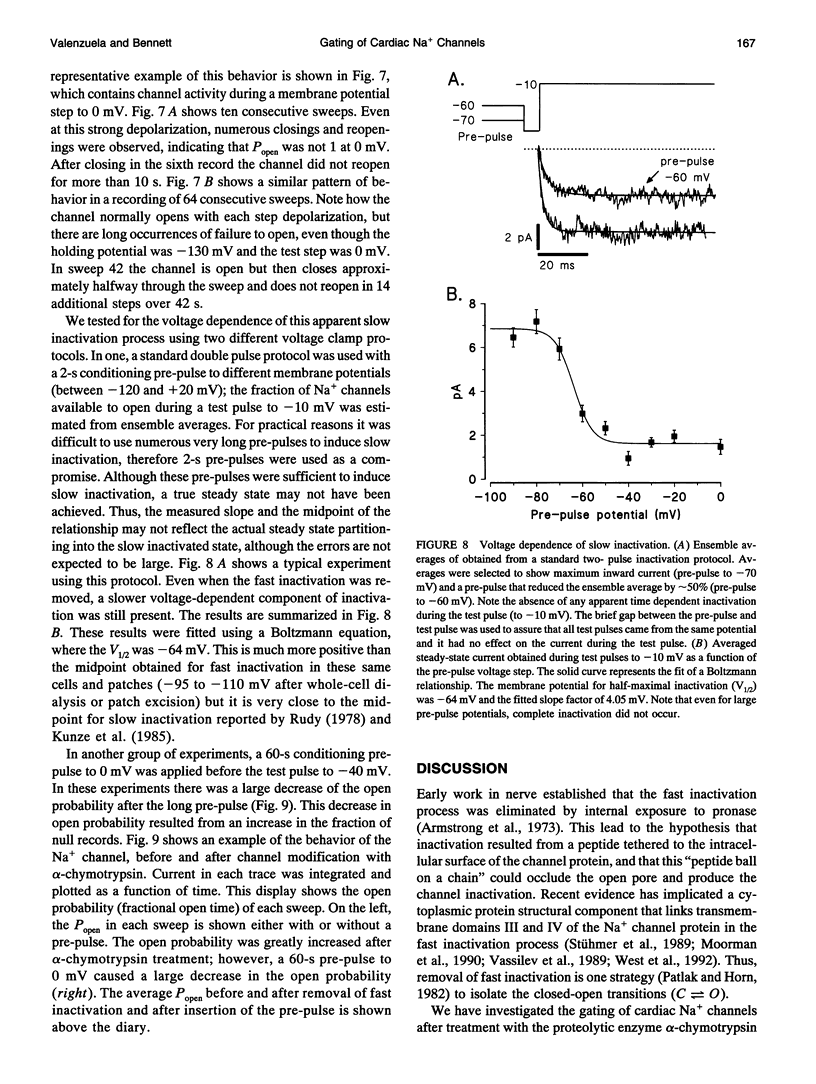
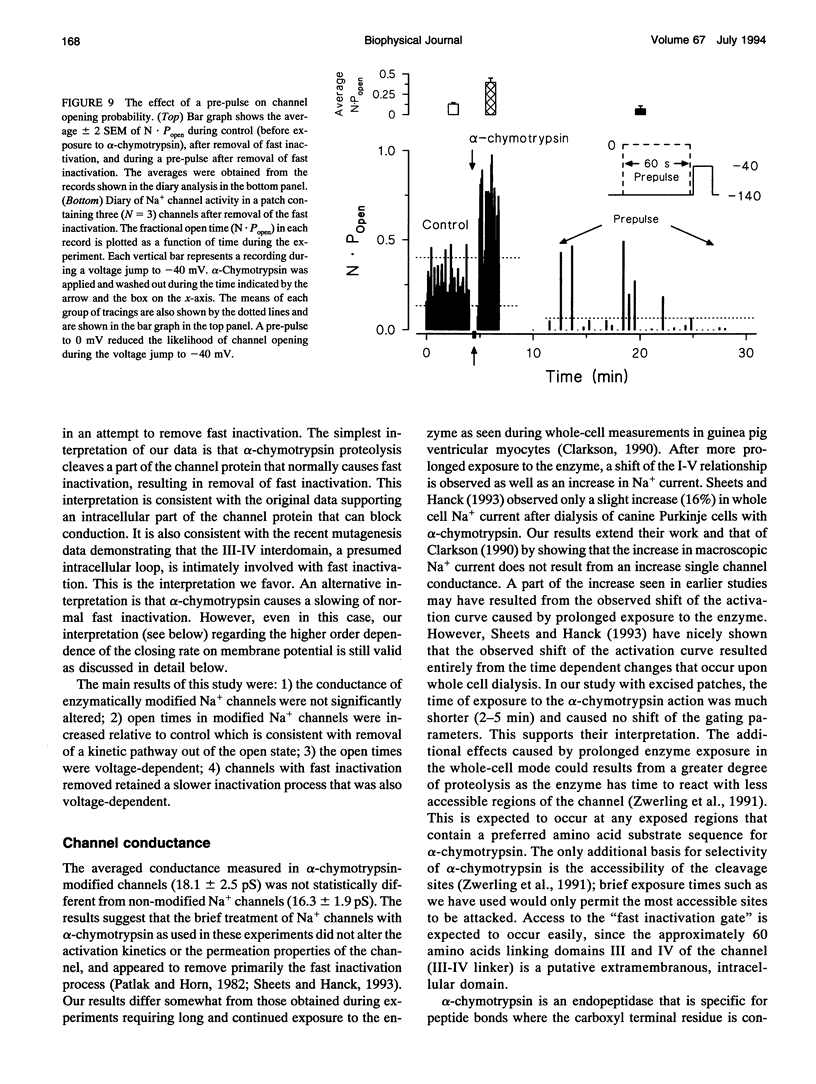
Abstract
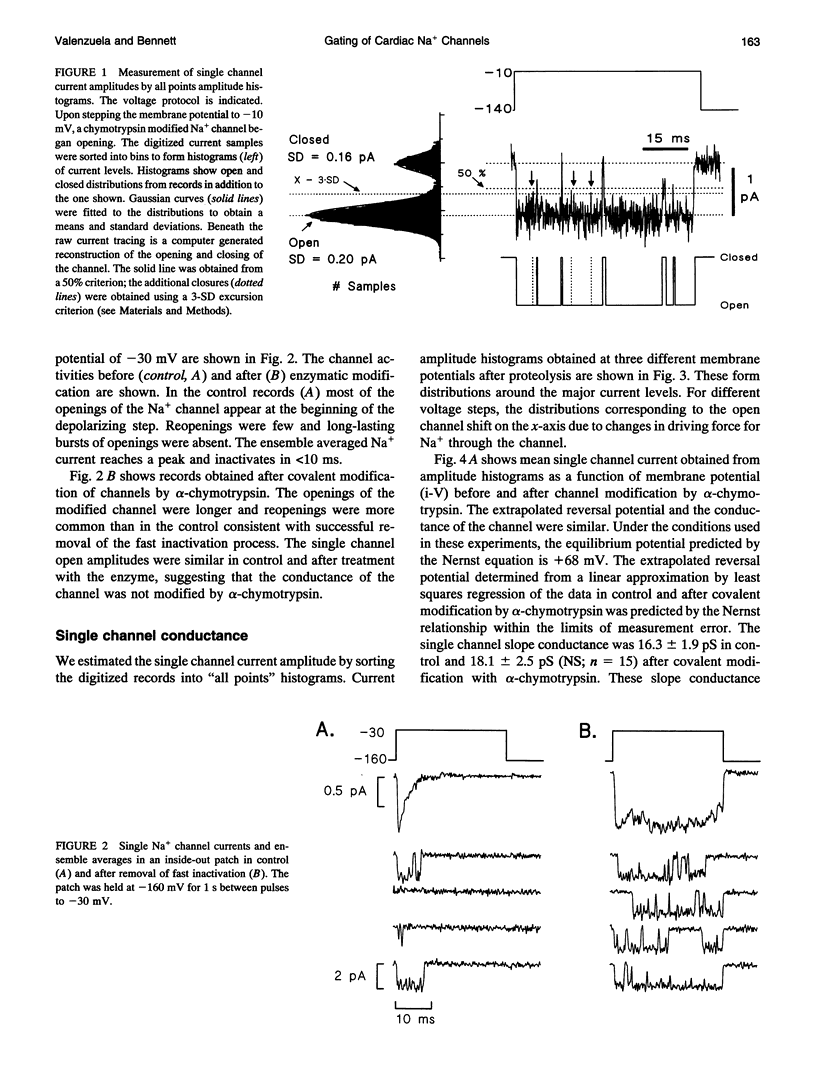
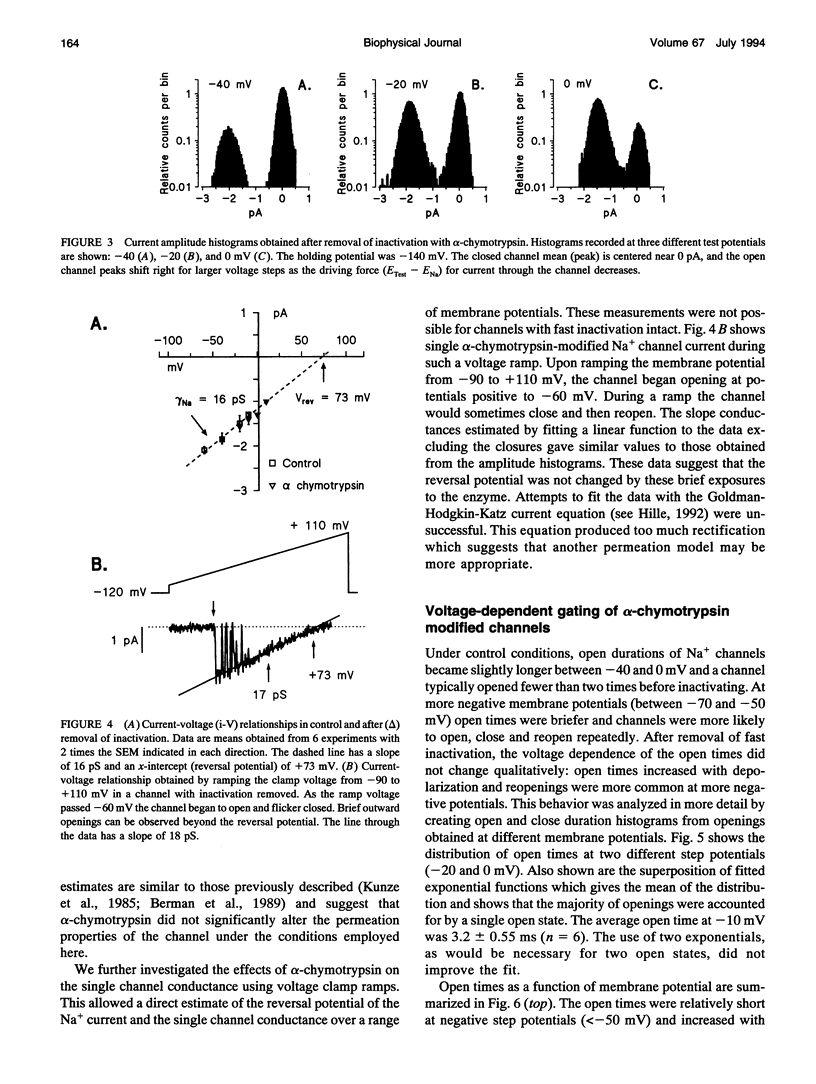
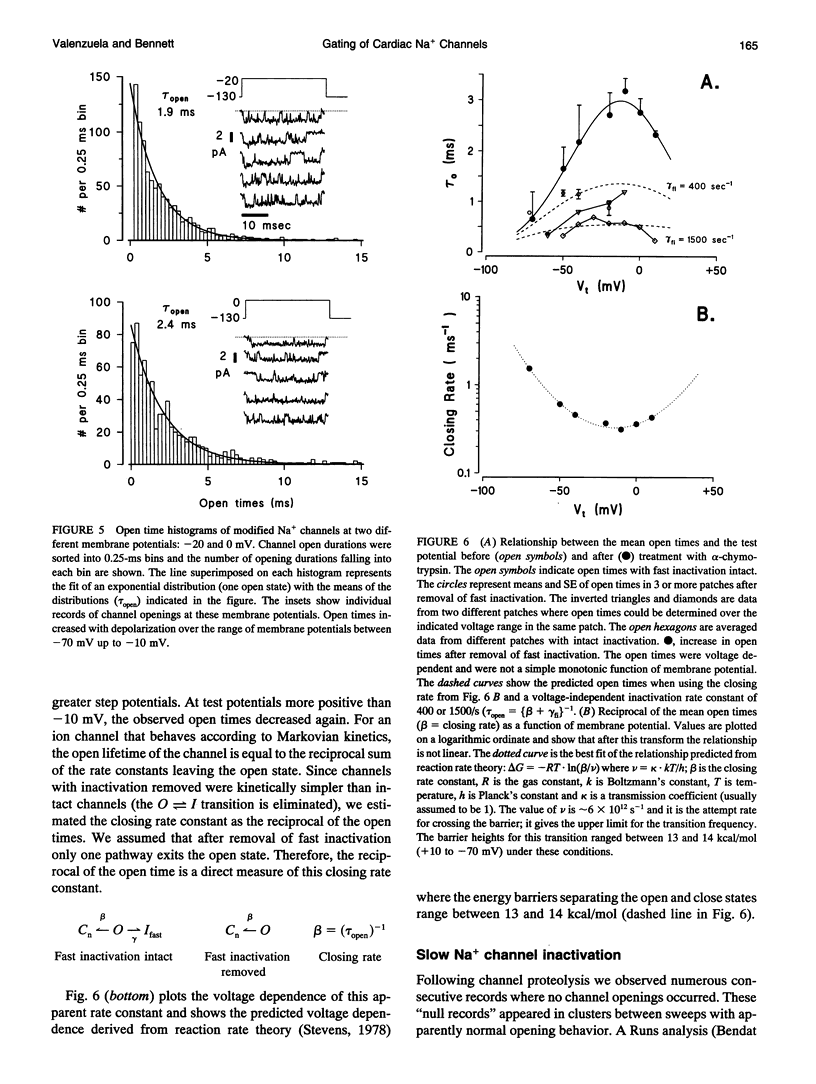
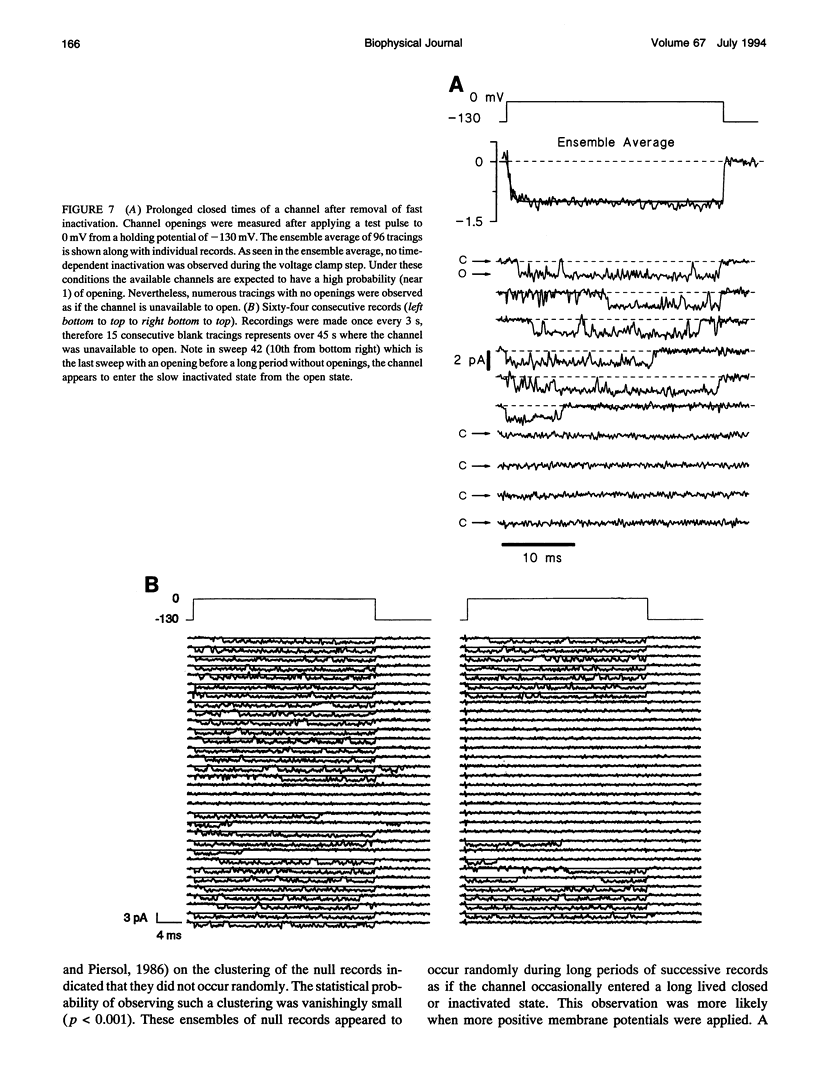
Single cardiac Na+ channels were investigated after intracellular proteolysis to remove the fast inactivation process in an attempt to elucidate the mechanisms of channel gating and the role of slow inactivation. Na+ channels were studied in inside-out patches excised from guinea-pig ventricular myocytes both before and after very brief exposure (2-4 min) to the endopeptidase, alpha-chymotrypsin. Enzyme exposure times were chosen to maximize removal of fast inactivation and to minimize potential nonspecific damage to the channel. After proteolysis, the single channel current-voltage relationship was approximately linear with a slope conductance of 18 +/- 2.5 pS. Na+ channel reversal potentials measured before and after proteolysis by alpha-chymotrypsin were not changed. The unitary current amplitude was not altered after channel modification suggesting little or no effect on channel conductance. Channel open times were increased after removal of fast inactivation and were voltage-dependent, ranging between 0.7 (-70 mV) and 3.2 (-10 mV) ms. Open times increased with membrane potential reaching a maximum at -10 mV; at more positive membrane potentials, open times decreased again. Fast inactivation appeared to be completely removed by alpha-chymotrypsin and slow inactivation became more apparent suggesting that fast and slow inactivation normally compete, and that fast inactivation dominates in unmodified channels. This finding is not consistent with a slow inactivated state