Abstract
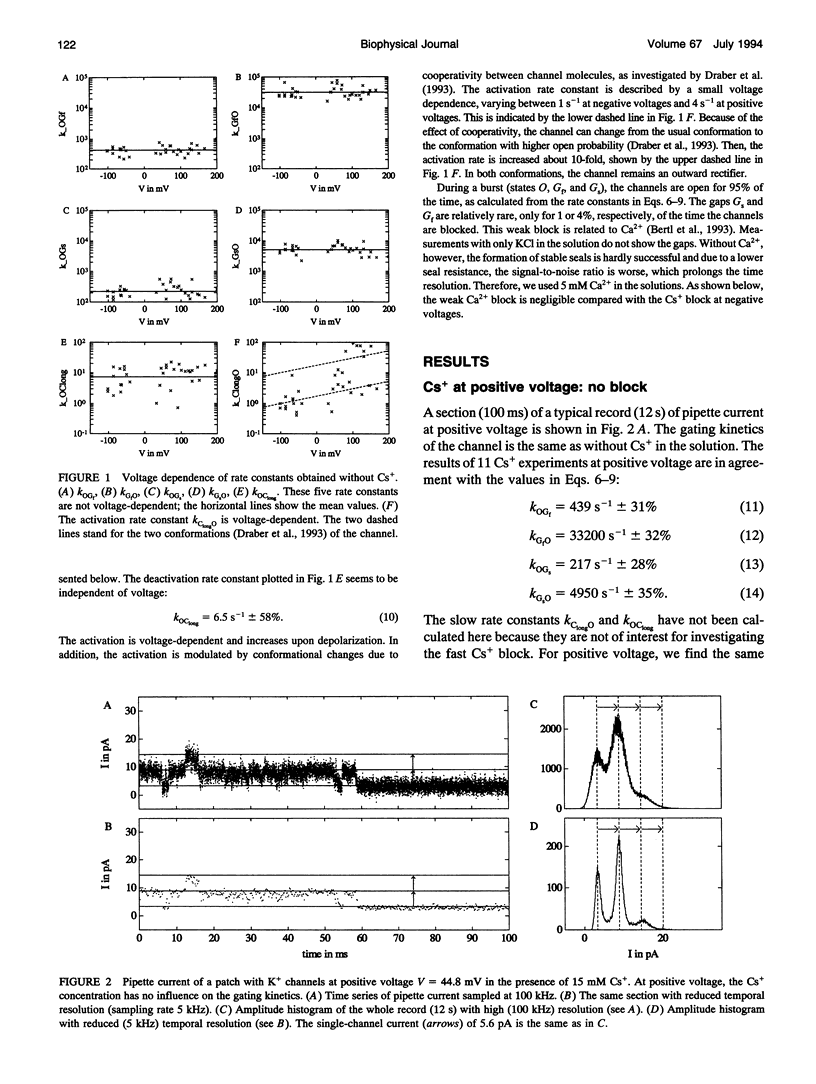
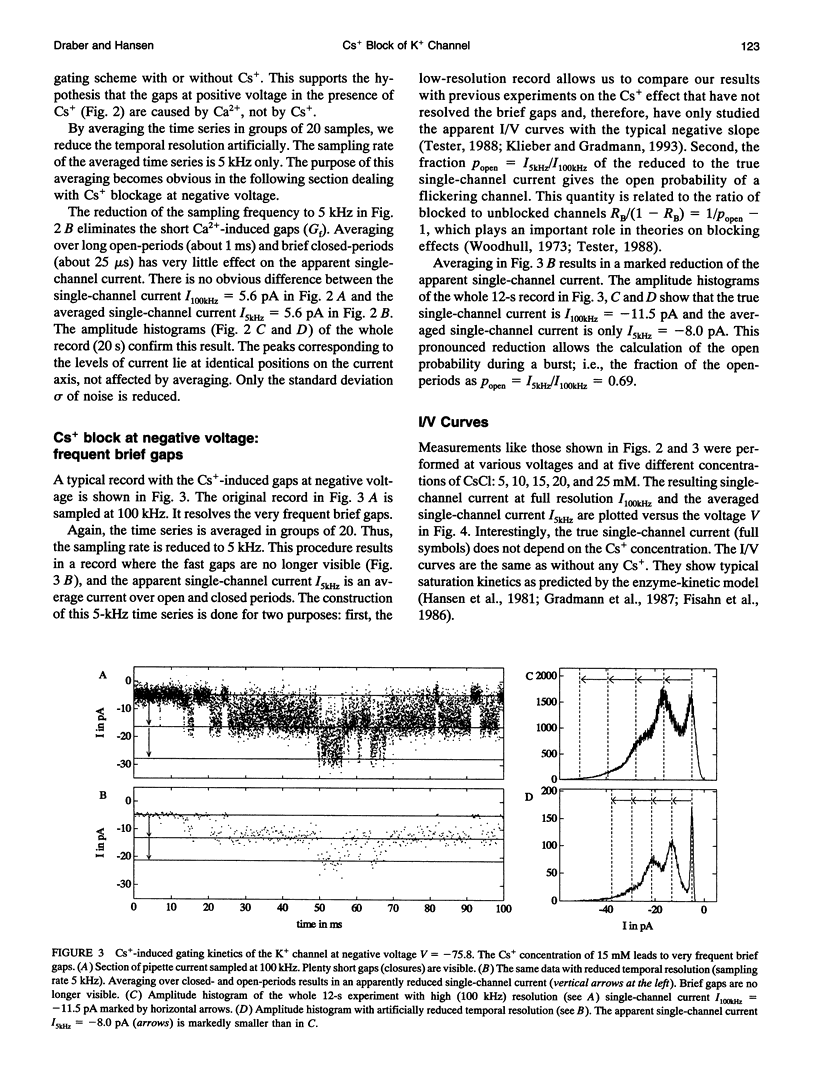
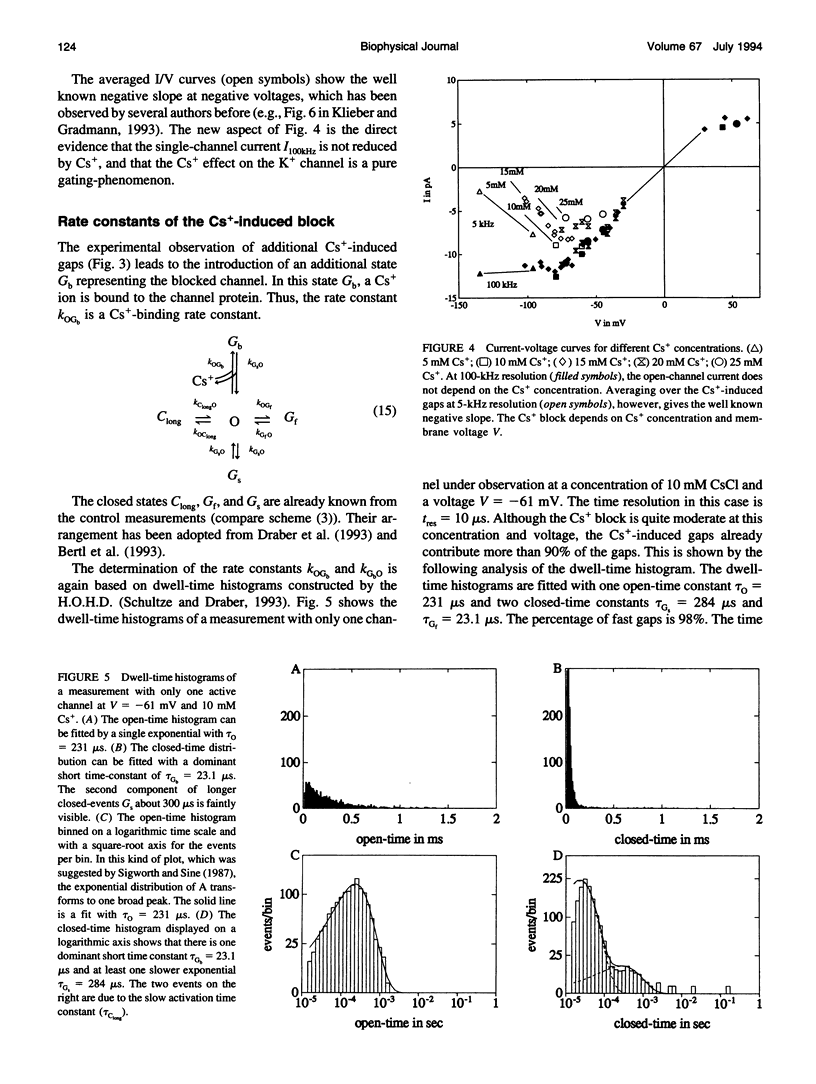
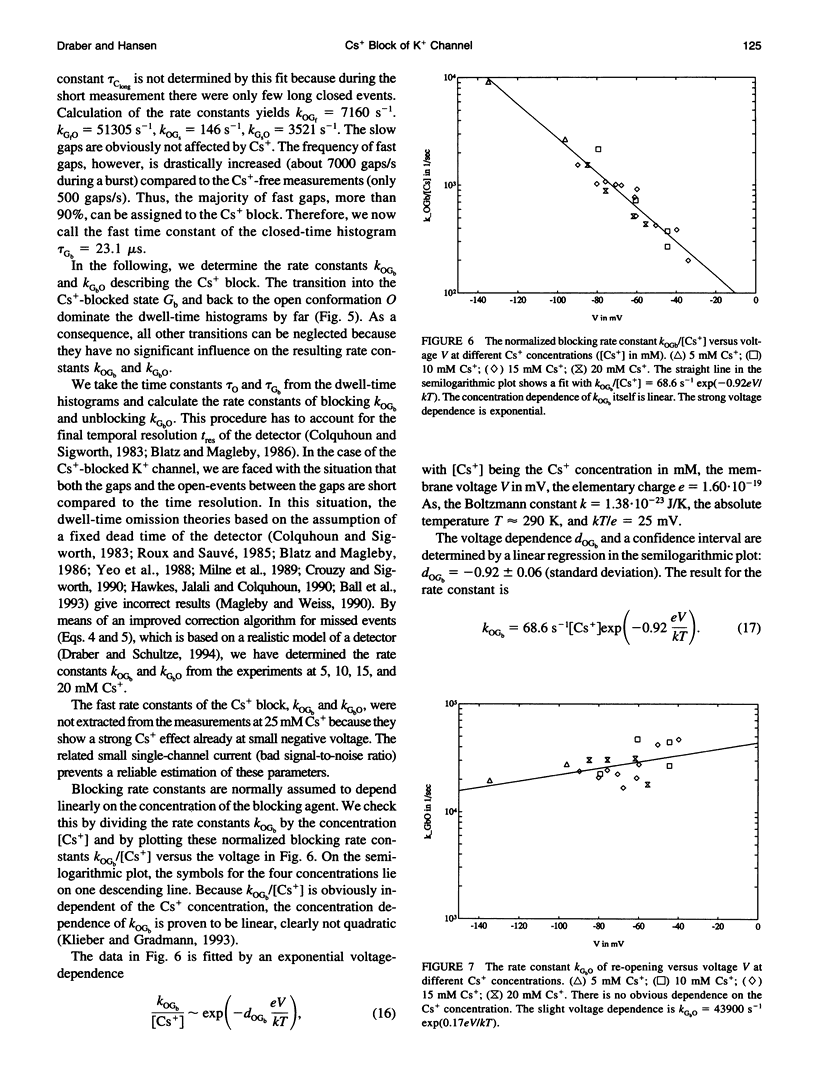
The Cs+ block of K+ channels has often been investigated by methods that allow only indirect estimation of the rate constants of blocking and re-opening. This paper presents single-channel records with high temporal resolution which make the direct observation of the fast transitions between the blocked and the unblocked state possible. The rate constants kOGb, kGbO of Cs(+)-dependent blocking and of re-opening are evaluated from the time constants found in the open-time and closed-time histograms. The blocking rate constant kOGb between 1000 and 50000 s-1 depends linearly on the Cs+ concentration and strongly on voltage, increasing by a factor of 1.44 per 10 mV hyperpolarization. The re-opening rate constant kGbO approximately 30000 s-1 is independent of Cs+ concentration and only slightly voltage-dependent. Formally, the results can be described by a Woodhull-model. The strong voltage dependence with d > 1, however, weakens its plausibility. The results are interpreted in terms of a molecular framework emerging from recent results on the structure of voltage-gated channels.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. A., Huprikar S. S., Kochian L. V., Lucas W. J., Gaber R. F. