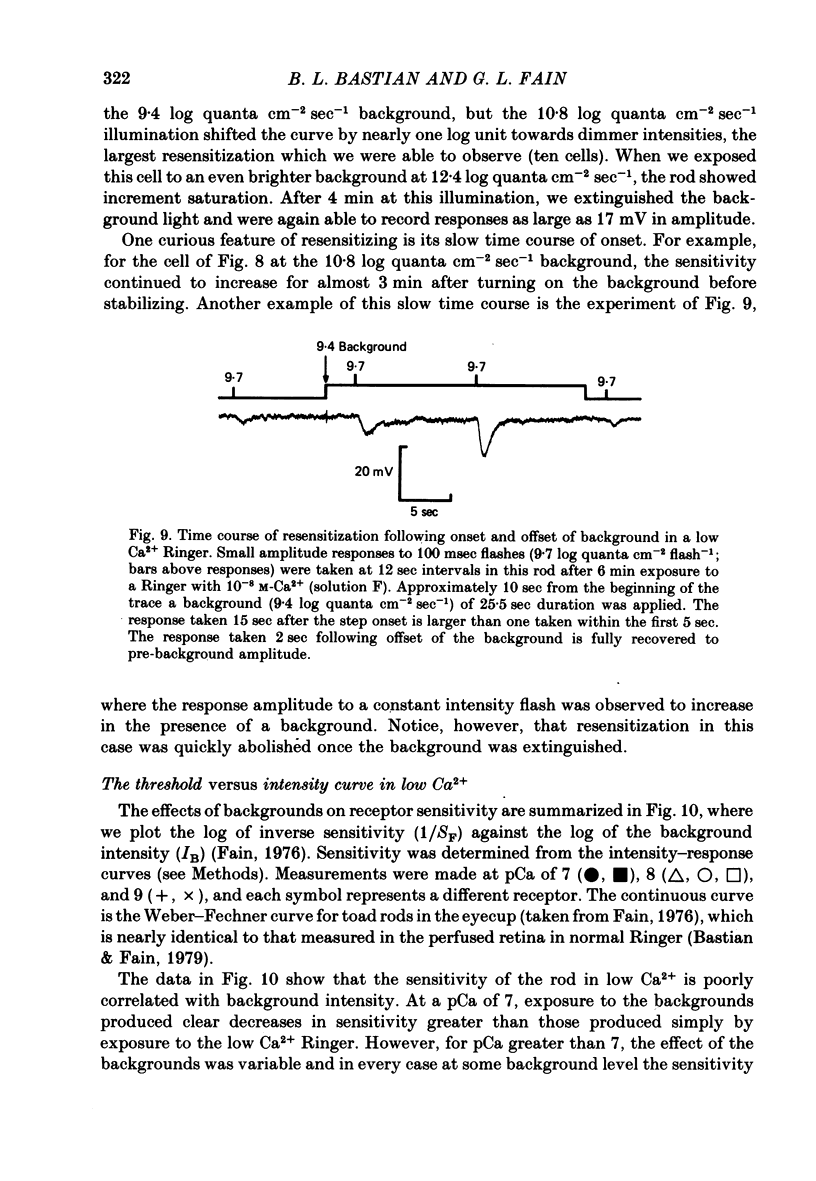
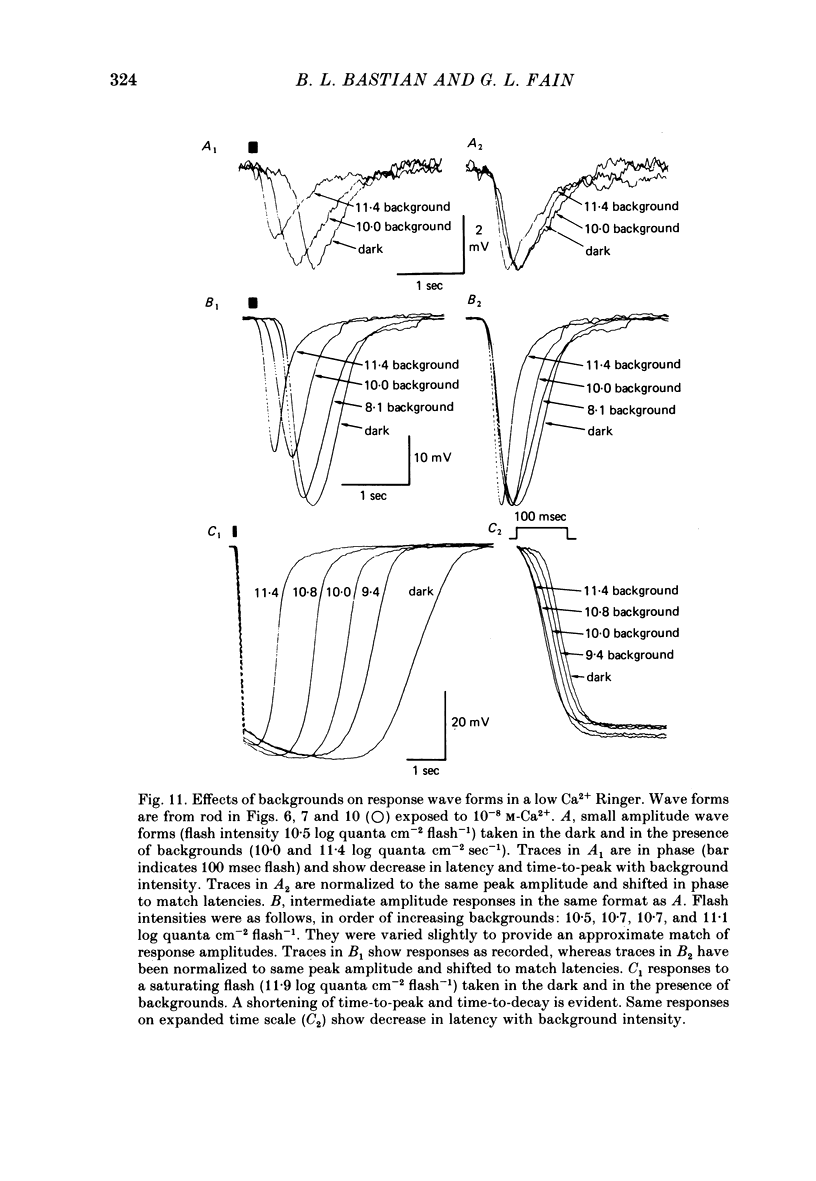
4. In 10-8 M-Ca2+, backgrounds which themselves produced no response in the rod and no changes in rod sensitivity produced large decreases in response latency for responses of all amplitudes, and pronounced changes in time-to-peak and time-to-decay for moderate and large amplitude responses.
5. Since the effects of background light and low Ca2+ on the wave form of the rod are distinct and in some cases antagonistic, and since the changes in receptor sensitivity produced by backgrounds and low Ca2+ are not additive, the decreases in sensitivity produced by exposure to low Ca2+ appear to be caused by a mechanism distinct from normal light adaptation. We suggest that they are caused by an increase in the buffering capacity of the receptor cytosol for Ca2+ and that Ca2+ is the excitatory messenger or `internal transmitter', as originally suggested by Yoshikami & Hagins (1971).
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F. Transport and metabolism of calcium ions in nerve. Prog Biophys Mol Biol. 1972;24:177–223. doi: 10.1016/0079-6107(72)90007-7. [DOI] [PubMed] [Google Scholar]
- Bastian B. L., Fain G. L. Light adaptation in toad rods: requirement for an internal messenger which is not calcium. J Physiol. 1979 Dec;297(0):493–520. doi: 10.1113/jphysiol.1979.sp013053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Changes in time scale and sensitivity in turtle photoreceptors. J Physiol. 1974 Nov;242(3):729–758. doi: 10.1113/jphysiol.1974.sp010732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L., Lamb T. D. Reconstruction of the electrical responses of turtle cones to flashes and steps of light. J Physiol. 1974 Nov;242(3):759–791. doi: 10.1113/jphysiol.1974.sp010733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Bertrand D., Fuortes M. G., Pochobradsky J. Actions of EGTA and high calcium on the cones in the turtle retina. J Physiol. 1978 Feb;275:419–437. doi: 10.1113/jphysiol.1978.sp012198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bownds M. D. Biochemical steps in visual transduction: roles for nucleotides and calcium ions. Photochem Photobiol. 1980 Oct;32(4):487–490. doi: 10.1111/j.1751-1097.1980.tb03792.x. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capovilla M., Cervetto L., Torre V. Effects of changing external potassium and chloride concentrations on the photoresponses of Bufo bufo rods. J Physiol. 