that can only be entered through the fast inactivated state, since removal of fast inactivation does not eliminate slow inactivation. The data indicate that cardiac Na+ channels can enter the slow inactivated state by a pathway that bypasses the fast inactivated state and that the likelihood of entering the slow inactivated state increases after removal of fast inactivation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich R. W., Corey D. P., Stevens C. F. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983 Dec 1;306(5942):436–441. doi: 10.1038/306436a0. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Bezanilla F., Rojas E. Destruction of sodium conductance inactivation in squid axons perfused with pronase. J Gen Physiol. 1973 Oct;62(4):375–391. doi: 10.1085/jgp.62.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Sodium channel inactivation in the crayfish giant axon. Must channels open before inactivating? Biophys J. 1981 Sep;35(3):595–614. doi: 10.1016/S0006-3495(81)84815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M. F., Camardo J. S., Robinson R. B., Siegelbaum S. A. Single sodium channels from canine ventricular myocytes: voltage dependence and relative rates of activation and inactivation. J Physiol. 1989 Aug;415:503–531. doi: 10.1113/jphysiol.1989.sp017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Lee K. S., Powell T. Sodium current in single rat heart muscle cells. J Physiol. 1981 Sep;318:479–500. doi: 10.1113/jphysiol.1981.sp013879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A. Molecular properties of voltage-sensitive sodium channels. Annu Rev Biochem. 1986;55:953–985. doi: 10.1146/annurev.bi.55.070186.004513. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-sensitive ion channels. Science. 