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Taylor S. R. Interaction of barium ions with potassium channels in squid giant axons. Biophys J. 1980 Jun;30(3):473–488. doi: 10.1016/S0006-3495(80)85108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball F. G., Yeo G. F., Milne R. K., Edeson R. O., Madsen B. W., Sansom M. S. Single ion channel models incorporating aggregation and time interval omission. Biophys J. 1993 Feb;64(2):357–374. doi: 10.1016/S0006-3495(93)81375-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertl A., Slayman C. L., Gradmann D. Gating and conductance in an outward-rectifying K+ channel from the plasma membrane of Saccharomyces cerevisiae. J Membr Biol. 1993 Mar;132(3):183–199. doi: 10.1007/BF00235737. [DOI] [PubMed] [Google Scholar]
- Blatz A. L., Magleby K. L. Correcting single channel data for missed events. Biophys J. 1986 May;49(5):967–980. doi: 10.1016/S0006-3495(86)83725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi X., Wolff D., Alvarez O., Latorre R. Mechanisms of Cs+ blockade in a Ca2+-activated K+ channel from smooth muscle. Biophys J. 1987 Nov;52(5):707–716. doi: 10.1016/S0006-3495(87)83265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzy S. C., Sigworth F. J. Yet another approach to the dwell-time omission problem of single-channel analysis. Biophys J. 1990 Sep;58(3):731–743. doi: 10.1016/S0006-3495(90)82416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi M., Drewe J. A., Kirsch G. E., Brown A. M. Histidine substitution identifies a surface position and confers Cs+ selectivity on a K+ pore. Biophys J. 1993 Sep;65(3):1235–1242. doi: 10.1016/S0006-3495(93)81154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demo S. D., Yellen G. Ion effects on gating of the Ca(2+)-activated