1980 Oct;307:529–551. doi: 10.1113/jphysiol.1980.sp013452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles J. A., Yamane S. Effects of adapting lights on the time course of the receptor potential of the anuran retinal rod. J Physiol. 1975 May;247(1):189–207. doi: 10.1113/jphysiol.1975.sp010927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUORTES M. G., HODGKIN A. L. CHANGES IN TIME SCALE AND SENSITIVITY IN THE OMMATIDIA OF LIMULUS. J Physiol. 1964 Aug;172:239–263. doi: 10.1113/jphysiol.1964.sp007415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Gerschenfeld H. M., Quandt F. N. Calcium spikes in toad rods. J Physiol. 1980 Jun;303:495–513. doi: 10.1113/jphysiol.1980.sp013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Lisman J. E. Membrane conductances of photoreceptors. Prog Biophys Mol Biol. 1981;37(2):91–147. doi: 10.1016/0079-6107(82)90021-9. [DOI] [PubMed] [Google Scholar]
- Fain G. L. Quantum sensitivity of rods in the toad retina. Science. 1975 Mar 7;187(4179):838–841. doi: 10.1126/science.1114328. [DOI] [PubMed] [Google Scholar]
- Fain G. L. Sensitivity of toad rods: Dependence on wave-length and background illumination. J Physiol. 1976 Sep;261(1):71–101. doi: 10.1113/jphysiol.1976.sp011549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Robinson W. E., Yoshikami S. Ionic aspects of excitation in rod outer segments. Ciba Found Symp. 1975;(31):169–189. doi: 10.1002/9780470720134.ch10. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Yoshikami S. Proceedings: A role for Ca2+ in excitation of retinal rods and cones. Exp Eye Res. 1974 Mar;18(3):299–305. doi: 10.1016/0014-4835(74)90157-2. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt J., Dowling J. E. Intracellular recordings from gecko photoreceptors during light and dark adaptation. J Gen Physiol. 1975 Nov;66(5):617–648. doi: 10.1085/jgp.66.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton S. A., Ostroy S. E., Dowling J. E. Electrical and adaptive properties of rod photoreceptors in Bufo marinus. I. Effects of altered extracellular Ca2+ levels. J Gen Physiol. 1977 Dec;70(6):747–770. doi: 10.1085/jgp.70.6.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Brown J. E. The effects of intracellular iontophoretic injection of calcium and sodium ions on the light response of Limulus ventral photoreceptors. J Gen Physiol. 1972 Jun;59(6):701–719. doi: 10.1085/jgp.59.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton P. A., Yau K. W., Lamb T. D. Spread of activation and desensitisation in rod outer segments. Nature. 1980 Jan 3;283(5742):85–87. doi: 10.1038/283085a0. [DOI] [PubMed] [Google Scholar]