1988 Oct 7;242(4875):50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- Clarkson C. W. Modification of Na channel inactivation by alpha-chymotrypsin in single cardiac myocytes. Pflugers Arch. 1990 Sep;417(1):48–57. doi: 10.1007/BF00370768. [DOI] [PubMed] [Google Scholar]
- Cota G., Armstrong C. M. Sodium channel gating in clonal pituitary cells. The inactivation step is not voltage dependent. J Gen Physiol. 1989 Aug;94(2):213–232. doi: 10.1085/jgp.94.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer B. B., Mancina M., Williams E. S., Watanabe A. M. Isolation of calcium tolerant myocytes from adult rat hearts: review of the literature and description of a method. Life Sci. 1983 Jul 4;33(1):1–18. doi: 10.1016/0024-3205(83)90706-3. [DOI] [PubMed] [Google Scholar]
- Fozzard H. A., January C. T., Makielski J. C. New studies of the excitatory sodium currents in heart muscle. Circ Res. 1985 Apr;56(4):475–485. doi: 10.1161/01.res.56.4.475. [DOI] [PubMed] [Google Scholar]
- Gillespie J. I., Meves H. The time course of sodium inactivation in squid giant axons. J Physiol. 1980 Feb;299:289–307. doi: 10.1113/jphysiol.1980.sp013125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gintant G. A., Datyner N. B., Cohen I. S. Slow inactivation of a tetrodotoxin-sensitive current in canine cardiac Purkinje fibers. Biophys J. 1984 Mar;45(3):509–512. doi: 10.1016/S0006-3495(84)84187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman L., Schauf C. L. Inactivation of the sodium current in Myxicola giant axons. Evidence for coupling to the activation process. J Gen Physiol. 1972 Jun;59(6):659–675. doi: 10.1085/jgp.59.6.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonoi T., Hille B. Gating of Na channels. Inactivation modifiers discriminate among models. J Gen Physiol. 