K+ channel correlate with occupancy of the pore. Biophys J. 1992 Mar;61(3):639–648. doi: 10.1016/S0006-3495(92)81869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draber S., Schultze R. Correction for missed events based on a realistic model of a detector. Biophys J. 1994 Jan;66(1):191–201. doi: 10.1016/S0006-3495(94)80756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draber S., Schultze R., Hansen U. P. Cooperative behavior of K+ channels in the tonoplast of Chara corallina. Biophys J. 1993 Oct;65(4):1553–1559. doi: 10.1016/S0006-3495(93)81194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draber S., Schultze R., Hansen U. P. Patch-clamp studies on the anomalous mole fraction effect of the K+ channel in cytoplasmic droplets of Nitella: an attempt to distinguish between a multi-ion single-file pore and an enzyme kinetic model with lazy state. J Membr Biol. 1991 Aug;123(2):183–190. doi: 10.1007/BF01998088. [DOI] [PubMed] [Google Scholar]
- Durell S. R., Guy H. R. Atomic scale structure and functional models of voltage-gated potassium channels. Biophys J. 1992 Apr;62(1):238–250. doi: 10.1016/S0006-3495(92)81809-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
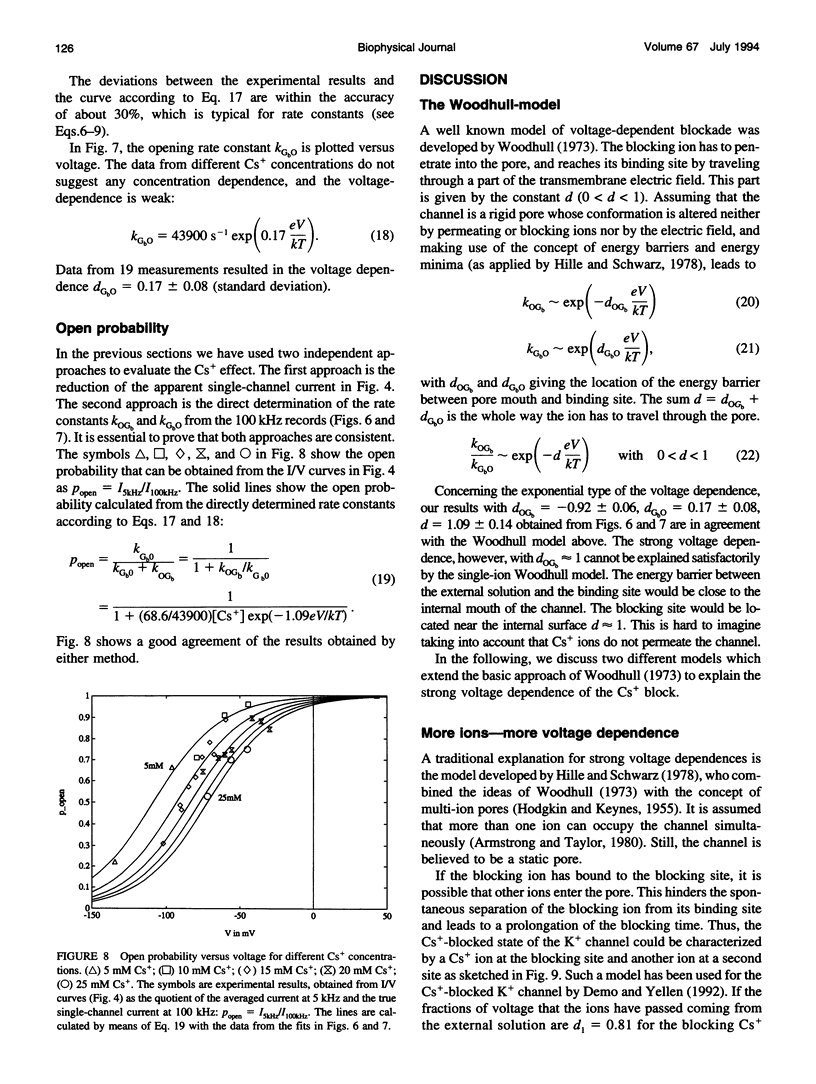
- Goldstein S. A., Miller C. A point mutation in a Shaker K+ channel changes its charybdotoxin binding site from low to high affinity. Biophys J. 1992 Apr;62(1):5–7. doi: 10.1016/S0006-3495(92)81760-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S. A., Miller C. Mechanism of charybdotoxin block of a voltage-gated K+ channel. Biophys