- Pinto L. H., Ostroy S. E. Ionizable groups and conductances of the rod photoreceptor membrane. J Gen Physiol. 1978 Mar;71(3):329–345. doi: 10.1085/jgp.71.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson P. R., Kawamura S., Abramson B., Bownds M. D. Control of the cyclic GMP phosphodiesterase of frog photoreceptor membranes. J Gen Physiol. 1980 Nov;76(5):631–645. doi: 10.1085/jgp.76.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnetkamp P. P. Calcium translocation and storage of isolated intact cattle rod outer segments in darkness. Biochim Biophys Acta. 1979 Jul 5;554(2):441–459. doi: 10.1016/0005-2736(79)90383-3. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P. P. Ion selectivity of the cation transport system of isolated intact cattle rod outer segments: evidence for a direct communication between the rod plasma membrane and the rod disk membranes. Biochim Biophys Acta. 1980 May 8;598(1):66–90. doi: 10.1016/0005-2736(80)90266-7. [DOI] [PubMed] [Google Scholar]
- Schröder W., Frings D., Stieve H. Measuring calcium uptake and release by invertebrate photoreceptor cells by laser microprobe mass spectroscopy. Scan Electron Microsc. 1980;(Pt 2):647-54, 606. [PubMed] [Google Scholar]
- Szuts E. Z. Calcium flux across disk membranes. Studies with intact rod photoreceptors and purified disks. J Gen Physiol. 1980 Sep;76(3):253–286. doi: 10.1085/jgp.76.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuts E. Z., Cone R. A. Calcium content of frog rod outer segments and discs. Biochim Biophys Acta. 1977 Jul 14;468(2):194–208. doi: 10.1016/0005-2736(77)90114-6. [DOI] [PubMed] [Google Scholar]
- Wormington C. M., Cone R. A. Ionic blockage of the light-regulated sodium channels in isolated rod outer segments. J Gen Physiol. 1978 Jun;71(6):657–681. doi: 10.1085/jgp.71.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane S. A possible model for the electrical responses of frog rods during light and dark adaptation. Biol Cybern. 1976 Aug 30;23(4):229–239. doi: 10.1007/BF00340339. [DOI] [PubMed] [Google Scholar]
- Yau K. W., McNaughton P. A., Hodgkin A. L. Effect of ions on the light-sensitive current in retinal rods. Nature. 1981 Aug 6;292(5823):502–505. doi: 10.1038/292502a0. [DOI] [PubMed] [Google Scholar]