1987 Feb;89(2):253–274. doi: 10.1085/jgp.89.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A. O., Starmer C. F. Mechanisms of closure of cardiac sodium channels in rabbit ventricular myocytes: single-channel analysis. Circ Res. 1987 Jun;60(6):897–913. doi: 10.1161/01.res.60.6.897. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klockner U. Calcium tolerant ventricular myocytes prepared by preincubation in a "KB medium". Pflugers Arch. 1982 Oct;395(1):6–18. doi: 10.1007/BF00584963. [DOI] [PubMed] [Google Scholar]
- Kirsch G. E., Brown A. M. Kinetic properties of single sodium channels in rat heart and rat brain. J Gen Physiol. 1989 Jan;93(1):85–99. doi: 10.1085/jgp.93.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze D. L., Lacerda A. E., Wilson D. L., Brown A. M. Cardiac Na currents and the inactivating, reopening, and waiting properties of single cardiac Na channels. J Gen Physiol. 1985 Nov;86(5):691–719. doi: 10.1085/jgp.86.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. H., Yue D. T., Rose W. C., Marban E. Sodium channel inactivation from resting states in guinea-pig ventricular myocytes. J Physiol. 1991 Nov;443:629–650. doi: 10.1113/jphysiol.1991.sp018855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman J. R., Kirsch G. E., Brown A. M., Joho R. H. Changes in sodium channel gating produced by point mutations in a cytoplasmic linker. Science. 1990 Nov 2;250(4981):688–691. doi: 10.1126/science.2173138. [DOI] [PubMed] [Google Scholar]