J. 1993 Oct;65(4):1613–1619. doi: 10.1016/S0006-3495(93)81200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Woolum J. C., Cornwall M. C. Selectivity of the Ca2+-activated and light-dependent K+ channels for monovalent cations. Biophys J. 1982 Jun;38(3):319–322. doi: 10.1016/S0006-3495(82)84565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradmann D., Klieber H. G., Hansen U. P. Reaction kinetic parameters for ion transport from steady-state current-voltage curves. Biophys J. 1987 Apr;51(4):569–585. doi: 10.1016/S0006-3495(87)83382-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. The potassium permeability of a giant nerve fibre. J Physiol. 1955 Apr 28;128(1):61–88. doi: 10.1113/jphysiol.1955.sp005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen U. P., Gradmann D., Sanders D., Slayman C. L. Interpretation of current-voltage relationships for "active" ion transport systems: I. Steady-state reaction-kinetic analysis of class-I mechanisms. J Membr Biol. 1981;63(3):165–190. doi: 10.1007/BF01870979. [DOI] [PubMed] [Google Scholar]
- Heinemann S. H., Sigworth F. J. Open channel noise. IV. Estimation of rapid kinetics of formamide block in gramicidin A channels. Biophys J. 1988 Oct;54(4):757–764. doi: 10.1016/S0006-3495(88)83013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Structural elements involved in specific K+ channel functions. Annu Rev Physiol. 1992;54:537–555. doi: 10.1146/annurev.ph.54.030192.002541. [DOI] [PubMed] [Google Scholar]