- Nilius B. Modal gating behavior of cardiac sodium channels in cell-free membrane patches. Biophys J. 1988 Jun;53(6):857–862. doi: 10.1016/S0006-3495(88)83166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Ikeda T., Kayano T., Suzuki H., Takeshima H., Kurasaki M., Takahashi H., Numa S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature. 1986 Mar 13;320(6058):188–192. doi: 10.1038/320188a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Shimizu S., Tanabe T., Takai T., Kayano T., Ikeda T., Takahashi H., Nakayama H., Kanaoka Y., Minamino N. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984 Nov 8;312(5990):121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- Oxford G. S. Some kinetic and steady-state properties of sodium channels after removal of inactivation. J Gen Physiol. 1981 Jan;77(1):1–22. doi: 10.1085/jgp.77.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J. B., Ortiz M. Slow currents through single sodium channels of the adult rat heart. J Gen Physiol. 1985 Jul;86(1):89–104. doi: 10.1085/jgp.86.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak J., Horn R. Effect of N-bromoacetamide on single sodium channel currents in excised membrane patches. J Gen Physiol. 1982 Mar;79(3):333–351. doi: 10.1085/jgp.79.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quandt F. N. Burst kinetics of sodium channels which lack fast inactivation in mouse neuroblastoma cells. J Physiol. 1987 Nov;392:563–585. doi: 10.1113/jphysiol.1987.sp016797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogart R. B., Cribbs L. L., Muglia L. K., Kephart D. D., Kaiser M. W. Molecular cloning of a putative tetrodotoxin-resistant rat heart Na+ channel isoform. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8170–8174. doi: 10.1073/pnas.86.20.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Inactivation in Myxicola giant axons responsible for slow and accumulative adaptation phenomena. J Physiol. 1981 Mar;312:531–549. doi: 10.1113/jphysiol.1981.sp013642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Slow inactivation of the sodium conductance in squid giant axons. Pronase resistance. J Physiol. 1978 Oct;283:1–21. doi: 10.1113/jphysiol.1978.sp012485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikawa T., Carmeliet E. Slow recovery of the maximal rate of rise (Vmax) of the action potential in sheep cardiac Purkinje fibers. Pflugers Arch. 1982 Jul;394(1):90–93. doi: 10.1007/BF01108313. [DOI] [PubMed] [Google Scholar]
- Scanley B. E., Hanck D. A., Chay T., Fozzard H. A. Kinetic analysis of single sodium channels from canine cardiac Purkinje cells. J Gen Physiol. 1990 Mar;95(3):411–437. doi: 10.1085/jgp.95.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets M. F., Hanck D. A. Modification of sodium channel inactivation by alpha-chymotrypsin in canine cardiac Purkinje cells. J Cardiovasc Electrophysiol. 1993 Dec;4(6):686–694. doi: 10.1111/j.1540-8167.1993.tb01254.x. [DOI] [PubMed] [Google Scholar]
- Stevens C. F. Interactions between intrinsic membrane protein and electric field. An approach to studying nerve excitability. Biophys J. 1978 May;22(2):295–306. doi: 10.1016/S0006-3495(78)85490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stühmer W., Conti F., Suzuki H., Wang X. D., Noda M., Yahagi N., Kubo H., Numa S. Structural parts involved in activation and inactivation of the sodium channel. Nature. 1989 Jun 22;339(6226):597–603. doi: 10.1038/339597a0. [DOI] [PubMed] [Google Scholar]
- Valenzuela C., Bennett P. B. Voltage- and use-dependent modulation of calcium channel current in guinea pig ventricular cells by amiodarone and des-oxo-amiodarone. J Cardiovasc Pharmacol. 1991 Jun;17(6):894–902. doi: 10.1097/00005344-199106000-00006. [DOI] [PubMed] [Google Scholar]