- Kirsch G. E., Drewe J. A., Taglialatela M., Joho R. H., DeBiasi M., Hartmann H. A., Brown A. M. A single nonpolar residue in the deep pore of related K+ channels acts as a K+:Rb+ conductance switch. Biophys J. 1992 Apr;62(1):136–144. doi: 10.1016/S0006-3495(92)81800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klieber H. G., Gradmann D. Enzyme kinetics of the prime K+ channel in the tonoplast of Chara: selectivity and inhibition. J Membr Biol. 1993 Mar;132(3):253–265. doi: 10.1007/BF00235742. [DOI] [PubMed] [Google Scholar]
- Laver D. R. Divalent cation block and competition between divalent and monovalent cations in the large-conductance K+ channel from Chara australis. J Gen Physiol. 1992 Aug;100(2):269–300. doi: 10.1085/jgp.100.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991 Mar 21;350(6315):232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- MacKinnon R., Miller C. Mutant potassium channels with altered binding of charybdotoxin, a pore-blocking peptide inhibitor. Science. 1989 Sep 22;245(4924):1382–1385. doi: 10.1126/science.2476850. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Weiss D. S. Estimating kinetic parameters for single channels with simulation. A general method that resolves the missed event problem and accounts for noise. Biophys J. 1990 Dec;58(6):1411–1426. doi: 10.1016/S0006-3495(90)82487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne R. K., Yeo G. F., Madsen B. W., Edeson R. O. Estimation of single channel kinetic parameters from data subject to limited time resolution. Biophys J. 1989 Apr;55(4):673–676. doi: 10.1016/S0006-3495(89)82865-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C. S., Miller C. Mapping function to structure in a channel-blocking peptide: electrostatic mutants of charybdotoxin. Biochemistry. 1992 Sep 1;31(34):7749–7755. doi: 10.1021/bi00149a002. [DOI] [PubMed] [Google Scholar]
- Roux B., Sauvé R. A general solution to the time interval omission problem applied to single channel analysis. Biophys J. 1985 Jul;48(1):149–158. doi: 10.1016/S0006-3495(85)83768-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze R., Draber S. A nonlinear filter algorithm for the detection of jumps in patch-clamp data. J Membr Biol. 1993 Feb;132(1):41–52. doi: 10.1007/BF00233050. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J., Sine S. M. Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J. 1987 Dec;52(6):1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T., Takeshima H., Mikami A., Flockerzi V., Takahashi H., Kangawa K., Kojima M., Matsuo H., Hirose T., Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987 Jul 23;328(6128):313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Woodhull A. M. Ionic blockage of sodium channels in nerve. J Gen Physiol. 1973 Jun;61(6):687–708. doi: 10.1085/jgp.61.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. J Gen Physiol. 1984 Aug;84(2):157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo G. F., Milne R. K., Edeson R. O., Madsen B. W. Statistical inference from single channel records: two-state Markov model with limited time resolution. Proc R Soc Lond B Biol Sci. 1988 Oct 22;235(1278):63–94. doi: 10.1098/rspb.1988.0063. [DOI] [PubMed] [Google Scholar]