- Vandenberg C. A., Horn R. Inactivation viewed through single sodium channels. J Gen Physiol. 1984 Oct;84(4):535–564. doi: 10.1085/jgp.84.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilev P., Scheuer T., Catterall W. A. Inhibition of inactivation of single sodium channels by a site-directed antibody. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8147–8151. doi: 10.1073/pnas.86.20.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J. W., Patton D. E., Scheuer T., Wang Y., Goldin A. L., Catterall W. A. A cluster of hydrophobic amino acid residues required for fast Na(+)-channel inactivation. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10910–10914. doi: 10.1073/pnas.89.22.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D. T., Lawrence J. H., Marban E. Two molecular transitions influence cardiac sodium channel gating. Science. 1989 Apr 21;244(4902):349–352. doi: 10.1126/science.2540529. [DOI] [PubMed] [Google Scholar]
- Zagotta W. N., Aldrich R. W. Voltage-dependent gating of Shaker A-type potassium channels in Drosophila muscle. J Gen Physiol. 1990 Jan;95(1):29–60. doi: 10.1085/jgp.95.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter Y. I., Motin L. G. Existence of two fast inactivation states in cardiac Na channels confirmed by two-stage action of proteolytic enzymes. Biochim Biophys Acta. 1991 Sep 10;1068(1):77–80. doi: 10.1016/0005-2736(91)90063-e. [DOI] [PubMed] [Google Scholar]
- Zwerling S. J., Cohen S. A., Barchi R. L. Analysis of protease-sensitive regions in the skeletal muscle sodium channel in vitro and implications for channel tertiary structure. J Biol Chem. 1991 Mar 5;266(7):4574–4580. [PubMed] [Google Scholar]


